Emerging Trends in Nanomaterial-Based Biomedical Aspects
Abstract
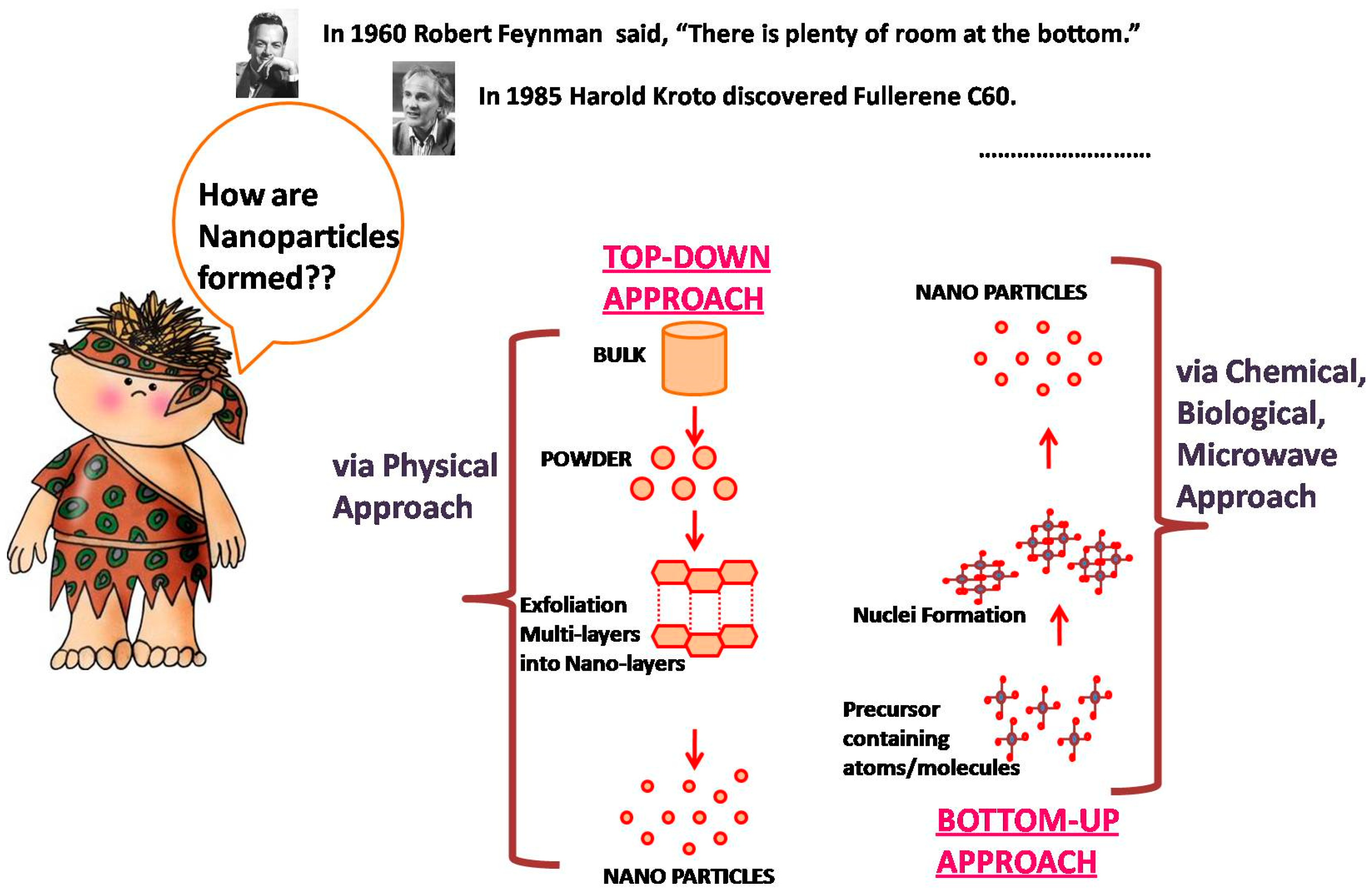
:1. Introduction
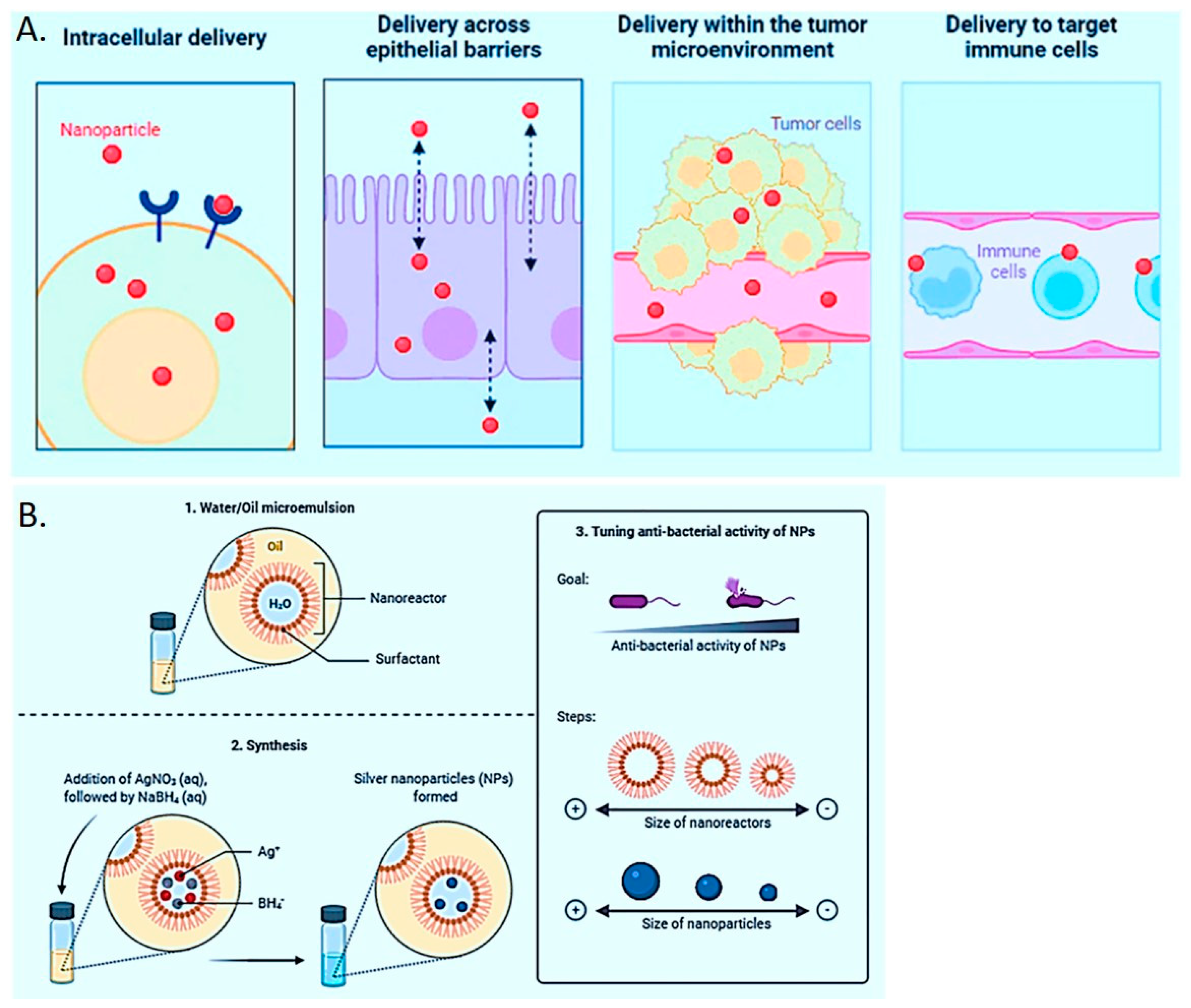
2. Combination of Biology and Nanomaterials
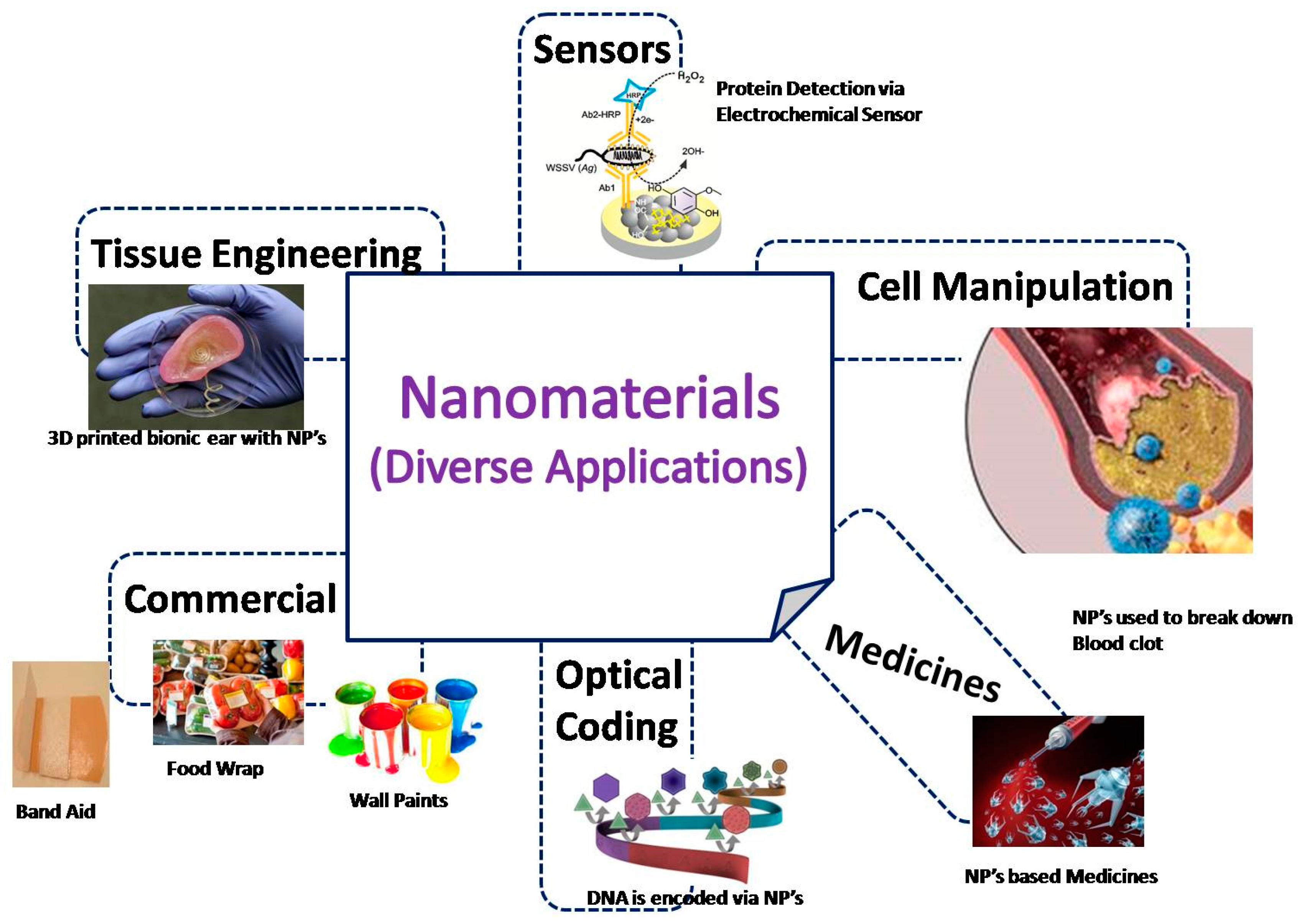
3. Involvement of Nanomaterials in the Domain of Healthcare
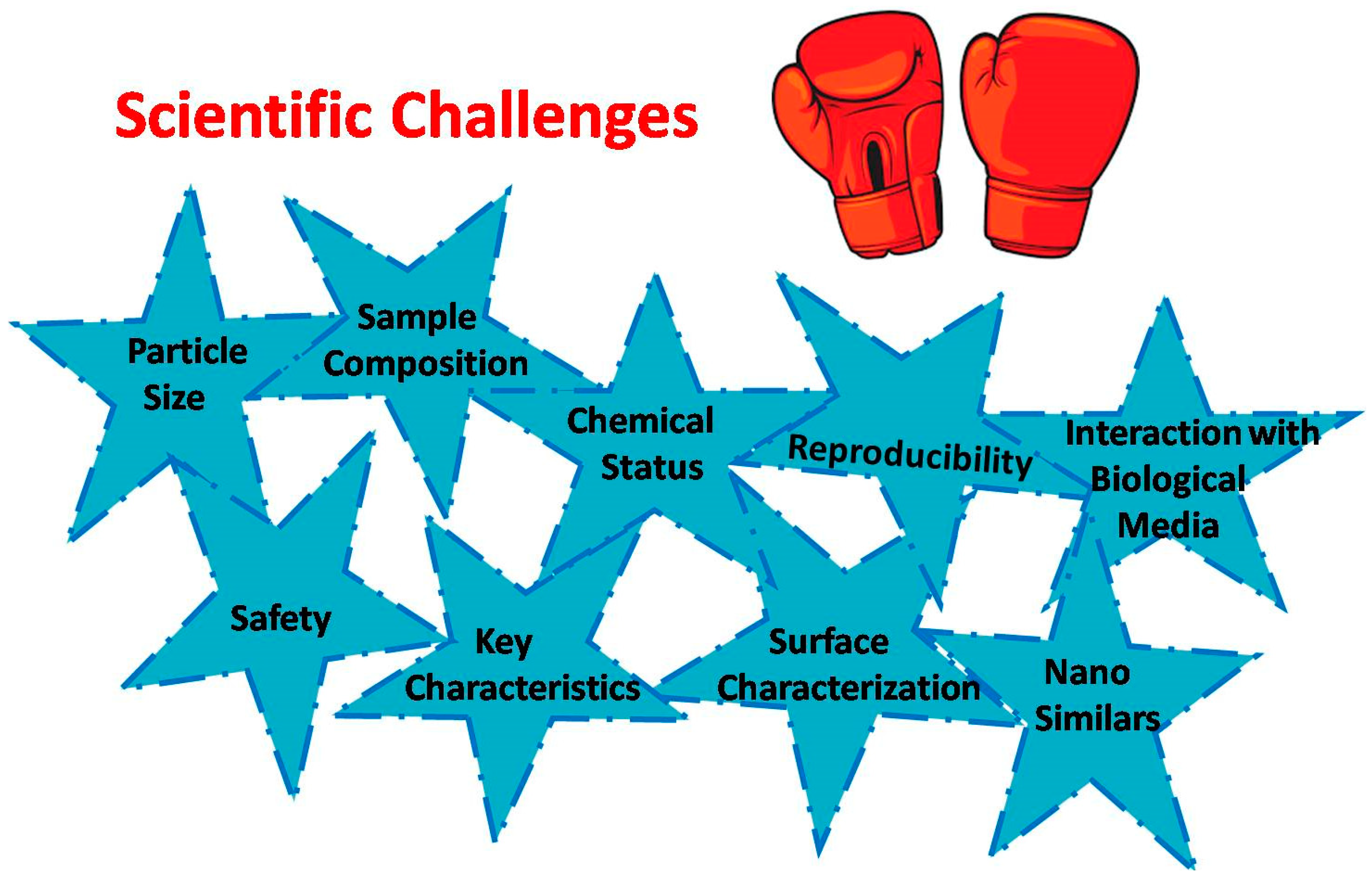
4. Nanomaterial Characterization Techniques
5. Nanotechnological Applications
Application in Sensor Technology
6. Drawbacks/Limitations
- A major shortcoming is the lack of work opportunities in the arenas of traditional farming with the manufacturing and engineering sector. These nanorobots and sophisticated machines have lessened the prominence of manpower but guarantee fast and accurate output;
- This technology has invoked an opportunity for atomic-scale evaluation devices, which would have been virtually impossible. Moreover, since they are very minute, they can be weaponized in due course. These nano-sized weapons would be comparatively easier to create, and therefore novel systems can be initiated. One of the likely prospective is a ‘smart-bullet’, a computerized bullet to facilitate the aim in a controlled and accurate manner. Such kinds of innovations might be a boon for the military, but if not produced thoughtfully and carefully, the consequences could be dire. These kinds of atomic weapon systems are much more powerful and destructive. The potential toxic effect due to industrial-scale manufacturing on human health is unforeseen;
- Mass production of nano-size formulations might not be possible. Such nanotechnological-based procurement for honest and true molecular-sized manufacturing might not be possible;
- There is the probable scope of mass poisoning over the time frame that could eventually lead to health complications for those who consume them, i.e., health deterioration. Inhaled nanoparticulates settle in the brain and lungs, which will lead to an increase in biomarkers causing inflammation and stress;
- Roughly more extravagant negative future scenarios are debunked by experts in nanotechnology. One such example is the so-called ‘gray go’ scenario. This concept was a hypothetical catastrophic scenario that involves molecular nanotechnology wherein self-replicating nanobots consume all the biomass on the Earth while replicating themselves. It was assumed that machines might somehow gain the capability of eating the habitation and self-replicating by accident. It might be due to potential new toxins and pollutants;
- The absence of complete understanding regarding nanotechnology makes it a rather challenging subject for manufacturers. The proper and essential impact of these products needs to be considered equally;
- This technological innovation is quite uneconomical for the generation and assembly of particles in various models and needs expertise. Moreover, technological advances prior to it had caused alterations in many ways with the trend of luxury products, thereby making the system obsolete and expensive. Change in the industrial sector had resulted in huge job losses;
- With the advancements and performance initiatives, certain markets like diamond and oil have fallen due to the introduction of various alternatives. These alternatives are better off with faster production, robustness and do not require fossil fuels as starting materials. This is one of the major reasons for the fall in the market value of diamonds, as quite massive amounts can be used as potential alternates;
- The risk factor involved in manufacturing accounts for huge money investments with upscaling of nanoplants and customer satisfaction risk could make them bear surplus losses. Maintaining such products is equally costly;
- Issues of practicality, including production from masses such as fossil fuels (like coal and petroleum), have led to the crashing of small-scale industrial sectors as nanotechnology does not leave a single particle unfurnished.
7. Future of Nanomaterials
8. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W. Application of Fe3O4 nanoparticles functionalized carbon nanotubes for electrochemical sensing of DNA hybridization. J. Appl. Electrochem. 2016, 46, 559–566. [Google Scholar] [CrossRef]
- Eldar-Boock, A.; Polyak, D.; Scomparin, A.; Satchi-Fainaro, R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr. Opin. Biotechnol. 2013, 24, 682–689. [Google Scholar] [CrossRef]
- Shivenduuranjan, N.; Bhartenduunathhmishraaeditors, E. (Eds.) Environmental Chemistry for a Sustainable World 37 Environmental Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 5. [Google Scholar]
- Parikh, A.N. Biologically Inspired Nanomaterials: A Conference Report Hybrid opto-plasmonic integration for nanoscale light & heat management View project. arXiv 2007, arXiv:0708.0164. [Google Scholar]
- Allara, D.L. A perspective on surfaces and interfaces. Nature 2005, 437, 638–639. [Google Scholar] [CrossRef]
- Yan, M.; Ren, J. Covalent immobilization of polypropylene thin films. J. Mater. Chem. 2005, 15, 523–527. [Google Scholar] [CrossRef]
- Pursel, S.; Horn, M.W.; Demirel, M.C.; Lakhtakia, A. Growth of sculptured polymer submicronwire assemblies by vapor deposition. Polymer 2005, 46, 9544–9548. [Google Scholar] [CrossRef]
- Vegting, I.L.; Nanayakkara, P.W.B.; van Dongen, A.E.; Vandewalle, E.; van Galen, J.; Kramer, M.H.H.; Bonjer, J.; Koole, G.M.; Visser, M.C. Analysing completion times in an academic emergency department: Coordination of care is the weakest link. Neth. J. Med. 2011, 69, 392–398. [Google Scholar]
- Muthukrishnan, G.; Roberts, C.A.; Chen, Y.C.; Zahn, J.D.; Hancock, W.O. Patterning surface-bound microtubules through reversible DNA hybridization. Nano Lett. 2004, 4, 2127–2132. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Yang, S.; Wang, C.; Sun, X.; Lu, J.; Yin, H.; Jiang, W.; Meng, H.; Rao, F. Bioactive Self-Assembling Peptide Hydrogels Functionalized with Brain-Derived Neurotrophic Factor and Nerve Growth Factor Mimicking Peptides Synergistically Promote Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2994–3005. [Google Scholar] [CrossRef]
- Hynonen, U.; Palva, A. Lactobacillus surface layer proteins: Structure, function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef] [Green Version]
- Pum, D.; Neubauer, A.; Gyoervary, E.; Sára, M.; Sleytr, U.B. S-layer proteins as basic biomolecular building blocks S-layer proteins as basic biomolecular building blocks. Adv. Packag. 2008, 100, 2–8. [Google Scholar]
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef] [Green Version]
- Messner, P.; Pum, D.; Sara, M.; Stetter, K.O.; Sleytr, U.B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J. Bacteriol. 1986, 166, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Allara, D.L.; Nuzzo, R.G. Spontaneously Organized Molecular Assemblies. 1. Formation, Dynamics, and Physical Properties of n-alkanoic acids adsorbed from solution on an oxidized aluminum surface. Langmuir 1985, 1, 45–52. [Google Scholar] [CrossRef]
- Yun, C.S.; Javier, A.; Jennings, T.; Fisher, M.; Hira, S.; Peterson, S.; Hopskin, B.; Reich, N.O.; Strouse, G.F. Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J. Am. Chem. Soc. 2005, 127, 3115–3119. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Reed, S.M.; Clegg, R.S.; Hutchison, J.E. Tuning the Electrical Properties of Monolayers by Using Internal Peptide Bonds. ACS Symp. Ser. 2003, 844, 36–50. [Google Scholar]
- You, C.C.; De, M.; Rotello, V.M. Monolayer-protected nanoparticle-protein interactions. Curr. Opin. Chem. Biol. 2005, 9, 639–646. [Google Scholar] [CrossRef]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into Enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1882. [Google Scholar] [CrossRef]
- Rice, M.K.; Ruder, W.C. Creating biological nanomaterials using synthetic biology. Sci. Technol. Adv. Mater. 2014, 15, 014401. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Press, D. IJN-16923-new-generation-of-cancer-treatment--carbon-nanotubes-with-fl. Dovepress 2011, 2011, 2963–2979. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The Advances of Carbon Nanotubes in Cancer Diagnostics and Therapeutics. J. Nanomater. 2017, 2017, 3418932. [Google Scholar] [CrossRef] [Green Version]
- Kam, N.W.S.; Dai, H. Single walled carbon nanotubes for transport and delivery of biological cargos. Phys Status Solidi B Basic Res. 2006, 243, 3561–3566. [Google Scholar] [CrossRef] [Green Version]
- da Silva Bruckmann, F.; Nunes, F.B.; da Rosa Salles, T.; Franco, C.; Cadoná, F.C.; Bohn Rhoden, C.R. Biological Applications of Silica-Based Nanoparticles. Magnetochemistry 2022, 8, 131. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria–“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Guru, E.; Chatterjee, S. Study of Synthetic Biomolecular Network in Escherichia Coli. Int. J. Biophys. 2013, 3, 38–50. [Google Scholar]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J. Nanotechnol. 2012, 2012, 270383. [Google Scholar] [CrossRef] [Green Version]
- DeLong, R.K.; Akhtar, U.; Sallee, M.; Parker, B.; Barber, S.; Zhang, J.; Caig, M.; Garrad, R.; Hickey, A.J.; Engstrom, E. Characterization and performance of nucleic acid nanoparticles combined with protamine and gold. Biomaterials 2009, 30, 6451–6459. [Google Scholar] [CrossRef] [Green Version]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85. [Google Scholar] [CrossRef]
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors 2023, 23, 2240. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, J.; Shis, D.; Gupta, C.; Long, J.; Wagner, D.; Ott, W.; Josic, K.; Bennett, M. Tuning the dynamic range of bacteria; promoters regulated by ligand-inducible transcription factors. Nat. Comm. 2018, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Rudge, T.J.; Brown, J.R.; Federici, F.; Dalchau, N.; Phillips, A.; Ajioka, J.W.; Haseloff, J. Characterization of Intrinsic Properties of Promoters. ACS Synth. Biol. 2016, 5, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, L.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Golbabaei, F.; Ebrahimi, A.; Shirkhanloo, H.; Koohpaei, A.; Faghihi-zarandi, A. Single-Walled Carbon Nanotubes (SWCNTs), as a Novel Sorbent for Determination of Mercury in Air. Glob. J. Heal. Sci. 2016, 8, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA A Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Methods for nanoemulsion and nanoencapsulation of food bioactives. Environ. Chem. Lett. 2019, 17, 1471–1483. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsions in food: Market demand. Environ. Chem. Lett. 2019, 17, 1003–1009. [Google Scholar] [CrossRef]
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Food-grade nanoencapsulation of vitamins. Environ. Chem. Lett. 2019, 17, 991–1002. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Silver nanoparticles engineered by thermal co-reduction approach induces liver damage in Wistar rats: Acute and sub-chronic toxicity analysis. 3 Biotech 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Singh, S.; Gandhi, M. Toxicity and regulations of food nanomaterials. Environ. Chem. Lett. 2018, 17, 929–944. [Google Scholar] [CrossRef]
- Sharma, A.K.; Keservani, R.K.; Kesharwani, R.K. Nanobiomaterials Applications in Drug Delivery; Apple Academic Press: Waretown, NJ, USA, 2021; ISBN 9781774636442. [Google Scholar]
- Sharmila, G.; Muthukumaran, C.; Sangeetha, E.; Saraswathi, H.; Soundarya, S.; Kumar, N.M. Green fabrication, characterization of Pisonia alba leaf extract derived MgO nanoparticles and its biological applications. Nano-Struct. Nano-Objects 2019, 20, 100380. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Fakayode, O.J.; Songca, S.P.; Oluwafemi, O.S. Evolution of gluconic acid capped paramagnetic iron oxide nanoparticles. Nano-Struct. Nano-Objects 2019, 20, 100389. [Google Scholar] [CrossRef]
- Bharti, D.K.; Gupta, M.K.; Srivastava, A.K. Temperature dependent dielectric and electric properties of zinc silicate nanorods. Nano-Struct. Nano-Objects 2019, 17, 123–128. [Google Scholar] [CrossRef]
- Knipe, J.; Peters, J.; Peppas, N. Theranostic agents for intracellular gene delivery with spatiotemporal imaging. Nano Today 2013, 8, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Ganson, N.J.; Kelly, S.J.; Scarlett, E.; Sundy, J.S.; Hershfield, M.S. Research article Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly (ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Thromb. Haemost. 2006, 8, R12. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2012, 12, 39–50. [Google Scholar] [CrossRef]
- Kamada, H.; Tsutsumi, Y.; Sato-Kamada, K.; Yamamoto, Y.; Yoshioka, Y.; Okamoto, T.; Nakagawa, S.; Nagata, S.; Mayumi, T. Synthesis of a poly (vinylpyrrolidone-co-dimethyl maleic anhydride) co-polymer and its application for renal drug targeting. Nat. Biotechnol. 2003, 21, 399–404. [Google Scholar] [CrossRef]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A. Co-delivery of paclitaxel and cisplatin with biocompatible PLGA-PEG nanoparticles enhances chemoradiotherapy in non-small cell lung cancel models. J. Mat. Chem. B 2017, 5, 6045–6057. [Google Scholar] [CrossRef]
- Farazuddin, M.; Sharma, B.; Khan, A.A.; Joshi, B.; Owais, M. Anticancer efficacy of perillyl alcohol-bearing PLGA microparticles. Int. J. Nanomed. 2012, 7, 35–47. [Google Scholar]
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 1–19. [Google Scholar] [CrossRef]
- Papasani, M.R.; Wang, G.; Hill, R.A. Gold nanoparticles: The importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine 2012, 8, 804–814. [Google Scholar] [CrossRef]
- McCarroll, J.; Baigude, H.; Yang, C.; Rana, T. Nanotubes functionalized with lipids and natural amino acid dendrimers: A new strategy to create nanomaterials for delivering systemic RNAi. Bioconjug. Chem. 2010, 1, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Toxicity of nanoparticles Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Hong, R.; Han, G.; Ferna, J.M.; Kim, B.; Forbes, N.S.; Rotello, V.M. Glutathione-Mediated Delivery and Release Using Monolayer Protected Nanoparticle Carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef]
- Polizzi, M.A.; Stasko, N.A.; Schoenfisch, M.H. Water-Soluble Nitric Oxide-Releasing Gold Nanoparticles. Langmuir 2007, 23, 4938–4943. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, Y.-P.; Wang, C.-W.; Tzeng, H.-C.; Wu, C.-H.; Chen, Y.-C.; Chen, C.-P.; Chen, L.-C.; Wu⊥, Y.-C. DNA−Gold Nanorod Conjugates for Remote Control of Localized Gene Expression by near Infrared Irradiation. J. Am. Chem. Soc. 2006, 128, 3709–3715. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potential in detection of liver cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar]
- Dreaden, E.; Mwakwari, S.; Sodji, Q.; Oyelere, A.; Sayed, M. Tamoxifen-Poly(ethylene glycol)-thiol gold nanoparticle conjugates: Enhanced potency and selective delivery for breast cancer treatment. Bioconjug Chem. 2010, 20, 2247–2253. [Google Scholar] [CrossRef] [Green Version]
- Ducan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Control. Release 2011, 148, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Elbakry, A.; Zaky, A.; Liebl, R.; Rachel, R.; Goepferich, A.; Breunig, M. Layer-by-Layer Assembled Gold Nanoparticles for siRNA Delivery. Nano Lett. 2009, 9, 2059–2064. [Google Scholar] [CrossRef]
- Song, W.; Du, J.; Sun, T.; Zhang, P.; Wang, J. Gold Nanoparticles Capped with Polyethyleneimine for Enhanced siRNA Delivery. Small 2010, 6, 239–246. [Google Scholar] [CrossRef]
- Biocompatibility, G.; Han, L.; Zhao, J.; Zhang, X.; Cao, W.; Hu, X.; Zou, G.; Duan, X.; Liang, X.-J. Enhanced siRNA Delivery and Silencing Gold À Chitosan Nanosystem with Surface Charge-Reversal Polymer. ACS Nano 2012, 6, 7340–7351. [Google Scholar]
- Fraguas-Sánchez, A.I.; Lozza, I.; Torres-Suárez, A.I. Actively Targeted Nanomedicines in Breast Cancer: From Pre-Clinal Investigation to Clinic. Cancers 2022, 14, 1198. [Google Scholar] [CrossRef]
- Obozina, A.S.; Komedchikova, E.N.; Kolesnikova, O.A.; Iureva, A.M.; Kovalenko, V.L.; Zavalko, F.A.; Rozhnikova, T.V.; Tereshina, E.D.; Mochalova, E.N.; Shipunova, V.O. Genetically Encoded Self-Assembling Protein Nanoparticles for the Targeted Delivery In Vitro and In Vivo. Pharmaceutics 2023, 15, 231. [Google Scholar] [CrossRef]
- Bonoiu, A.C.; Mahajan, S.D.; Ding, H.; Roy, I.; Yong, K.; Kumar, R.; Hu, R.; Bergey, E.L.; Schwartz, S.A.; Prasad, P.N. Nanotechnology approach for drug addiction therapy: Gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc. Nalt. Acad. Sci. USA 2009, 106, 5546–5550. [Google Scholar] [CrossRef]
- Massich, M.D.; Giljohann, D.A.; Seferos, D.S.; Ludlow, L.E.; Horvath, C.M.; Mirkin, C.A. Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates. Mol. Pharmaceutics 2009, 6, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Meade, B.R.; Dowdy, S.F. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv. Drug Deliv. Rev. 2008, 60, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Salvador-morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green, M.L.H.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Chen, S.; Ray, S.S.; Jaiswal, N.; Ranjan, S. Phyto-nanosensors: Advancement of phytochemicals as an electrochemical platform for various biomedical applications. Environ. Nanotechnol. 2021, 5, 311–338. [Google Scholar] [CrossRef]
- Zhao, P.; Tian, Y.; You, J.; Hu, X.; Liu, Y. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Chubarov, A.; Dmitrienko, E. pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment. Pharmaceutics 2023, 15, 771. [Google Scholar] [CrossRef] [PubMed]
- Chevallet, M.; Veronesi, G.; Fuchs, A.; Mintz, E.; Michaud-Soret, I.; Deniaud, A. Impact of labile metal nanoparticles on cellular homeostasis. Current developments in imaging, synthesis and applications. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, X.; Nakayama-ratchford, N.; Dai, H. Supramolecular Chemistry on Water- Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Gassara, F.; Brar, S.K. Nanoparticles—Production and Role in Biotransformation Copyright: American Scientific Publishers. J. Nanosci. Nanotechnol. 2011, 11, 899–918. [Google Scholar] [CrossRef]
- Sitharaman, B.; Wilson, L.J. Gadofullerenes and gadonanotubes: A new paradigm for high-performance magnetic resonance imaging contrast agent probes. J. Biomed. Nanotechnol. 2007, 3, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Sitharaman, B.; Bolskar, R.D.; Rusakova, I.; Wilson, L.J. Gd@C 60 [C(COOH)2 ]10 and Gd@C60(OH)x: Nanoscale Aggregation Studies of Two Metallofullerene MRI Contrast Agents in Aqueous Solution. Nano Lett. 2004, 4, 2373–2378. [Google Scholar] [CrossRef]
- Galanis, A.; Rauch, E.; Arenal, R.; Portillo, J.; Casablanca, J.; Margiolaki, I. A novelty for cultural heritage material analysis: Transmission Electron Microscope (TEM) 3D electron diffraction tomography applied to Roman glass tesserae. Microchem. J. 2017, 138, 19–25. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, J.-K.; Jeong, Y.-M.; Cheong, S.-I.; Ahn, Y.-C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Suriamoorthy, P.; Zhang, X.; Hao, G. Folic acid-CdTe quantum dot conjugates and their applications for cancer cell targeting. Cancer Nanotechnol. 2010, 1, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.C.; Jin, R.; Nam, J.; Thaxton, C.S.; Mirkin, C.A. Raman Dye-Labeled Nanoparticle Probes for Proteins. J. Am. Chem. Soc. 2003, 125, 14676–14677. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Brenza, T.M.; McMillan, J.M.; Narasimhan, B.; Sakaguchi, D.S.; Sharma, A.D.; Zbarska, S.; Gendelman, H.E. Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 715–729. [Google Scholar] [CrossRef] [Green Version]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-Based Nanoparticles Entrapping Water-Insoluble Photosensitizing Anticancer Drugs: A Novel Drug—Carrier System for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Radtke, A.; Tomasz, J. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Claro, A.P.R.A.; Konatu, R.T.; Escada, A.L.D.A.; Nunes, M.C.D.S.; Maurer-Morelli, C.V.; Dias-Netipanyj, M.F.; Popat, K.C.; Mantovani, D. Incorporation of Silver Nanoparticles on Ti7.5Mo Alloy Surface Containing TiO2 Nanotubes Arrays for Promoting Antibacterial Coating—In Vitro And In Vivo Study. Appl. Surf. Sci. 2018, 455, 780–788. [Google Scholar] [CrossRef]
- Bark, D.; Vahabi, H.; Bui, H.; Movafaghi, H.; Kota, A.; Popat, K.; Dasi, L.P. Hemodynamic performance and thrombogenic properties of superhydrophobic bileaflet mechanical heart valve. Annala Biomed. Eng. 2018, 45, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.C.W. Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding. J. Biomed. Opt. 2002, 7, 532–537. [Google Scholar] [CrossRef]
- Gertz, F.; Khitun, A.; Gertz, F.; Khitun, A. Biological cell manipulation by magnetic nanoparticles Biological cell manipulation by magnetic nanoparticles. AIP Adv. 2016, 6, 025308. [Google Scholar] [CrossRef] [Green Version]
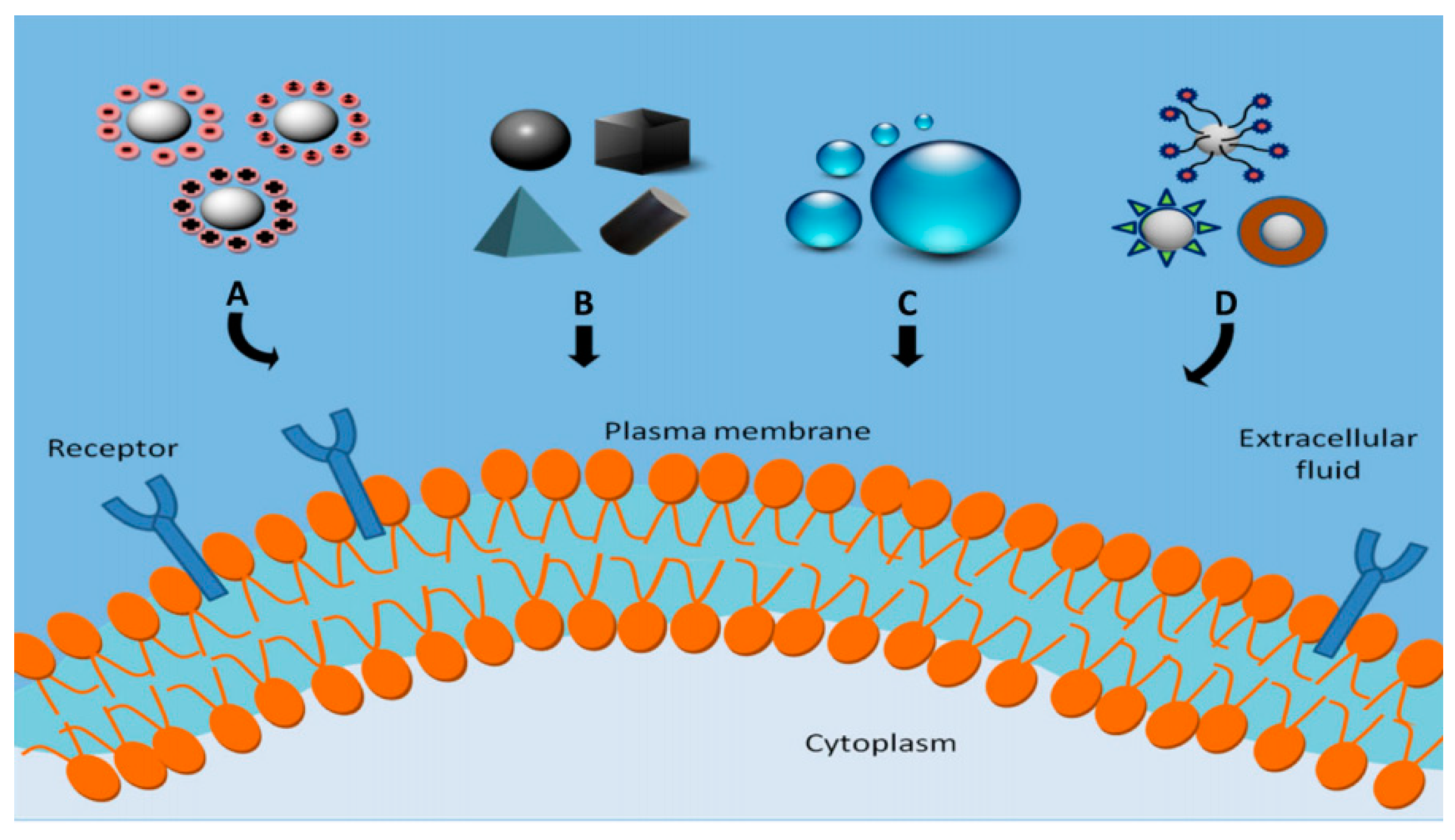
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Gandhi, M.; Chen, S.-S.; Chang, H.-M.; Cao, D.-T.; Huy, L.Q. Anti-wetting behaviour of Superhydrophobic Octadecyltrimethoxysilane blended PVDF/Recycled Carbon Black Composite Membrane for enhanced desalination. Environ. Sci. Water Res. Technol. 2018, 4, 1612–1623. [Google Scholar] [CrossRef]
- Ma, W.; Aboagye-Mensah, D.; Soloviev, M.; Davletov, B.; Ferrari, E. ScienceDirect Symposium on Flexible Organic Electronics Protein Conjugation to Nanoparticles by Designer Affinity Tags. Mater. Today Proc. 2017, 4, 6923–6929. [Google Scholar] [CrossRef]
- Bruchez, M.P.; Weiss, S.; Angeles, L. Semiconductor Nanocrystals as Fluorescent Biological Labels Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 2013, 281, 2013–2016. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Rajagopal, D.; Parthasarathy, S.; Raja, S.; Huang, S.; Kumar, A.S. In Situ Immobilized Sesamol-Quinone / Carbon Nanoblack-Based Electrochemical Redox Platform for Efficient Bioelectrocatalytic and Immunosensor Applications. ACS Omega 2018, 3, 10823–10835. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Retout, M.; Valkenier, H.; Triffaux, E.; Doneux, T.; Bartik, K.; Bruylants, G. Rapid and Selective Detection of Proteins by Dual Trapping using Gold Nanoparticles functionalized with Peptide Aptamers. ACS Sensors 2016, 1, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Yao, R.; Mao, S.; Chen, X.; Na, J.; Sun, W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015, 7, 044101. [Google Scholar] [CrossRef]
- Mahtab, R.; Murphy, C.J. Probing DNA Structure With Nanoparticles. Method Mol. Biol. 2005, 303, 179–190. [Google Scholar]
- Chatterjee, S.; Basu, S.; Paul, T.; Bandyopadhyay, A.; Chattopadhyay, D.; Sarkar, K. A superparamagnetic nanoparticle based approach towards the purification of biomolecules. In Proceedings of the 4th International Conference on Advanced Nano Materials, IIT-Madras, Chennai, India, 17–19 October 2012. [Google Scholar]
- Parak, W.J.; Boudreau, R.; Le Gros, M.; Gerion, D.; Zanchet, D.; Micheel, C.M.; Willaims, S.C.; Alivisatos, A.P.; Larabell, C. Cell Motility and Metastatic Potential Studies Based on Quantum Dot Imaging of Phagokinetic Tracks. Adv. Mater. 2002, 4095, 10–14. [Google Scholar]
- Mishra, S.; Sundaram, B. Fate, transport and toxicity of nanoparticles: An emerging pollutant on biotic factors. Process Saf. Environ. 2023, 174, 595–607. [Google Scholar] [CrossRef]
- Gandhi, M.; Rajagopal, D.; Senthil Kumar, A. Facile Electrochemical Demethylation of 2-Methoxyphenol to Surface-Confined Catechol on the MWCNT and Its Efficient Electrocatalytic Hydrazine Oxidation and Sensing Applications. ACS Omega 2020, 5, 16208–16219. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M. RSC Advances In situ electro-organic synthesis of hydroquinone using anisole on MWCNT / Na fi on modi fi ed electrode surface and its heterogeneous. RSC Adv. 2021, 11, 4062–4076. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Rajagopal, D.; Senthil Kumar, A. Molecularly wiring of Cytochrome c with carboxylic acid functionalized hydroquinone on MWCNT surface and its bioelectrocatalytic reduction of H2O2 relevance to biomimetic electron-transport and redox signalling. Electrochimica Acta 2021, 368, 137596. [Google Scholar] [CrossRef]
- Gandhi, M.; Rajagopal, D.; Senthil Kumar, A. In-situ Electro-organic Conversion of Lignocellulosic-Biomass Product-Syringaldehyde to a MWCNT Surface-Confined Hydroquinone Electrocatalyst for Biofuel Cell and Sensing of Ascorbic acid Applications. Appl. Surf. Sci. 2021, 562, 150158. [Google Scholar] [CrossRef]
- Vishnu, N.; Gandhi, M.; Badhulika, S.; Kumar, A.S. Tea quality testing using 6B pencil lead as an electrochemical sensor. Anal. Methods 2018, 10, 2327–2336. [Google Scholar] [CrossRef]
- Pujalte, I.; Passagne, I.; Daculsi, R.; Portal, C.; Courtes, C.; Azous, B. Cytotoxic effects and cellular oxidative mechanisms of metallic nanoparticles on renal tubular cells: Impact of particle solubility. Toxicol. Res. 2014, 4, 409–422. [Google Scholar] [CrossRef]
- Badrealam, K.F.; Owais, M. Nanoscale Drug Delivery Systems: An Updated View. 2014, pp. 180–204. Available online: https://api.semanticscholar.org/CorpusID:33201981 (accessed on 12 June 2023).
- Nirmala, A.; Sonar, G.P.; Bhardwaj, P.; Chakravorty, A. Materials Horizons: From Nature to Nanomaterials Handbook of Porous Carbon Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2023; ISBN-10:9811971870. [Google Scholar]
- Nebu, J.; Anu, K.S.; Devi, J.A.; Aparna, R.; Aswathy, A.; Lekha, G.; Sony, G. Pottasium triiodide enhanced turn-off sensing of tyrosine in carbon dot platform. Microchem. J. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Gandhi, M.; Amreen, K.; Tiwari, B.K. Recent Advances in the Electro-Active Therapeutic Phytochemical-Based Sensors. Electrochem 2022, 3, 613–632. [Google Scholar] [CrossRef]
- Gandhi, M.; Indiramma, J.; Jayaprakash, N.S.; Kumar, A.S. An efficient electrochemical sandwich ELISA for urinary human serum albumin-biomarker based on highly redox-active thionine surface-confined MWCNT/PEDOT.PSS platform. J. Electroanal. Chem. 2022, 906, 116018. [Google Scholar] [CrossRef]



| Companies | Work of Interest | Nanoparticles Used | Technological B |
|---|---|---|---|
| Advectus Life Science, Inc., West Vancouver, BC, Canada | Drug Delivery Approach | poly (D,L-lactide-co-glycolide) (PLGA) | Polymeric nanoparticles engineered to carry anti-tumor drugs across the blood–brain barrier |
| Alnis Biosciences, Inc., Bancroft, Berkeley, CA, USA | Bio-Pharmaceutical | - | Biodegradable nanoparticles for drug delivery |
| Argonide, Sanford, FL, USA | Membrane Filtration | - | Nanoporous ceramic material for endotoxin filtration, orthopedics and dental implants, DNA and protein separation |
| Biophan Technologies, Inc., NY, USA | MRI shielding | - | Nanomagnetic/carbon composite material to shield medical devices from RF field |
| BASF, Ludwigshafen, Germany | Toothpaste | Hydroxyapatite NPs | Hydroxyapatite NPs improve dental surface |
| Capsulation Nanoscience AG, Berlin, Germany | Improve drug solubility | - | Layer-by-layer poly-electrolyte coating, 8–50 nm |
| Eiffel Biotech, Taiwan, ROC | Drug delivery | - | Size reduction of drug particles to 50–100 nm |
| Enviro Systems, Inc., OK, USA | Surface disinfection | - | Nano-emulsions |
| Dynal Biotech, Carlsbad, CA, USA | - | Magnetic beads | |
| Immunicon, PA, USA | Tracking and separation of different cell types | - | Magnetic core surrounded by a polymeric layer coated with antibody for capturing cells |
| KES Science and Technology, Inc., USA | Airocide Filters | Nano-TiO2 | To destroy airborne pathogens |
| Evident Technologies, NY, USA | Luminescent biomarkers | - | Semiconductor QDs with amine, carboxyl functional groups having emissions from 350–2500 nm |
| Nanobio corporation, USA | Pharmaceutical | - | Antimicrobial nano-emulsion |
| Nanocarrier Co., Ltd., Tokyo, Japan | Drug delivery | - | Micellar NPs for encapsulation of drugs, proteins, and DNA |
| NanoPharma AG, Czech Republic | Drug delivery | Polybutylcyanoacrylate NPs | NP coated with drugs and later with surfactant to go across blood–brain barrier |
| Nanoplex Technologies, Inc., CA, USA | Nanobar codes for bioanalysis | - | |
| Nanoprobes, Inc., Yaphank, NY, USA | Biological Markers | AuNPs | Bio-conjugates for TEM and fluorescent microscopy |
| Nanosphere, Inc., Northbrook, IL, USA | Gold biomarkers | Au and Ag NPs | DNA barcode attached to nanoprobe for identification purposes; PCR to amplify the signal; catalytic silver deposition to amplify signal using surface plasmon resonance |
| Nanomed Pharmaceutical, Inc., MI, USA | Drug delivery | - | NP for drug delivery |
| Oxonica Ltd., UK | Sunscreens | - | Doped transparent NPs to absorb harmful UV and convert to heat |
| PSi Vida Ltd., MA, USA | Tissue engineering, implants, drug and gene delivery, bio-filtration | - | Exploiting material properties of nanostructures porous silicone |
| Smith & Nephew, Watford, UK | Acticoatbandages | Ag nanocrystal | Nanocrystal silver is highly toxic to pathogens |
| Quantum Dot corporation, CA, USA | Luminescent biomarkers | - | Bio-conjugated semiconductor QD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, M.; Amreen, K. Emerging Trends in Nanomaterial-Based Biomedical Aspects. Electrochem 2023, 4, 365-388. https://doi.org/10.3390/electrochem4030024
Gandhi M, Amreen K. Emerging Trends in Nanomaterial-Based Biomedical Aspects. Electrochem. 2023; 4(3):365-388. https://doi.org/10.3390/electrochem4030024
Chicago/Turabian StyleGandhi, Mansi, and Khairunnisa Amreen. 2023. "Emerging Trends in Nanomaterial-Based Biomedical Aspects" Electrochem 4, no. 3: 365-388. https://doi.org/10.3390/electrochem4030024
APA StyleGandhi, M., & Amreen, K. (2023). Emerging Trends in Nanomaterial-Based Biomedical Aspects. Electrochem, 4(3), 365-388. https://doi.org/10.3390/electrochem4030024







