1. Introduction
Chronic rhinosinusitis (CRS) is estimated to affect over 12% of adults in the United States [
1]. The clinical manifestations of CRS are variable, though in nearly all cases quality of life (QOL) is diminished [
2], with associations with impaired productivity, lost workdays, more healthcare visits, and increased spending on treatment. Validated metrics such as the 22-item Sinonasal Outcomes Test (SNOT-22) have been created to determine the severity of disease, to monitor progression, and to demonstrate the response to medical and surgical treatment [
3].
Endoscopic sinus surgery (ESS) has a role in the management of CRS to improve sinus ventilation and mucociliary clearance as well as to facilitate the topical administration of medication [
4]. Adjunct, long-term medical therapy is necessary for the effective treatment and maintenance of CRS [
5]. The beneficial effect of steroids is well-known in CRS; however, the side effects of long-term systemic steroid use are not desirable and can lead to serious complications [
6]. Topical intranasal steroids can minimize systemic effects and are an integral component in treatment; however, penetration into the middle meatus and sinus cavities can be limited by postoperative edema and crusting [
7]. Current efforts are focused on improving steroid delivery to the diseased sinuses while limiting their systemic effects [
8].
Bioabsorbable steroid-eluting implants are a relatively new technology that may be utilized within the ethmoid sinus lumen following ESS [
9,
10]. Only one such implant is currently available, marketed under the trade name Propel (Intersect ENT, Menlo Park, CA, USA), which combines the release of 370 μg of mometasone furoate with a spring-like spacer activity that is designed for gradual release over 30 days [
11]. This offers the potential benefits of decreasing postoperative inflammation and mucosal edema, reducing polyposis and adhesions, securing the middle turbinate in a medialized position, and separating raw mucosal edges [
8,
10].
Previous studies examining the utility of adjuvant use of a bioabsorbable steroid-eluting implant in ESS have demonstrated its safety and clinical effectiveness in CRS patients in general [
6,
7,
8,
12]. However, it is unclear which patient groups are most likely to benefit from this technology. Study of patient-reported QOL outcomes with use of this device has been limited, and the differential effect of this device upon phenotypes of CRS has not been fully studied. The aim of the present study was to determine if changes in patient-reported QOL after ESS with implant placement are related to the severity of baseline sinonasal inflammation. Objective measures of tissue eosinophilia, serum eosinophilia, polyposis and degree of radiographic sinus opacification were used as indicators of inflammation severity. A secondary aim was to determine if surgeon-reported endoscopic appearance showed similar postoperative improvements in patients with mild versus severe baseline inflammation.
2. Methods
A single-cohort before-after study design was utilized to evaluate outcomes of ESS for CRS performed by a single surgeon (EDM) from October 2014 to March 2016. During this time period, 151 consecutive adult patients undergoing ethmoidectomy for CRS had placement of a Propel implant. This implant is composed of a bioabsorbable polymer, poly-(ll-lactide-co-glycolide), woven into a scaffold and impregnated with 370 μg of mometasone furoate, and is deployed into the sinus cavity using an specialized catheter. ESS cases performed for other indications besides CRS did not receive an implant and were not included in the study. Patients were excluded who received postoperative systemic corticosteroids (nine cases), who had a diagnosis of cystic fibrosis (five cases), or who received an implant but were found incidentally to have a sinonasal neoplasm (one case). All included participants underwent unilateral or bilateral total (anterior and posterior) ethmoidectomy, and each operated ethmoid cavity received an implant. Treatment of other paranasal sinuses was permitted, as was concurrent septoplasty or inferior turbinate reduction. In addition to ethmoidectomy, 96.3% (131/136) underwent concurrent maxillary antrostomy, 83.8% (114/136) underwent frontal sinus exploration, and 56.6% (77/136) underwent sphenoidotomy.
CRS was defined as symptomatic mucosal inflammation of the paranasal sinuses of at least 12 consecutive weeks duration. Pre-operative sinus computerized tomography (CT) within three months of the procedure was reviewed. Opacification was evaluated for each patient using the Lund-Mackay score (LMS) on all preoperative CT scans, with LMS = 6 considered the median score in this cohort. Pre-operative Lund-Kennedy Endoscopic score (LKES) was determined on all patients with in-office nasal endoscopy performed by the senior author. The presence of preoperative serum eosinophilia (>6.0% on peripheral smear) and presence of polyps were recorded. The presence of tissue eosinophilia (>10 cells/hpf) was documented on histopathologic examination of ethmoid tissue specimens. Relevant comorbidities were obtained from the medical history provided by the patient or recorded in the medical record.
Patients were seen approximately seven days and 28 days postoperatively for nasal endoscopy and debridement. If stent fragments were present on post-operative day 28, they were removed during the office visit. At 28 days all patients were then started on a daily application of an intranasal topical steroid. LKES was reported during nasal endoscopy at the three- and six-month post-operative visits. Patient-reported SNOT-22 scores were gathered preoperatively and at three and six months postoperatively, and were grouped for analysis relative to the median score of 45. The study was approved by the institutional review board of the senior author’s primary institution.
Sample size calculation was based on the expected mean (SD) postoperative change score, previously reported in a large cohort as 16.2 (20.0) [
13]. Assuming an alpha level of 0.05 and a power of 0.8, the calculated sample size was 48. A larger cohort was sampled to account for dropouts. Pre- and post-operative continuous variables were compared using two-tailed paired
t-tests. Nonparametric variables were compared using Fisher’s exact test.
p-values less than 0.05 were considered significant. Statistical analysis was completed using SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA).
3. Results
One hundred and thirty-six patients met inclusion criteria. Of these, 50 (36.8%) had polyposis, 21 (15.4%) had serum eosinophilia and 87 (64.0%) had tissue eosinophilia. None of these cases received systemic steroids during the study period. Baseline characteristics of eosinophilic (serum eosinophilia >6.0% on peripheral smear) and non-eosinophilic (serum eosinophilia ≤ 6.0%) groups were comparable, although more males presented with high-grade LMS and lower preoperative SNOT-22 scores (
Table 1). Comorbid conditions that could affect QOL were equally distributed. Patients with serum eosinophilia had a mean LMS of 11.9 versus 7.4 in patients without eosinophilia (
p = 0.003), whereas those with and without tissue eosinophilia had LMS scores of 9.11 and 6.96, respectively (
p = 0.211). Tissue eosinophilia and serum eosinophilia were weakly correlated (Spearman rho = 0.265). Two patients (1.5%) required revision ESS during the study period.
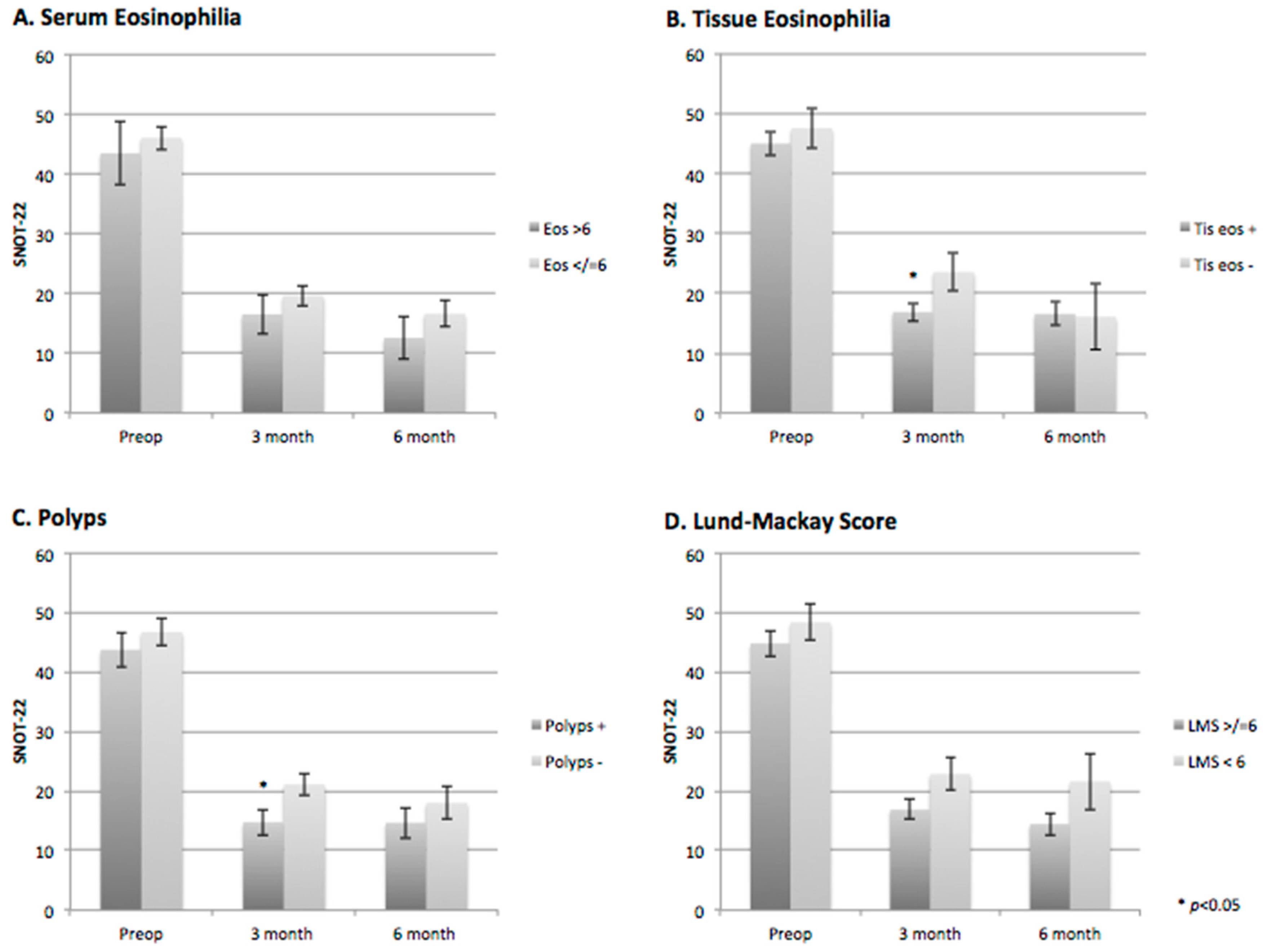
The mean (standard deviation) SNOT-22 score for all patients was 45.5 (19.4) preoperatively, which improved postoperatively to 18.8 (14.1) at three months (
p < 0.001), and to 16.5 (14.0) at six months (
p < 0.001). Similar results were found in the subgroup analysis of tissue eosinophilia, serum eosinophilia, the presence of polyps, and high-grade presentation of disease on CT (LMS > 6) (
Figure 1A–D,
Table 2). Three and six-month SNOT-22 scores were significantly lower than the preoperative SNOT-22 scores in all subgroups (
Figure 1A–D). The presence or absence of serum eosinophilia and the grade of disease on CT did not significantly affect postoperative SNOT-22 scores (
Figure 1A,D,
Table 2). Three-month postoperative SNOT-22 scores were significantly higher in patients with tissue eosinophilia and polyps compared to those without; however, at six months, there was no significant difference (
Figure 1B,C,
Table 2).
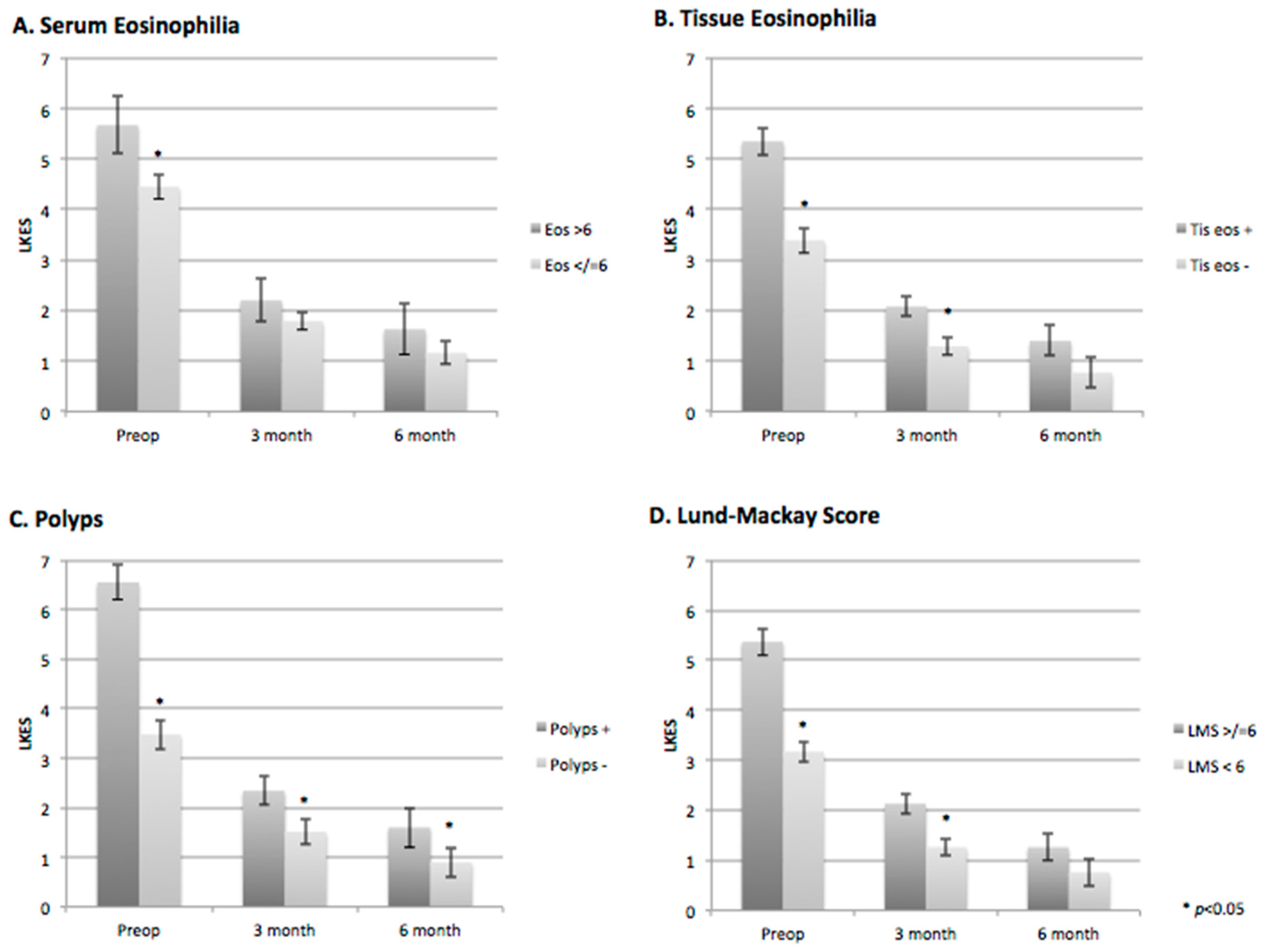
The baseline LKES scores were significantly higher for patients with serum and tissue eosinophilia, polyps and high-grade CT disease (
Figure 2A–D;
Table 3). The three- and six-month LKES scores were significantly lower for patients with and without serum eosinophilia, with no significant difference between the groups (
Figure 2A,
Table 3). Patients with tissue eosinophilia and high-grade CT disease had significantly higher three-month postoperative LKES scores (
p = 0.017 and
p = 0.006, respectively); however, this difference was not seen at six months postoperatively (
p = 0.189 and
p = 0.144, respectively) (
Figure 2B,D;
Table 3).
4. Discussion
CRS is a heterogeneous disease consisting of multiple variants with different underlying pathophysiologies [
14]. In the United States and Europe, patients with CRS are classified into two phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps [
15]. CRSwNP patients that have recurrence of nasal polyps after surgery are more likely to have pronounced eosinophilic infiltration of the nasal mucosa [
15,
16]. Eosinophilic CRS is a subtype of CRS that predicts less post-operative improvement in patient-reported QOL and disease-specific measures [
17,
18]. Peripheral blood eosinophilia and tissue eosinophilia are associated with more severe CRSwNP, higher recurrence rates of nasal polyps after surgery [
14,
15], and higher revision surgery rates [
19].
The availability of bioabsorbable steroid-eluting implants in the treatment of CRS offers the potential for decreased inflammation, adhesions, recurrent polyposis, and improved sinus ostia patency in the postoperative period after ESS [
10,
20]. However, few studies have focused on patient-reported QOL outcomes with the use of this device, and the relative efficacy in the treatment of different phenotypes of CRS has not been analyzed in previous studies [
6,
7,
8,
12]. The goal of this study was to determine if a bioabsorbable steroid-eluting implant would have a differential effect on patient-reported QOL relative to the status of baseline eosinophilia. We found that QOL scores showed comparable improvement among patients with eosinophilic CRS and those without. Similar results were found when comparing low- versus high-grade sinus CT opacification, as well as patients with CRSwP versus those without polyps. These findings suggest that the benefit from these implants may not be limited to one particular CRS phenotype, and could be beneficial to a wider range of patients undergoing ESS.
In addition to patient-reported outcomes, the clinician assessment of endoscopic appearance may be a useful outcome to assess disease control. Patients with eosinophilic CRS are often noted to have persistent postoperative edema and polypoid changes on endoscopy during the postoperative period. As expected, our data showed higher preoperative LKES for patients with polyposis, eosinophilia or higher-grade CT opacification. Moreover, subsequent improvement in postoperative LKES was observed for patients with both low- and high-grade disease. At six months postoperatively, patients with preoperative eosinophilia or high-grade CT opacification were found to have an endoscopic appearance comparable to those without eosinophilia or with low-grade CT opacification. Though future controlled studies are necessary to better examine this effect, our data suggest that the steroid-eluting implant in conjunction with ESS might assist with suppression of inflammation that persists for several months after implant degradation. Further studies are required to determine whether these effects are significantly better than surgery performed without placement of a bioabsorbable steroid-eluting implant.
Revision ESS rates as reported in the literature are variable. Patients with serum and tissue eosinophilia often require systemic steroids and have significantly higher recurrence and revision rates [
15,
21,
22,
23]. In a previous study of the steroid-eluting implant, revision ESS was indicated in 2.2% (2/90) of cases with use of the Propel stent, which is consistent with the revision rate (1.5%) in the present study [
6]. Of this, only one patient of 21 (4.8%) with serum and tissue eosinophilia required revision surgery after one year, which is significantly less than revision rates reported in the literature, though long-term follow up is necessary.
Several notable limitations are relevant in the present study. As a single-armed study without a comparison treatment group, conclusions about causation and comparative effectiveness are not possible. Additionally, although approximately 80% of patients continued to follow up six months from the time of surgery, there is a risk of follow-up bias, as postoperative outcomes could have influenced both follow-up and completion of the forms. Finally, some patients in this study also received a septoplasty and/or inferior turbinate reduction, which may be a confounding variable that overestimates the improvement in QOL measures.
Although the present study indicates that improvements occur regardless of the severity of preoperative inflammation, it remains unclear how these effects would compare to cases in which an implant was not utilized. Future studies with controlled trials of patient-reported QOL following ESS with bioabsorbable steroid-eluting implants are needed, which may utilize postoperative objective markers of inflammation to supplement the effects on patient-reported QOL. Investigation of the effect of simultaneous additional symptom scores or QOL measures may help elucidate the confounding potential of septoplasty and inferior turbinate reduction in conjunction with ESS. Lastly, examination into specific items of the SNOT-22 score that are most affected by implant placement may result in better preoperative counseling and patient selection.
5. Conclusions
Irrespective of the presence of polyposis or eosinophilia, patient-reported QOL scores are improved up to six months after placement of a steroid-eluting implant during ESS for patients with CRS. Endoscopic appearance shows comparable normalization over time regardless of the extent of preoperative inflammation. Controlled studies are necessary to determine the comparative effectiveness of the steroid-eluting implant.
Author Contributions
Edward D. McCoul conceived and designed the study; Jason D. Pou, Charles A. Riley, Anna K. Bareiss, Kiranya E. Tipirneni and Edward D. McCoul collected the data; Jason D. Pou, Charles A. Riley and Edward D. McCoul analyzed the data; Jason D. Pou, Charles A. Riley and Edward D. McCoul wrote and revised the paper; all authors approved the final manuscript.
Conflicts of Interest
There was no financial or material support for the research of this work. Edward D. McCoul is a consultant for Acclarent, which is unrelated to the current study. The other authors have no financial affiliations to disclose. The authors declare no conflict of interest.
References
- Pleis, J.R.; Ward, B.W.; Lucas, J.W. Summary health statistics for U.S. adults: National health interview survey, 2009. Vital Health Stat. 2010, 10, 1–207. [Google Scholar]
- Schlosser, R.J.; Gage, S.E.; Kohli, P.; Soler, Z.M. Burden of illness: A systematic review of depression in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item sinonasal outcome test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.; Wormald, P.J. Are routine dissolvable nasal dressings necessary following endoscopic sinus surgery? Laryngoscope 2010, 120, 1920–1921. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Kennedy, D.W. Mometasone implant for chronic rhinosinusitis. Med. Devices 2012, 5, 75–80. [Google Scholar]
- Forwith, K.D.; Chandra, R.K.; Yun, P.T.; Miller, S.K.; Jampel, H.D. ADVANCE: A multisite trial of bioabsorbable steroid-eluting sinus implants. Laryngoscope 2011, 121, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Marple, B.F.; Smith, T.L.; Han, J.K.; Gould, A.R.; Jampel, H.D.; Stambaugh, J.W.; Mugglin, A.S. Advance II: A prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants. Otolaryngol. Head Neck Surg. 2012, 146, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Murr, A.H.; Smith, T.L.; Hwang, P.H.; Bhattacharyya, N.; Lanier, B.J.; Stambaugh, J.W.; Mugglin, A.S. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int. Forum Allergy Rhinol. 2011, 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.G.; Kennedy, D.W. What is new and promising with drug-eluting stents in sinus surgery? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, K.A.; Kuhn, F.A. Stents and drug-eluting stents. Otolaryngol. Clin. N. Am. 2009, 42, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Intersect ENT Inc. Propel Mometasone Implant. Available online: http://propelopens.com/the-propel-advantage/how-it-works (accessed on 10 September 2016).
- Forwith, K.D.; Han, J.K.; Stolovitzky, J.P.; Yen, D.M.; Chandra, R.K.; Karanfilov, B.; Matheny, K.E.; Stambaugh, J.W.; Gawlicka, A.K. RESOLVE: Bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis after sinus surgery: 6-month outcomes from a randomized, controlled, blinded study. Int. Forum Allergy Rhinol. 2016, 6, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Rudmik, L.; Lund, V.J. The predictive value of the preoperative sinonasal outcome test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2015, 125, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, G.E.; Lee, B.J.; Kwon, S.W.; Lee, S.H.; Kim, H.S.; Jang, Y.J. Natural killer cells regulate eosinophilic inflammation in chronic rhinosinusitis. Sci. Rep. 2016, 6, 27615. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Bachert, C.; Cingi, C.; Dykewicz, M.S.; Hellings, P.W.; Naclerio, R.M.; Schleimer, R.P.; Ledford, D. Endotypes and phenotypes of chronic rhinosinusitis: A PRACTALL document of the European academy of allergy and clinical immunology and the American academy of allergy, asthma & immunology. J. Allergy Clin. Immunol. 2013, 131, 1479–1490. [Google Scholar] [PubMed]
- Shah, S.A.; Ishinaga, H.; Takeuchi, K. Pathogenesis of eosinophilic chronic rhinosinusitis. J. Inflamm. 2016, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Eosinophilic mucin rhinosinusitis: A distinct clinicopathological entity. Laryngoscope 2000, 110, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Soler, Z.M.; Sauer, D.; Mace, J.; Smith, T.L. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol. Head Neck Surg. 2010, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Vlaminck, S.; Vauterin, T.; Hellings, P.W.; Jorissen, M.; Acke, F.; Van-Cauwenberge, P.; Bachert, C.; Gevaert, P. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: A 3-year prospective observational study. Am. J. Rhinol. Allergy 2014, 28, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, A.; Lee, J.T. In-office use of a steroid-eluting implant for maintenance of frontal ostial patency after revision sinus surgery. Allergy Rhinol 2015, 6, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tansavatdi, K.P.; McGill, L.; Riggs, S.; Orlandi, R.R. Development of an animal model for wound healing in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Svendstrup, F. Functional endoscopic sinus surgery in chronic sinusitis—A series of 237 consecutively operated patients. Acta Otolaryngol. Suppl. 2000, 543, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.J.; Lee, J.W.; Yoon, Y.H.; Kim, Y.M.; Rha, K.S. Categorization and clinicopathological features of chronic rhinosinusitis with eosinophilic mucin in a Korean population. Clin. Exp. Otorhinolaryngol. 2015, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).