Antioxidant Activity and Cytotoxicity of Baru Nut Oil (Dipteryx alata Vogel) Nanoemulsion in Human Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Baru Nut Oil
2.2. Baru Nut Oil Nanoemulsion (BNON)
2.3. Physicochemical Characterization
2.4. Cytotoxicity to Human Keratinocytes
2.5. Hemolytic Activity Assay
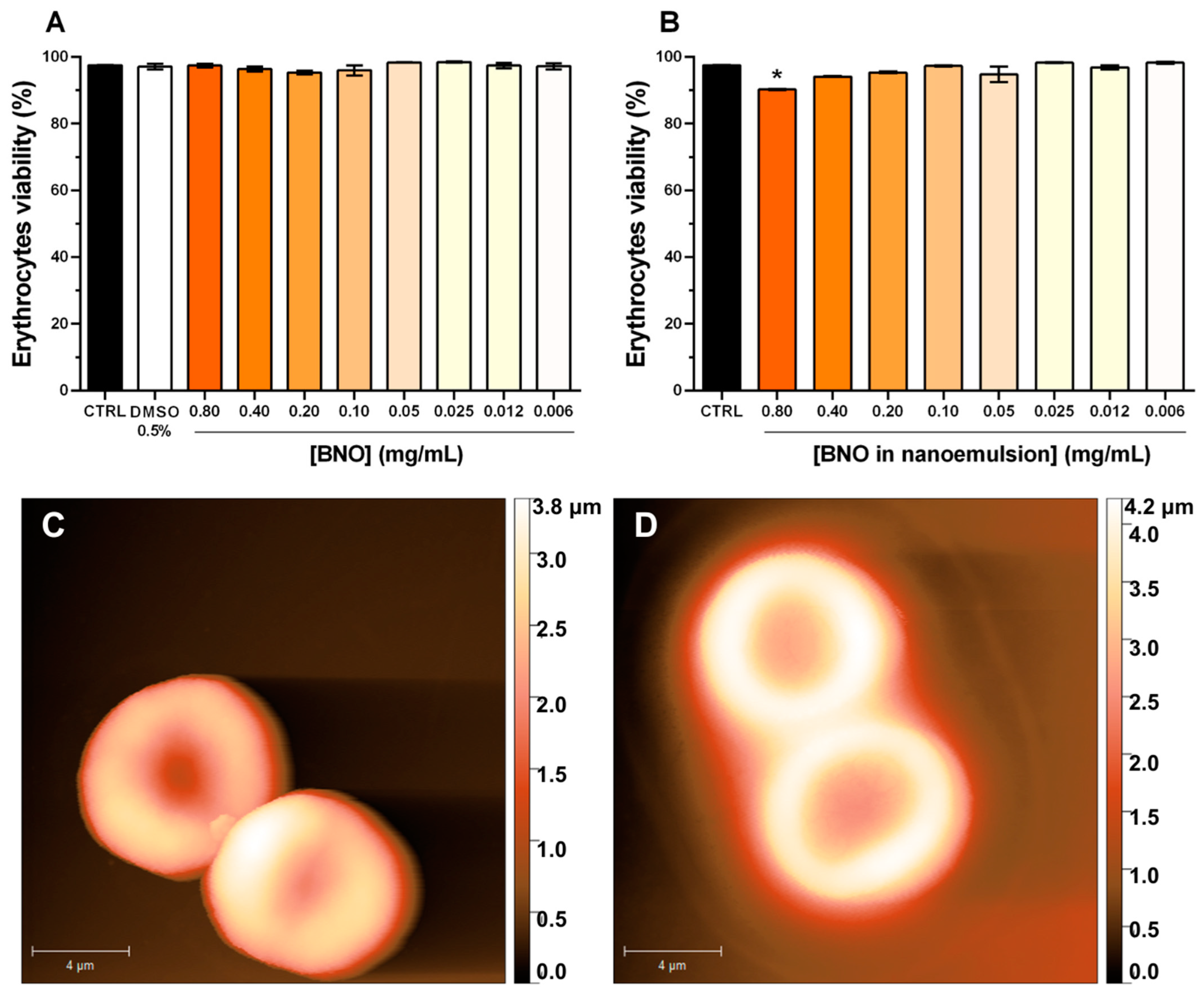
2.6. Human Erythrocyte Morphology by Atomic Force Microscopy (AFM)
2.7. Free Radical Scavenging Activity
2.8. Activity of BNON Against AAPH-Induced Oxidative Stress
2.9. Statistical Analysis
3. Results
3.1. Characterization of Baru Nut Oil Nanoemulsion (BNON)
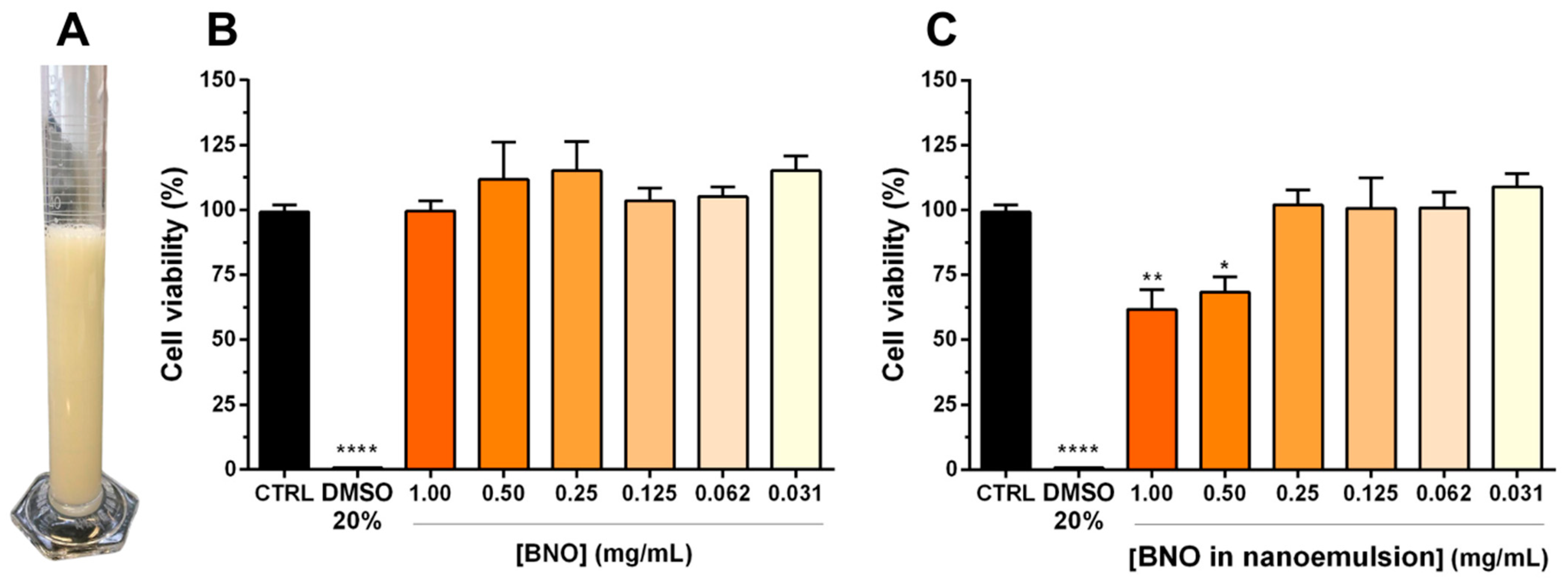
3.2. Cytotoxicity on Human Keratinocytes
3.3. Hemolytic Activity and Erythrocyte Morphology
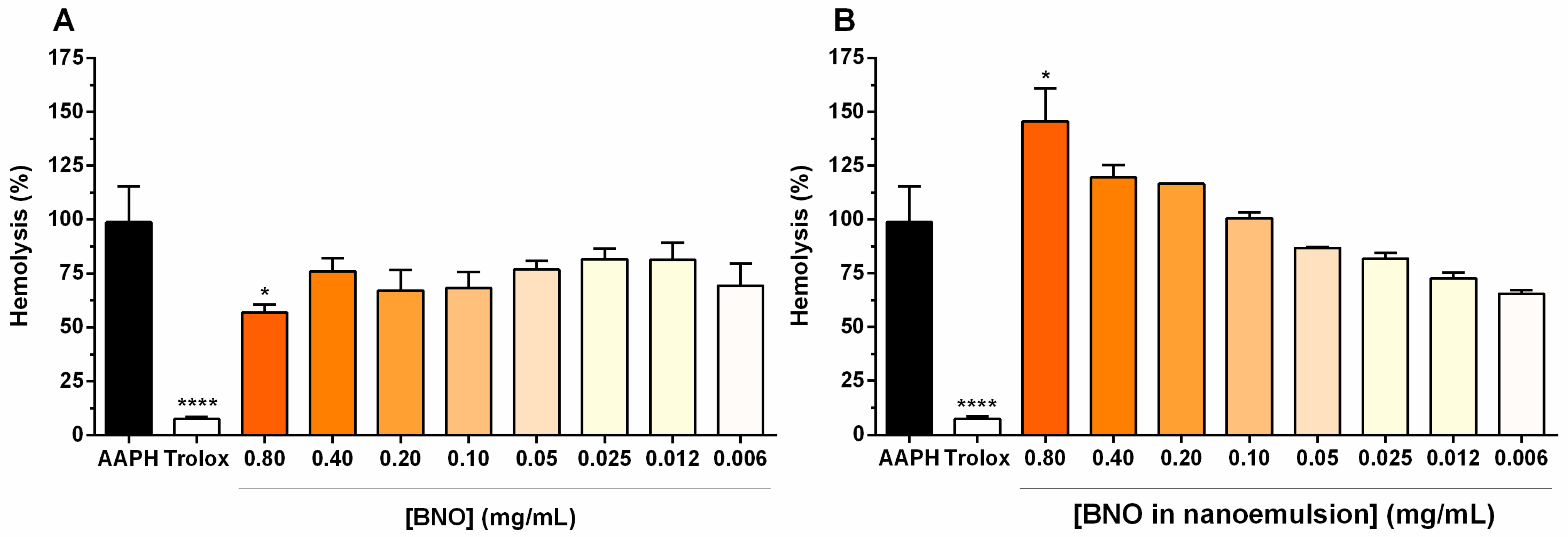
3.4. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, F.B.; Costa, A.C.; Müller, C.; Nascimento, K.T.; Batista, P.F.; Vital, R.G.; Megguer, C.A.; Jakelaitis, A.; Domingos, M. Dipteryx Alata, a Tree Native to the Brazilian Cerrado, Is Sensitive to the Herbicide Nicosulfuron. Ecotoxicology 2020, 29, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.W.L.D.; Machado, S.S.; Faria, K.D.C.; Oliveira, F.A.D.; Souza, A.P.D.; Menezes, I.P.P.D.; Silva, J.M.D. Molecular Insight for Baru Dipteryx Alata (Fabaceae) Populations Based on Novel SSRs. Acta Bot. Bras. 2023, 37, e20220168. [Google Scholar] [CrossRef]
- Lima, D.C.; Alves, M.D.R.; Noguera, N.H.; Nascimento, R.D.P.D. A Review on Brazilian Baru Plant (Dipteryx alata Vogel): Morphology, Chemical Composition, Health Effects, and Technological Potential. Future Foods 2022, 5, 100146. [Google Scholar] [CrossRef]
- Paulo, L.; Fernandes, R.; Gandra, K.; Minim, V.; Minim, L.; Grimaldi, R.; Vidigal, M. Baru Seed Extracted Oil (Dipteryx alata Vog.): Chemical Composition and Thermal and Oxidative Stability. J. Braz. Chem. Soc. 2023, 34, 664–672. [Google Scholar] [CrossRef]
- Scapin, E.; Sarri, D.R.A.; Augusco, M.A.C.; Rodrigues, M.A.M.; Fernandes, R.M.N.; Silva, J.F.M.; Cardoso, C.A.L.; Rambo, M.K.D. Phytochemical Analysis, Toxicity and Evaluation of Antioxidant and Antimicrobial Activities of Leaves of Dipteryx Alata Vogel. Braz. J. Biol. 2024, 84, e278004. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.M.; Marangoni Faoro, J.A.; Fava De Souza, M.; De Matos Balsalobre, N.; Leite Kassuya, C.A.; Kappel Trichez, V.D.; Mussury Franco Da Silva, R.M.; Formagio, A.S.N. Anti-Arthritic Potential and Antioxidant Properties of Infusion, Fractions and Flavonoid Glycosides from Dipteryx alata (Baru) Leaves. J. Ethnopharmacol. 2025, 338, 118973. [Google Scholar] [CrossRef]
- Leite, N.R.; Araújo, L.C.A.D.; Rocha, P.D.S.D.; Agarrayua, D.A.; Ávila, D.S.; Carollo, C.A.; Silva, D.B.; Estevinho, L.M.; De Picoli Souza, K.; Dos Santos, E.L. Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis Elegans via SOD-3 and DAF-16. Biomolecules 2020, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Silva-Luis, C.C.; De Brito Alves, J.L.; De Oliveira, J.C.P.L.; De Sousa Luis, J.A.; Araújo, I.G.A.; Tavares, J.F.; Do Nascimento, Y.M.; Bezerra, L.S.; Araújo De Azevedo, F.D.L.A.; Sobral, M.V.; et al. Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries. Nutrients 2022, 14, 2098. [Google Scholar] [CrossRef]
- Reis, M.Á.; Novaes, R.D.; Baggio, S.R.; Viana, A.L.M.; Salles, B.C.C.; Duarte, S.M.D.S.; Rodrigues, M.R.; Paula, F.B.D.A. Hepatoprotective and Antioxidant Activities of Oil from Baru Almonds (Dipteryx alata Vog.) in a Preclinical Model of Lipotoxicity and Dyslipidemia. Evid.-Based Complement. Altern. Med. 2018, 2018, 8376081. [Google Scholar] [CrossRef] [PubMed]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation of Factors Influencing Formation of Nanoemulsion by Spontaneous Emulsification: Impact on Droplet Size, Polydispersity Index, and Stability. Bioengineering 2022, 9, 384. [Google Scholar] [CrossRef]
- Malode, M.G.P.; Chauhan, S.A.; Bartare, S.A.; Malode, L.M.; Manwar, J.V.; Bakal, R.L. A Critical Review on Nanoemulsion: Advantages, Techniques and Characterization. J. App. Pharm. Sci. Res. 2022, 4, 6–12. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Himanath, G.; Shruthy, R.; Preetha, R.; Sreejit, V. Nanoemulsion with Coconut Oil and Soy Lecithin as a Stable Delivery System for Lycopene and Its Incorporation into Yogurt to Enhance Antioxidant Properties and Maintain Quality. ACS Food Sci. Technol. 2021, 1, 1538–1549. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C. Cytotoxic Activity of Poly-ɛ-Caprolactone Lipid-Core Nanocapsules Loaded with Lycopene-Rich Extract from Red Guava (Psidium guajava L.) on Breast Cancer Cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahian, M.; Saadatfar, A.; Masoumipour, F. Formulation and Characterization of Nanoemulsion from Alhagi Maurorum Essential Oil and Study of Its Antimicrobial, Antibiofilm, and Plasmid Curing Activity against Antibiotic-Resistant Pathogenic Bacteria. J. Environ. Health Sci. Eng. 2020, 18, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Azarnezhad, A.; Samadian, H.; Jaymand, M.; Sobhani, M.; Ahmadi, A. Toxicological Profile of Lipid-Based Nanostructures: Are They Considered as Completely Safe Nanocarriers? Crit. Rev. Toxicol. 2020, 50, 148–176. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Valenta, C. Lecithin-Based Nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-Emulsion Formation by Emulsion Phase Inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the Preparation and the Recent Advances in Their Food Applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Sharifi, F.; Jahangiri, M.; Nazir, I.; Asim, M.H.; Ebrahimnejad, P.; Hupfauf, A.; Gust, R.; Bernkop-Schnürch, A. Zeta Potential Changing Nanoemulsions Based on a Simple Zwitterion. J. Colloid Interface Sci. 2021, 585, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Thiemicke, A.; Neuert, G. Kinetics of Osmotic Stress Regulate a Cell Fate Switch of Cell Survival. Sci. Adv. 2021, 7, eabe1122. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Narayanan, S.; Dalrymple, D.; Cheng, X.; Serajuddin, A.T.M. Cytotoxicity Assessment of Lipid-Based Self-Emulsifying Drug Delivery System with Caco-2 Cell Model: Cremophor EL as the Surfactant. Eur. J. Pharm. Sci. 2016, 91, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.A.; Adorne, M.D.; Colomé, L.M.; Abdalla, D.S.P.; Guterres, S.S.; Pohlmann, A.R. Hemocompatibility of Poly(ɛ-Caprolactone) Lipid-Core Nanocapsules Stabilized with Polysorbate 80-Lecithin and Uncoated or Coated with Chitosan. Int. J. Pharm. 2012, 426, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alves, S.C.; Pereira, R.S.; Pereira, A.B.; Ferreira, A.; Mecha, E.; Silva, A.B.; Serra, A.T.; Bronze, M.R. Identification of Functional Compounds in Baru (Dipteryx alata Vog.) Nuts: Nutritional Value, Volatile and Phenolic Composition, Antioxidant Activity and Antiproliferative Effect. Food Res. Int. 2020, 131, 109026. [Google Scholar] [CrossRef] [PubMed]
- Marsup, P.; Yeerong, K.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-anun, C.; Chaiyana, W. Enhancement of Chemical Stability and Dermal Delivery of Cordyceps Militaris Extracts by Nanoemulsion. Nanomaterials 2020, 10, 1565. [Google Scholar] [CrossRef] [PubMed]

| Month | Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 0 | 530.1 ± 20.48 | 0.496 ± 0.057 | −35.7 ± 2.19 |
| 3 | 467.1 ± 38.08 | 0.726 ± 0.235 * | −33.5 ± 1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, J.; Figueredo, A.; Sá, B.S.; Moreira, D.C.; Bueno Nunes, J.; Eaton, P.; Leite, J.R.S.d.A.; Vasconcelos, A.G. Antioxidant Activity and Cytotoxicity of Baru Nut Oil (Dipteryx alata Vogel) Nanoemulsion in Human Cells. Appl. Nano 2025, 6, 3. https://doi.org/10.3390/applnano6010003
Queiroz J, Figueredo A, Sá BS, Moreira DC, Bueno Nunes J, Eaton P, Leite JRSdA, Vasconcelos AG. Antioxidant Activity and Cytotoxicity of Baru Nut Oil (Dipteryx alata Vogel) Nanoemulsion in Human Cells. Applied Nano. 2025; 6(1):3. https://doi.org/10.3390/applnano6010003
Chicago/Turabian StyleQueiroz, José, Arthur Figueredo, Bruno Silva Sá, Daniel Carneiro Moreira, João Bueno Nunes, Peter Eaton, José Roberto Souza de Almeida Leite, and Andreanne Gomes Vasconcelos. 2025. "Antioxidant Activity and Cytotoxicity of Baru Nut Oil (Dipteryx alata Vogel) Nanoemulsion in Human Cells" Applied Nano 6, no. 1: 3. https://doi.org/10.3390/applnano6010003
APA StyleQueiroz, J., Figueredo, A., Sá, B. S., Moreira, D. C., Bueno Nunes, J., Eaton, P., Leite, J. R. S. d. A., & Vasconcelos, A. G. (2025). Antioxidant Activity and Cytotoxicity of Baru Nut Oil (Dipteryx alata Vogel) Nanoemulsion in Human Cells. Applied Nano, 6(1), 3. https://doi.org/10.3390/applnano6010003










