Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report
Abstract
1. Introduction
2. Case Description
2.1. Patient #1
2.2. Patient #2
3. Results
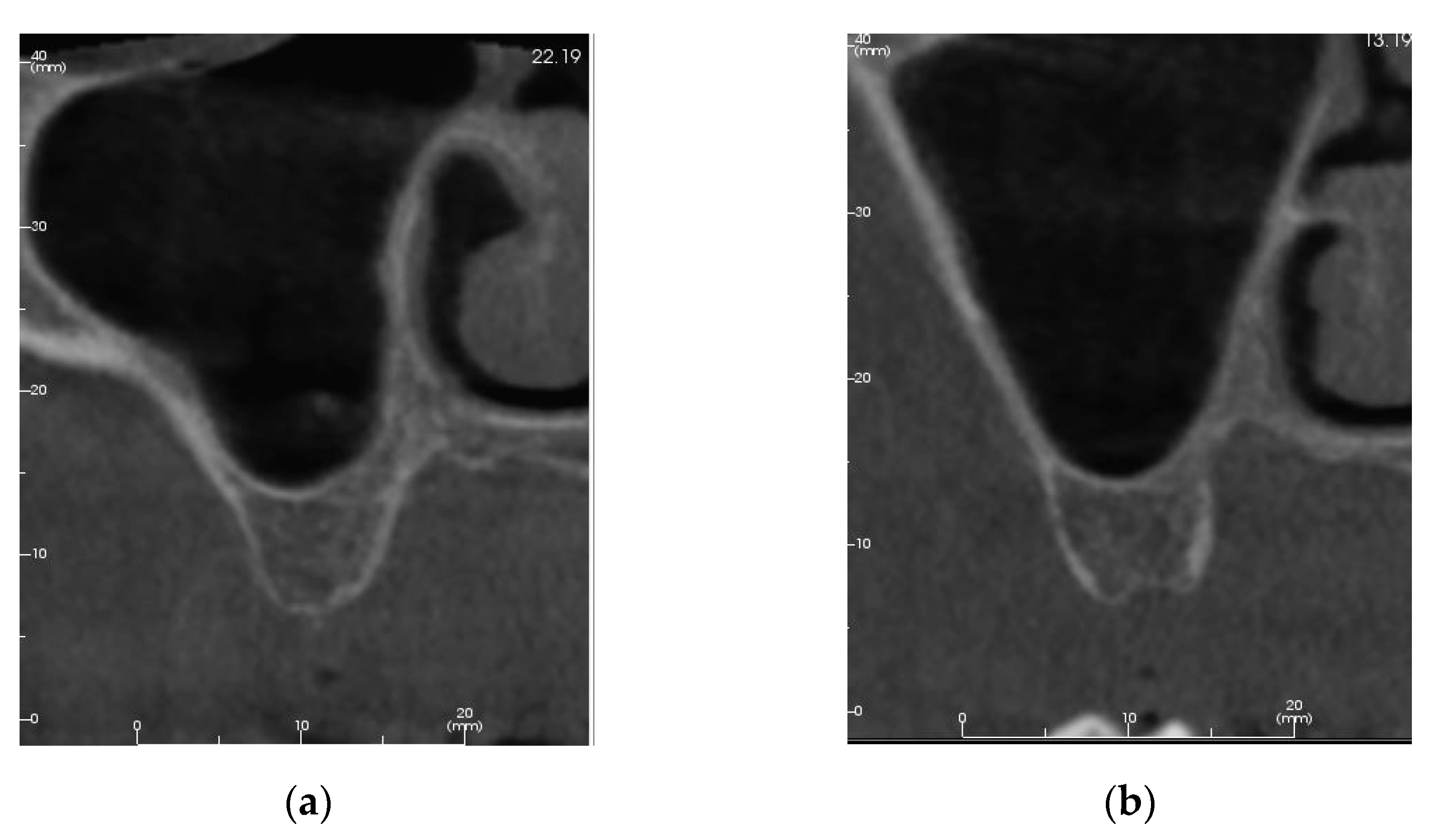
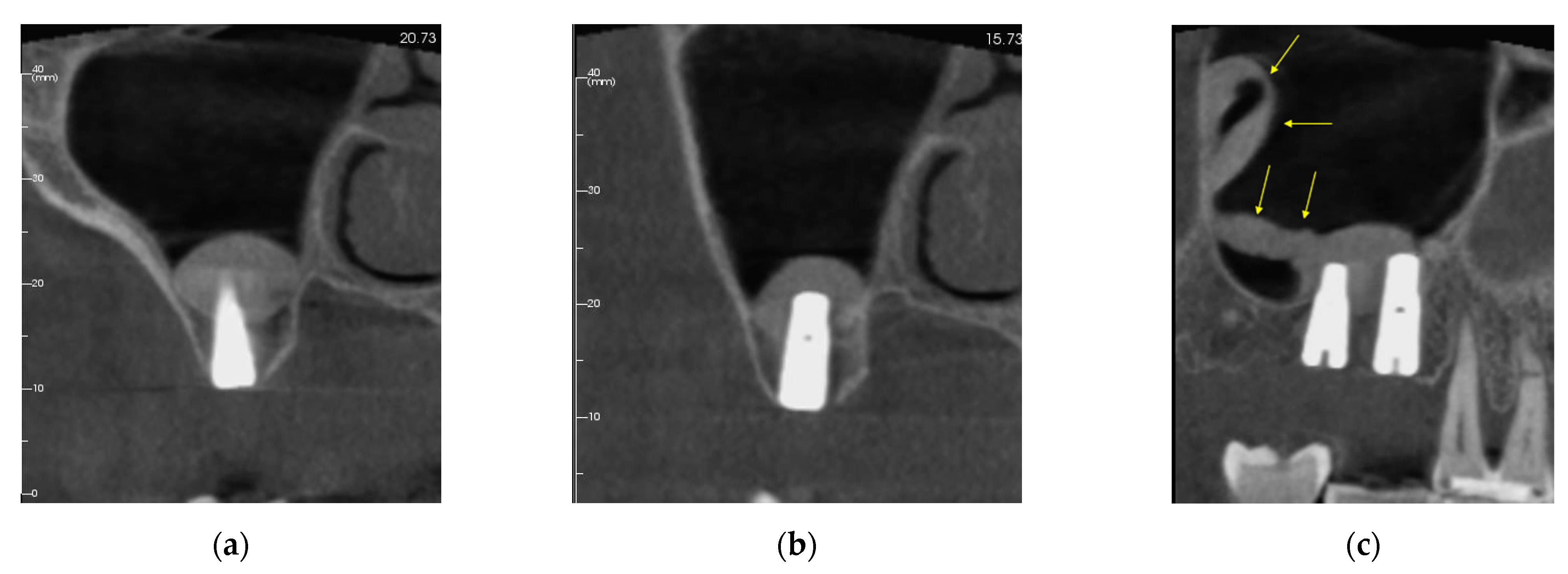
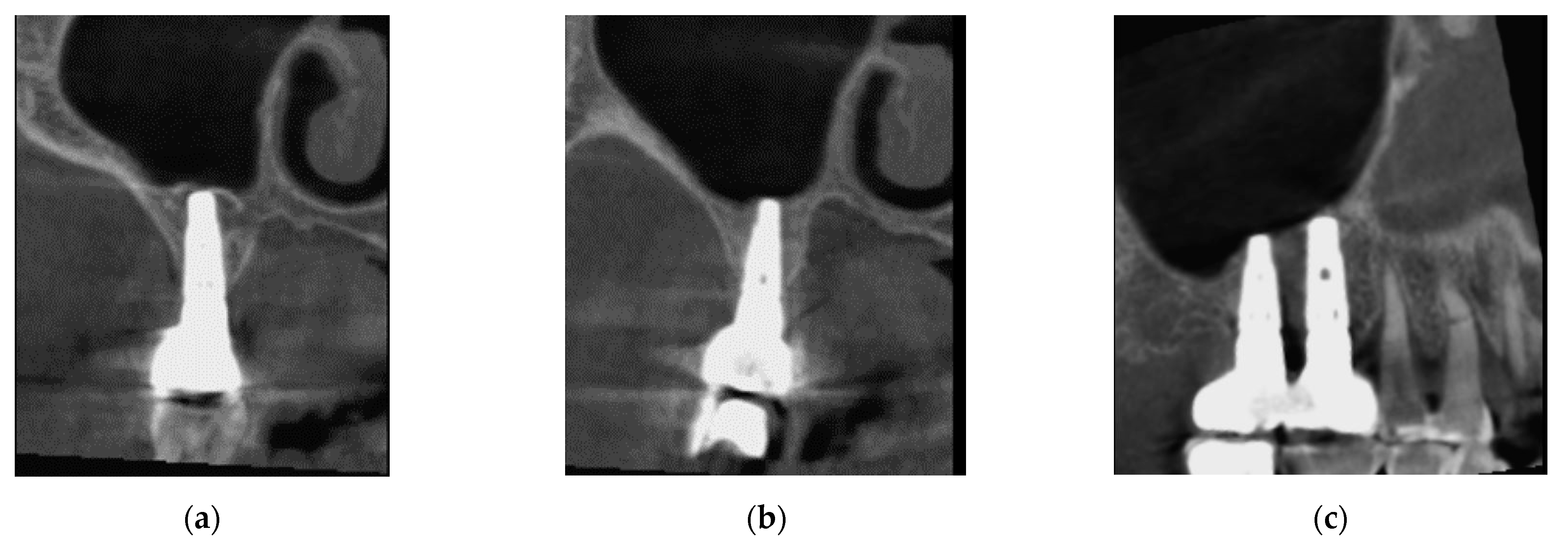
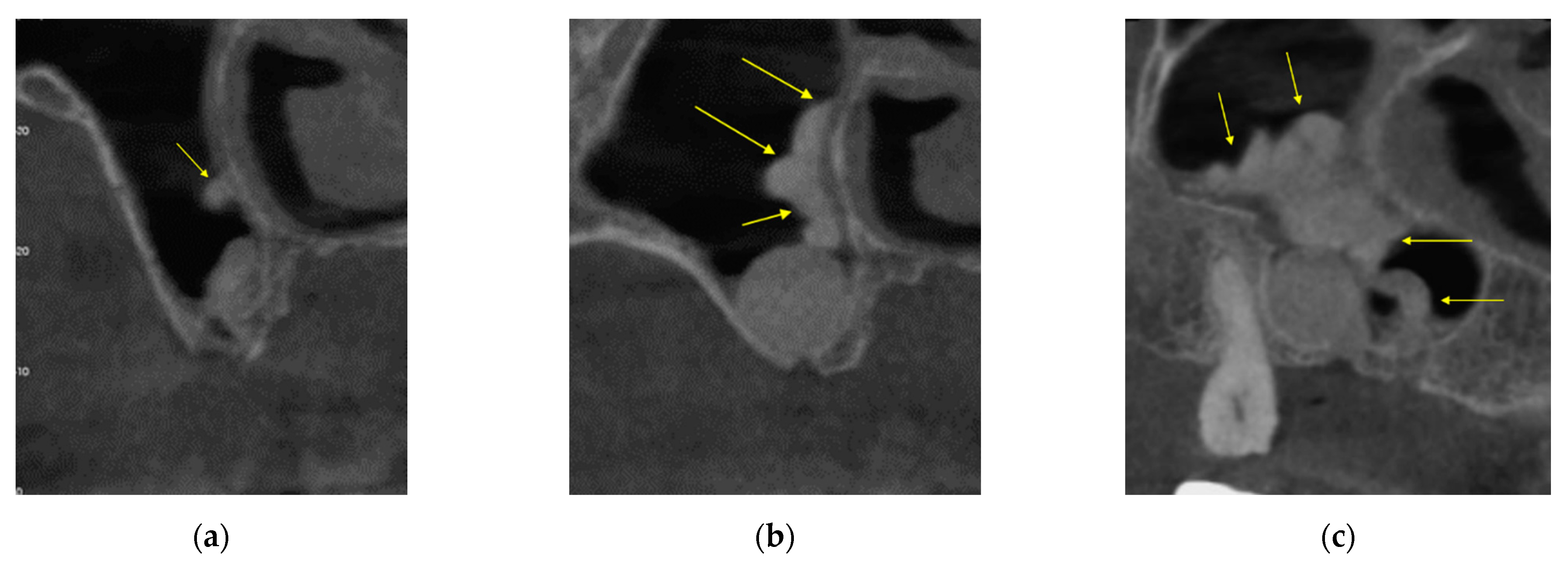
3.1. Patient #1
3.1.1. Surgery
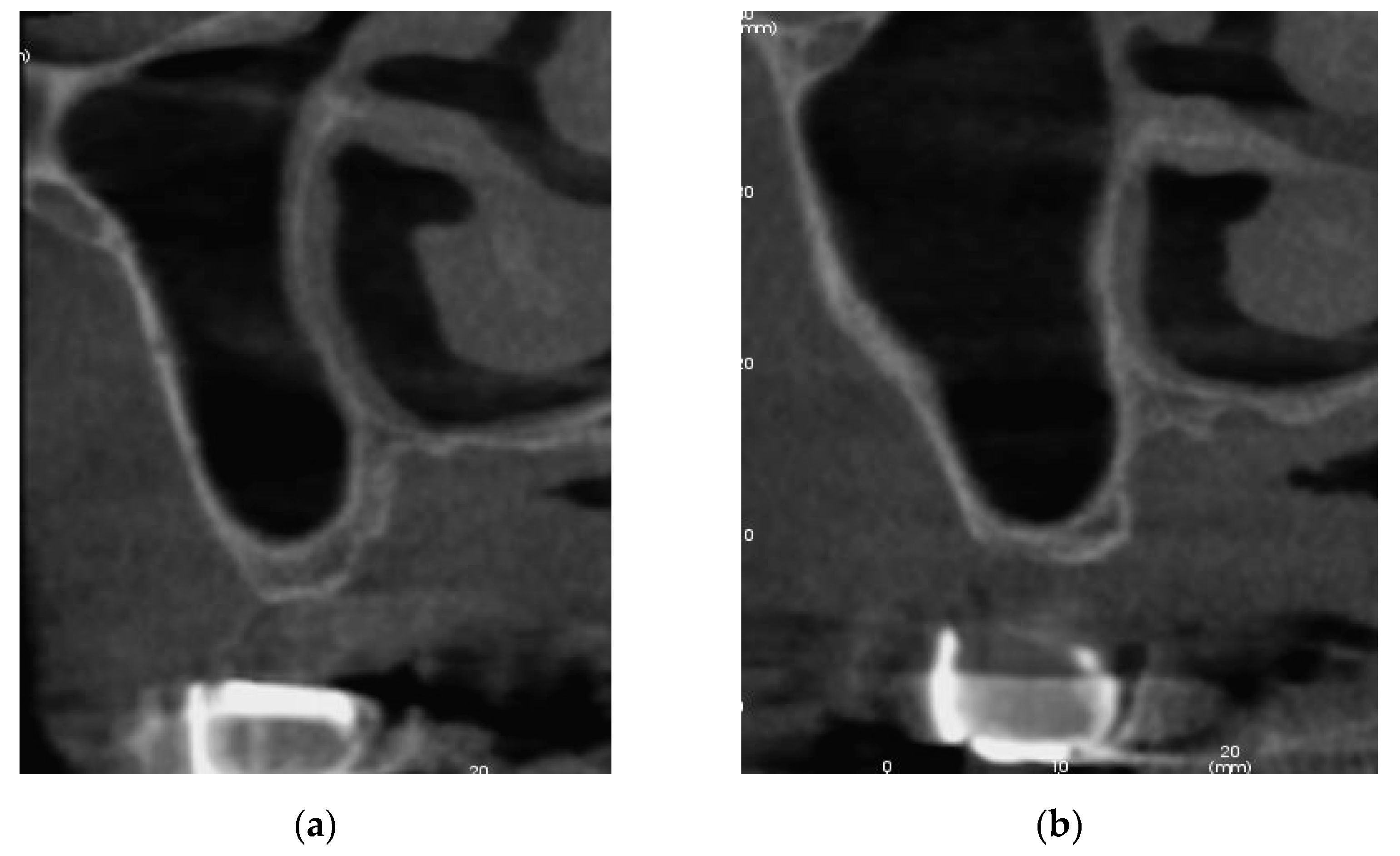
3.1.2. Follow Up
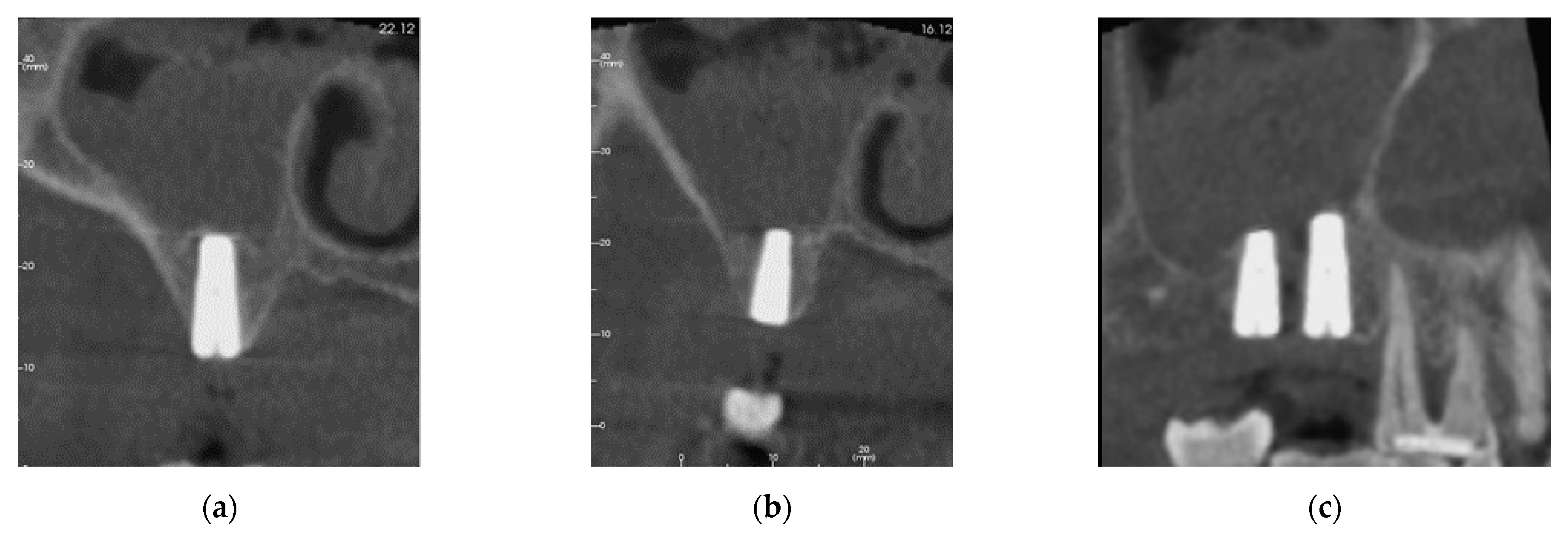
3.2. Patient Two
3.2.1. Surgery
3.2.2. Follow Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative complications during sinus floor elevation with lateral approach: A systematic review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef]
- Tan, W.C.; Lang, N.P.; Zwahlen, M.; Pjetursson, B.E. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: Transalveolar technique. J. Clin. Periodontol. 2008, 35, 241–254. [Google Scholar] [CrossRef]
- Boyacıgil, D.U.; Er, N.; Karaca, Ç.; Koç, O. The effect of residual bone height and membrane thickness on sinus membrane perforation in crestal sinus grafting: A prospective clinical study. Int. J. Oral Maxillofac. Surg. 2021, 50, 251–257. [Google Scholar] [CrossRef]
- Hernández-Alfaro, F.; Torradeflot, M.M.; Marti, C. Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin. Oral Implant. Res. 2008, 19, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Robiony, M.; Tenani, G.; Sbuelz, M.; Casadei, M. A simple method for repairing membrane sinus perforation. Open J. Stomatol. 2012, 2, 348–351. [Google Scholar] [CrossRef][Green Version]
- Falah, M.; Srouji, S. Use of buccal fat pad for closure of perforation and graft material in a maxillary sinus elevation procedure: A preliminary study. Int. J. Oral Maxillofac. Implant. 2016, 31, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Aricioglu, C.; Dolanmaz, D.; Esen, A.; Isik, K.; Avunduk, M.C. Histological evaluation of effectiveness of platelet-rich fibrin on healing of sinus membrane perforations: A preclinical animal study. J. Cranio-Maxillofac. Surg. 2017, 45, 1150–1157. [Google Scholar] [CrossRef]
- Barbu, H.M.; Iancu, S.A.; Jarjour Mirea, I.; Mignogna, M.D.; Samet, N.; Calvo-Guirado, J.L. Management of Schneiderian membrane perforations during sinus augmentation procedures: A preliminary comparison of two different approaches. J. Clin. Med. 2019, 8, 1491. [Google Scholar] [CrossRef]
- Proussaefs, P.; Lozada, J.L.; Kim, J.; Rohrer, M. Repair of the perforated sinus membrane with a resorbable collagen membrane: A human study. Int. J. Oral Maxillofac. Implant. 2004, 19, 413–420. [Google Scholar]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Mordenfeld, A.; Becktor, J.P.; Jensen, S.S. Maxillary sinus floor augmentation with synthetic bone substitutes compared with other grafting materials: A systematic review and meta-analysis. Implant Dent. 2018, 27, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Piattelli, A.; Caputi, S.; Degidi, M.; Mangano, C.; Scarano, A.; Perrotti, V.; Iezzi, G. Regeneration of human bone using different bone substitute biomaterials. Clin. Implant Dent. Relat. Res. 2015, 17, 150–162. [Google Scholar] [CrossRef]
- Stacchi, C.; Berton, F.; Fiorillo, L.; Nicolin, V.; Lombardi, T.; Cicciù, M.; Di Lenarda, R. Fresh frozen allogeneic bone block in maxillary sinus floor elevation: Histomorphometric analysis of a bone specimen retrieved 15 years after grafting procedure. Appl. Sci. 2019, 9, 1119. [Google Scholar] [CrossRef]
- Felisati, G.; Saibene, A.M.; Lenzi, R.; Pipolo, C. Late recovery from foreign body sinusitis after maxillary sinus floor augmentation. BMJ Case Rep. 2012, 2012, bcr2012007434. [Google Scholar] [CrossRef]
- Hunter, W.L.; Bradrick, J.P.; Houser, S.M.; Patel, J.B.; Sawady, J. Maxillary sinusitis resulting from ostium plugging by dislodged bone graft: Case report. J. Oral Maxillofac. Surg. 2009, 67, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Drago, L.; Wallace, S.S.; Capelli, M.; Galli, F.; Zuffetti, F.; Parenti, A.; Deflorian, M.; Fumagalli, L.; Weinstein, R.L.; et al. Prevention and treatment of postoperative infections after sinus elevation surgery: Clinical consensus and recommendations. Int. J. Dent. 2012, 2012, 365809. [Google Scholar] [CrossRef]
- Chiapasco, M.; Felisati, G.; Maccari, A.; Borloni, R.; Gatti, F.; Di Leo, F. The management of complications following displacement of oral implants in the paranasal sinuses: A multicenter clinical report and proposed treatment protocols. Int. J. Oral Maxillofac. Surg. 2009, 38, 1273–1278. [Google Scholar] [CrossRef]
- Santagata, M.; Guariniello, L.; Rauso, R.; Tartaro, G. Immediate loading of dental implant after sinus floor elevation with osteotome technique: A clinical report and preliminary radiographic results. J. Oral Implantol. 2010, 36, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.A.; Lico, S.; Casale, M.; Ormanier, Z.; Carinci, F. The use of various biomaterials in computer-guided crestal sinus lift procedures. A report on two case studies with volume comparison. Oral Implantol. 2016, 9, 89–97. [Google Scholar]
- Lopez, M.A.; Casale, M.; Candotto, V.; Papalia, R.; Bressi, F.; Carinci, F. The use of hyaluronic acid as a support of two different micronized biomaterials in crestal sinus lift procedures. A report on two case studies with volume comparison. J. Biol. Regul. Homeost. Agents 2017, 31, 129–138. [Google Scholar]
- Cossellu, G.; Farronato, G.; Farronato, D.; Ceschel, G.; Angiero, F. Space-maintaining management in maxillary sinus lifting: A novel technique using a resorbable polymeric thermo-reversible gel. Int. J. Oral Maxillofac. Surg. 2017, 46, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implant. Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally invasive management of implant-supported rehabilitation in the posterior maxilla, Part II. Surgical techniques and decision tree. Int. J. Periodontics Restor. Dent. 2020, 40, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Luccioli, M. A new sinus lift technique in conjunction with placement of 265 implants: A 6-year retrospective study. Implant Dent. 2000, 9, 363–368. [Google Scholar] [CrossRef]
- Mangano, C.; Piattelli, A.; Perrotti, V.; Iezzi, G. Dense hydroxyapatite inserted into postextraction sockets: A histologic and histomorphometric 20-year case report. J. Periodontol. 2008, 79, 929–933. [Google Scholar] [CrossRef]
- Valentini, P.; Bosshardt, D.D. 20-year follow-up in maxillary sinus floor elevation using bovine-derived bone mineral: A case report with histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implant. 2018, 33, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.P.; Siow, J.K.; Tan, H.M. Migration of a foreign body in the maxillary sinus illustrating natural mucociliary action. Med. J. Malays. 2005, 60, 383–385. [Google Scholar]
- Slavin, R.G.; Spector, S.L.; Bernstein, I.L. The diagnosis and management of sinusitis: A practice parameter update. J. Allergy Clin. Immunol. 2005, 116, S13–S47. [Google Scholar] [CrossRef]
- Arikan, O.K.; Onaran, Z.; Muluk, N.B.; Yilmazbaş, P.; Yazici, I. Enophthalmos due to atelectasis of the maxillary sinus: Silent sinus syndrome. J. Craniofacial Surg. 2009, 20, 2156–2159. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.S.; Nunez, D.A. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2006, 3, CD004458. [Google Scholar] [CrossRef]
- Berengo, M.; Sivolella, S.; Majzoub, Z.; Cordioli, G. Endoscopic evaluation of the bone-added osteotome sinus floor elevation procedure. Int. J. Oral Maxillofac. Surg. 2004, 33, 189–194. [Google Scholar] [CrossRef]
- Elian, S.; Barakat, K. Crestal endoscopic approach for evaluating sinus membrane elevation technique. Int. J. Implant. Dent. 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lee, C.H.; Won, T.B.; Kim, S.W.; Kim, J.W.; Rhee, C.S.; Min, Y.G. Functional recovery of rabbit maxillary sinus mucosa in two different experimental injury models. Laryngoscope 2008, 118, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Coutinho, T.M.; Carvalho Ferreira, D.; Souza, R.C.; Gonçalves, L.S. Odontogenic sinusitis: A comprehensive review. Acta Odontol. Scand. 2017, 75, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Ferri, M.; Imai, H.; Mesa, N.F.; Victorio, D.J.B.; Alccayhuaman, K.A.A.; Botticelli, D. Involvement of the maxillary sinus ostium (MSO) in the edematous processes after sinus floor augmentation: A cone-beam computed tomographic study. Int. J. Implant Dent. 2020, 6, 35. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardello, F.; Lombardi, T.; Stacchi, C. Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report. Sinusitis 2021, 5, 132-140. https://doi.org/10.3390/sinusitis5020014
Bernardello F, Lombardi T, Stacchi C. Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report. Sinusitis. 2021; 5(2):132-140. https://doi.org/10.3390/sinusitis5020014
Chicago/Turabian StyleBernardello, Fabio, Teresa Lombardi, and Claudio Stacchi. 2021. "Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report" Sinusitis 5, no. 2: 132-140. https://doi.org/10.3390/sinusitis5020014
APA StyleBernardello, F., Lombardi, T., & Stacchi, C. (2021). Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report. Sinusitis, 5(2), 132-140. https://doi.org/10.3390/sinusitis5020014







