Environment-Friendly Ascorbic Acid Fuel Cell
Abstract
1. Introduction
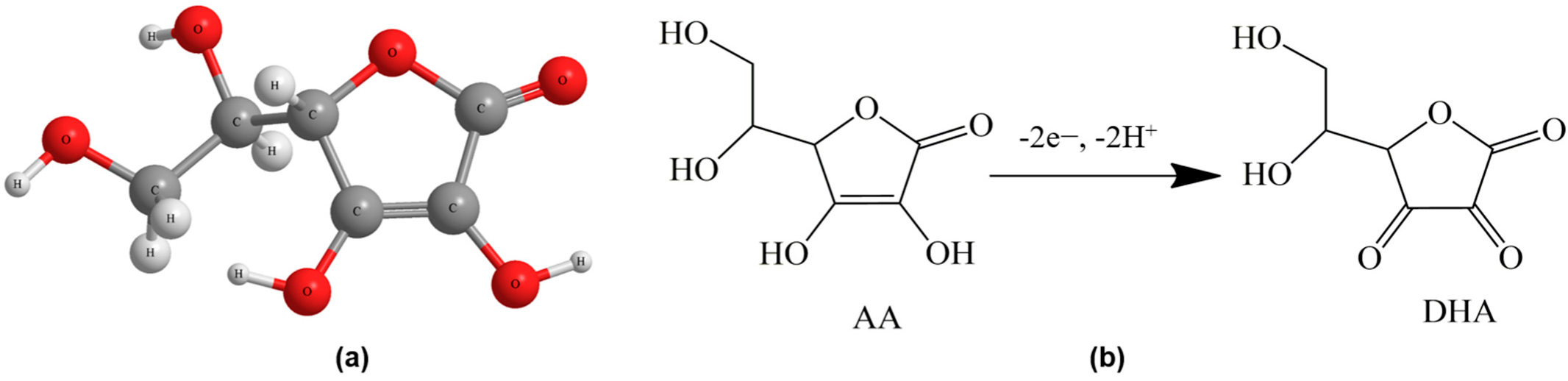
2. An Overview of Ascorbic Acid
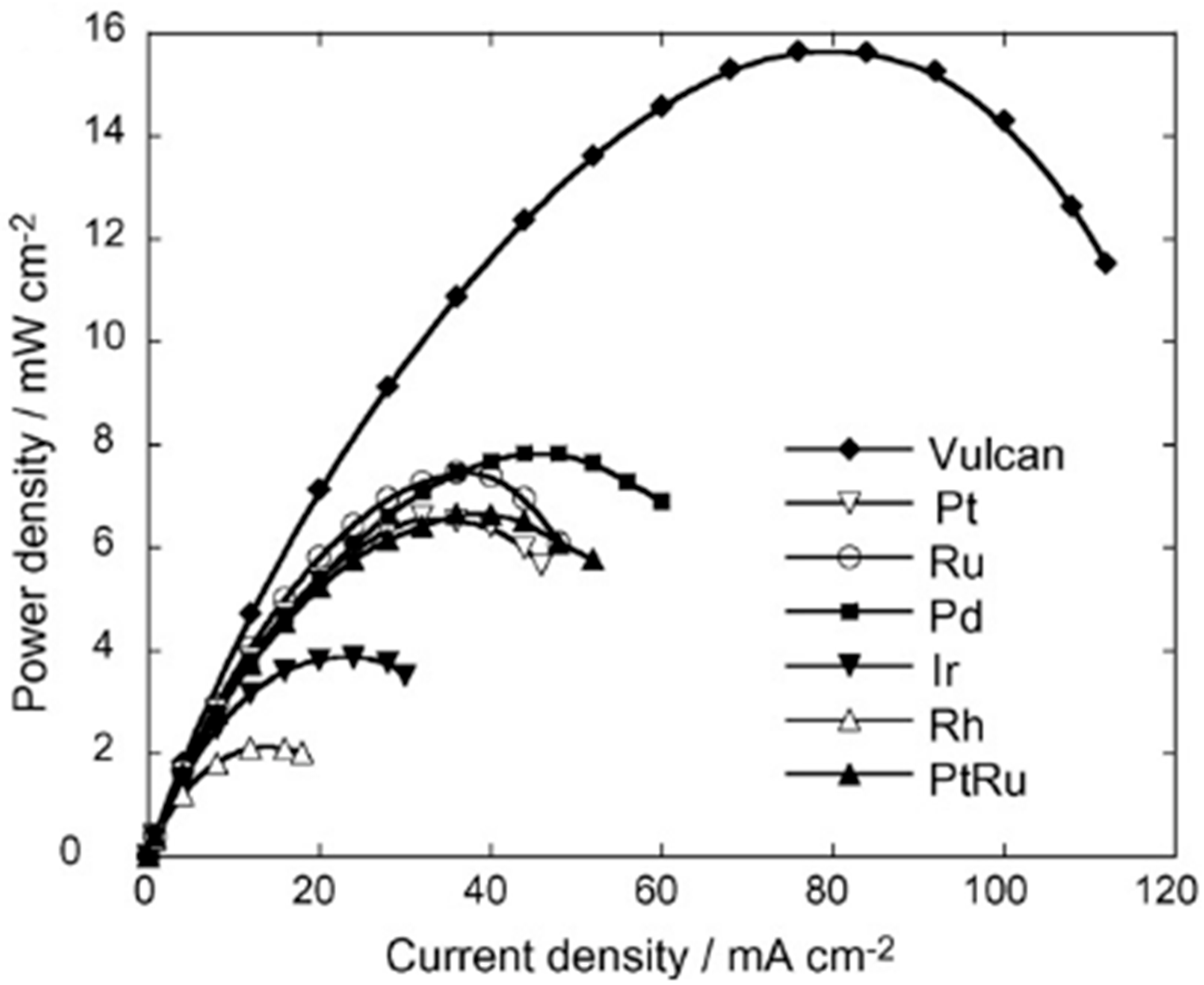
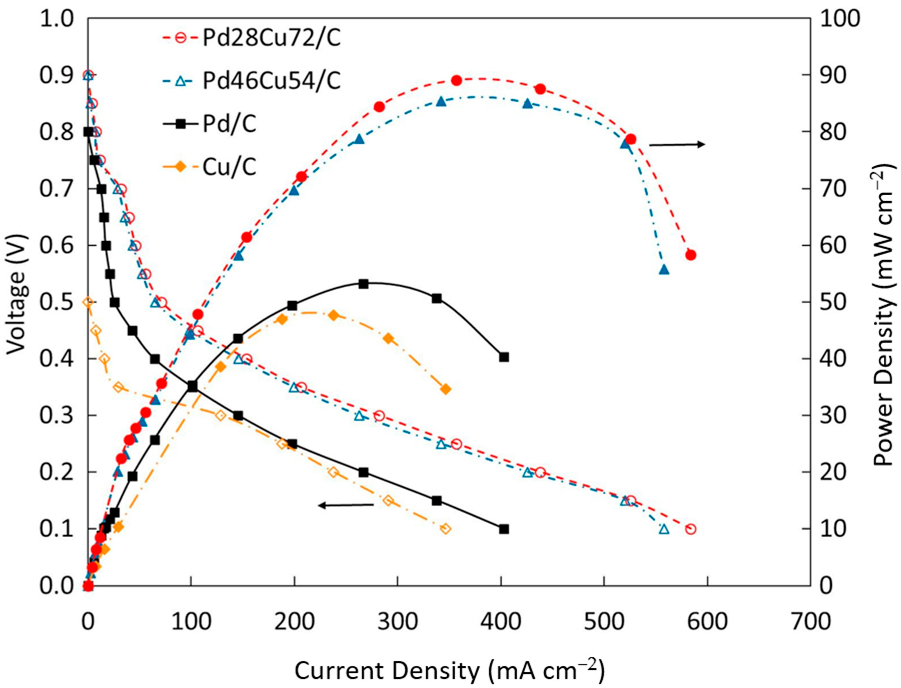
3. Catalysts for Ascorbic Acid Electrooxidation
4. Ascorbic Acid as Fuel
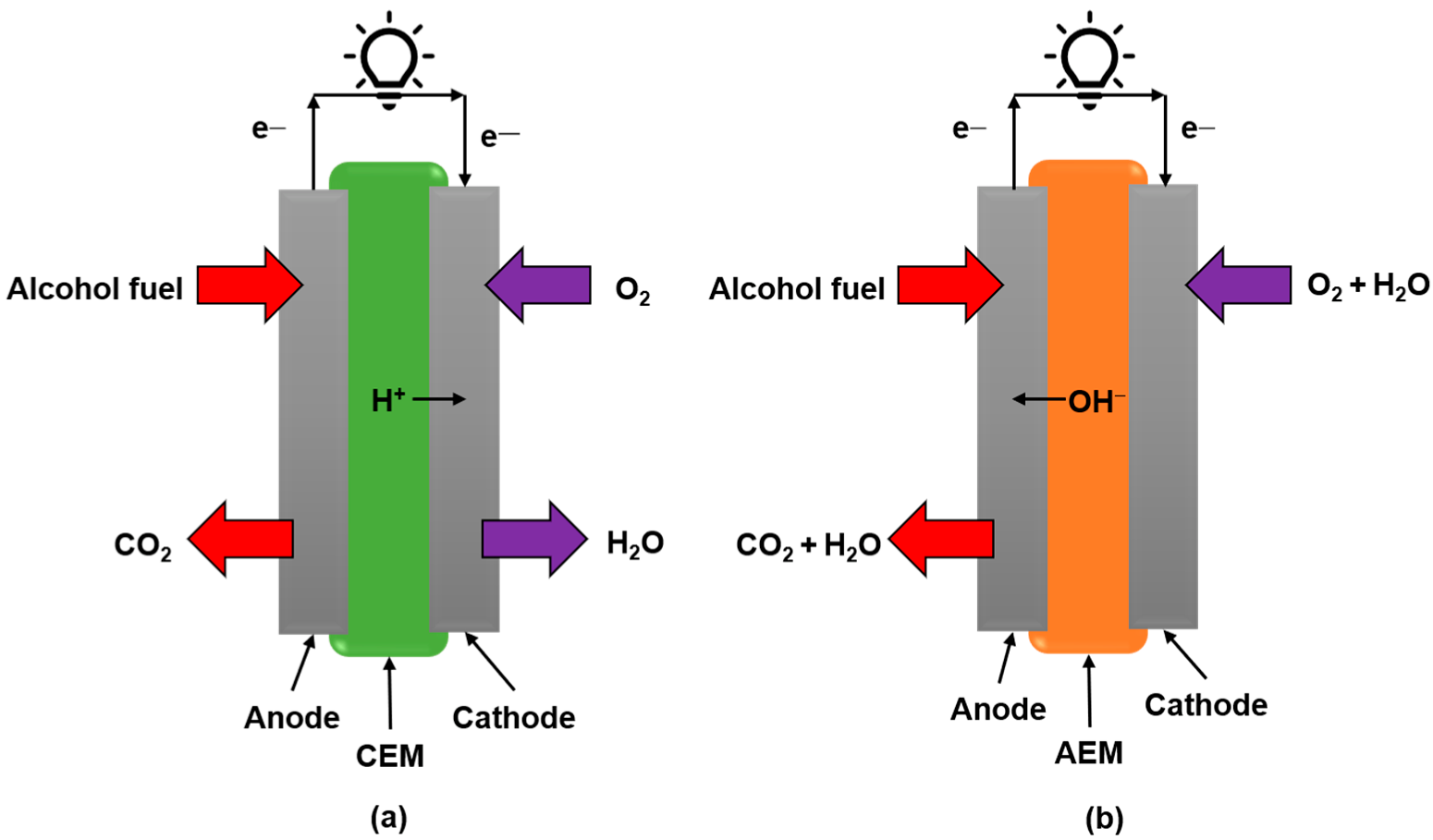
4.1. Acidic Fuel Cell
4.2. Alkaline Fuel Cell
4.3. Split-pH (Alkaline–Acid) Fuel Cells
- Anode: A2− = DHA + 2e−; Eanode = −0.42 V
- Cathode: H2O2 + 2H+ +2e− = 2H2O; Ecathode= +1.78 V
- Overall: A2− + H2O2 = DHA + 2H2O; Eoverall= +2.18 V
5. Challenges and Future Scope
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 133, anie.202016977. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst Approaches and Challenges for Automotive Fuel Cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct Liquid Fuel Cells: A Review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakub, Z. Nanocatalyst for Direct Methanol Fuel Cell (DMFC). Int. J. Hydrogen Energy 2010, 35, 7957–7970. [Google Scholar] [CrossRef]
- Haan, J.L.; Stafford, K.M.; Masel, R.I. Effects of the Addition of Antimony, Tin, and Lead to Palladium Catalyst Formulations for the Direct Formic Acid Fuel Cell. J. Phys. Chem. C 2010, 114, 11665–11672. [Google Scholar] [CrossRef]
- Antolini, E. Catalysts for Direct Ethanol Fuel Cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H. Alkaline Fuel Cell Technology—A Review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rakib, R.H.; Hasnat, M.A.; Nagao, Y. Electroless Deposition of Silver Dendrite Nanostructure onto Glassy Carbon Electrode and Its Electrocatalytic Activity for Ascorbic Acid Oxidation. ACS Appl. Energy Mater. 2020, 3, 2907–2915. [Google Scholar] [CrossRef]
- Hasan, M.M.; Nagao, Y. Christmas-Tree-Shaped Palladium Nanostructures Decorated on Glassy Carbon Electrode for Ascorbic Acid Oxidation in Alkaline Condition. ChemistrySelect 2021, 6, 5885–5892. [Google Scholar] [CrossRef]
- Cathcart, R.F. A Unique Function for Ascorbate. Med. Hypotheses 1991, 35, 32–37. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical Methods for Ascorbic Acid Determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Prieto, F.; Coles, B.A.; Compton, R.G. Mechanistic Determination Using Arrays of Variable-Sized Channel Microband Electrodes: The Oxidation of Ascorbic Acid in Aqueous Solution. J. Phys. Chem. B 1998, 102, 7442–7447. [Google Scholar] [CrossRef]
- Rueda, M.; Aldaz, A.; Sanchez-Burgos, F. Oxidation of L-Ascorbic Acid on a Gold Electrode. Electrochim. Acta 1978, 23, 419–424. [Google Scholar] [CrossRef]
- Hu, I.F.; Kuwana, T. Oxidative Mechanism of Ascorbic Acid at Glassy Carbon Electrodes. Anal. Chem. 1986, 58, 3235–3239. [Google Scholar] [CrossRef]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A Theoretical Study of Ascorbic Acid Oxidation and HOO˙/O2˙− Radical Scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Dhara, K.; Debiprosad, R.M. Review on Nanomaterials-Enabled Electrochemical Sensors for Ascorbic Acid Detection. Anal. Biochem. 2019, 586, 113415. [Google Scholar] [CrossRef]
- Kuss, S.; Compton, R.G. Electrocatalytic Detection of Ascorbic Acid Using N,N,N′,N′-Tetramethyl-Para-Phenylene-Diamine (TMPD) Mediated Oxidation at Unmodified Gold Electrodes; Reaction Mechanism and Analytical Application. Electrochim. Acta 2017, 242, 19–24. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; He, W.; Uyama, H.; Xie, Q.; Zhang, L.; Yang, F. Electrochemical Sensor Based on Carbon-Supported NiCoO2 Nanoparticles for Selective Detection of Ascorbic Acid. Biosens. Bioelectron. 2014, 55, 446–451. [Google Scholar] [CrossRef]
- Xi, L.; Ren, D.; Luo, J.; Zhu, Y. Electrochemical Analysis of Ascorbic Acid Using Copper Nanoparticles/Polyaniline Modified Glassy Carbon Electrode. J. Electroanal. Chem. 2010, 650, 127–134. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Baghayeri, M. A Selective Sensor Based on a Glassy Carbon Electrode Modified with Carbon Nanotubes and Ruthenium Oxide/Hexacyanoferrate Film for Simultaneous Determination of Ascorbic Acid, Epinephrine and Uric Acid. Anal. Methods 2011, 3, 2367. [Google Scholar] [CrossRef]
- Lee, Y.G.; Liao, B.X.; Weng, Y.C. Ruthenium Oxide Modified Nickel Electrode for Ascorbic Acid Detection. Chemosphere 2017, 173, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, S.; Chen, S. Preparation and Characterization of PtAu Hybrid Film Modified Electrodes and Their Use in Simultaneous Determination of Dopamine, Ascorbic Acid and Uric Acid. Talanta 2007, 74, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A Highly Sensitive and Stable Electrochemical Sensor for Simultaneous Detection towards Ascorbic Acid, Dopamine, and Uric Acid Based on the Hierarchical Nanoporous PtTi Alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Bae, I.T.; Shao, M.; Liu, C.C. Electro-Oxidation of l-Ascorbic Acid on Platinum in Acid Solutions: An In-Situ FTIRRAS Study. J. Electroanal. Chem. 1993, 346, 309–321. [Google Scholar] [CrossRef]
- Oko, D.N.; Garbarino, S.; Zhang, J.; Xu, Z.; Chaker, M.; Ma, D.; Guay, D.; Tavares, A.C. Dopamine and Ascorbic Acid Electro-Oxidation on Au, AuPt and Pt Nanoparticles Prepared by Pulse Laser Ablation in Water. Electrochim. Acta 2015, 159, 174–183. [Google Scholar] [CrossRef]
- Islam, M.T.; Hasan, M.M.; Shabik, M.F.; Islam, F.; Nagao, Y.; Hasnat, M.A. Electroless Deposition of Gold Nanoparticles on a Glassy Carbon Surface to Attain Methylene Blue Degradation via Oxygen Reduction Reactions. Electrochim. Acta 2020, 360, 136966. [Google Scholar] [CrossRef]
- Raj, C.R.; Ohsaka, T. Electroanalysis of Ascorbate and Dopamine at a Gold Electrode Modified with a Positively Charged Self-Assembled Monolayer. J. Electroanal. Chem. 2001, 496, 44–49. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Saldaña-Botín, A.; Arcos-Martínez, M.J. Determination of Ascorbic Acid in Serum Samples by Screen-Printed Carbon Electrodes Modified with Gold Nanoparticles. Talanta 2017, 174, 733–737. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Y.; Liu, X.; Rong, M.; Chen, X.; Chen, X. An Electrochemical Ascorbic Acid Sensor Based on Palladium Nanoparticles Supported on Graphene Oxide. Anal. Chim. Acta 2012, 745, 33–37. [Google Scholar] [CrossRef]
- Jiang, J.; Du, X. Sensitive Electrochemical Sensors for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid Based on Au@Pd-Reduced Graphene Oxide Nanocomposites. Nanoscale 2014, 6, 11303–11309. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Taheri, A. Preparation of Silver Hexacyanoferrate Nanoparticles and Its Application for the Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Talanta 2010, 80, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Majari Kasmaee, L.; Gobal, F. A Preliminary Study of the Electro-Oxidation of l-Ascorbic Acid on Polycrystalline Silver in Alkaline Solution. J. Power Sources 2010, 195, 165–169. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Borzoo, M. Green Synthesis of Silver Nanoparticles Using Onion Extract and Their Application for the Preparation of a Modified Electrode for Determination of Ascorbic Acid. J. Food Drug Anal. 2016, 24, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Gobal, F.; Majari Kasmaee, L. Silver Selenide as a Potential Electro-Catalyst for l-Ascorbic Acid Electro-Oxidation in Alkaline Solution. Electrocatalysis 2011, 2, 331–336. [Google Scholar] [CrossRef]
- Harraz, F.A.; Faisal, M.; Al-Salami, A.E.; El-Toni, A.M.; Almadiy, A.A.; Al-Sayari, S.A.; Al-Assiri, M.S. Silver Nanoparticles Decorated Stain-Etched Mesoporous Silicon for Sensitive, Selective Detection of Ascorbic Acid. Mater. Lett. 2019, 234, 96–100. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Nezhad, M.R.H.; Khodavesi, J.; Javadi, S. Silver Nanoparticles Modified Carbon Nanotube Paste Electrode for Simultaneous Determination of Dopamine and Ascorbic Acid. J. Electroanal. Chem. 2009, 633, 85–91. [Google Scholar] [CrossRef]
- Muneeb, O.; Do, E.; Boyd, D.; Perez, J.; Haan, J.L. PdCu/C Anode Catalysts for the Alkaline Ascorbate Fuel Cell. Appl. Energy 2019, 235, 473–479. [Google Scholar] [CrossRef]
- Uhm, S.; Choi, J.; Chung, S.T.; Lee, J. Electrochemically Oxidized Carbon Anode in Direct L-Ascorbic Acid Fuel Cells. Electrochim. Acta 2007, 53, 1731–1736. [Google Scholar] [CrossRef]
- Choun, M.; Lee, H.J.; Lee, J. Positively Charged Carbon Electrocatalyst for Enhanced Power Performance of L-Ascorbic Acid Fuel Cells. J. Energy Chem. 2016, 25, 793–797. [Google Scholar] [CrossRef]
- Sathe, B.R. A Scalable and Facile Synthesis of Carbon Nanospheres as a Metal Free Electrocatalyst for Oxidation of l -Ascorbic Acid: Alternate Fuel for Direct Oxidation Fuel Cells. J. Electroanal. Chem. 2017, 799, 609–616. [Google Scholar] [CrossRef]
- Muneeb, O.; Chino, I.; Saenz, A.; Haan, J.L. An Ascorbate Fuel Cell with Carbon Black Nanoparticles as Anode and Cathode. J. Power Sources 2019, 413, 216–221. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, K.; Xiang, L.; Lin, Y.; Su, L.; Mao, L. Carbon Nanotube-Modified Carbon Fiber Microelectrodes for In Vivo Voltammetric Measurement of Ascorbic Acid in Rat Brain. Anal. Chem. 2007, 79, 6559–6565. [Google Scholar] [CrossRef]
- Qiu, C.; Chen, H.; Liu, H.; Zhai, Z.; Qin, J.; Lv, Y.; Gao, Z.; Song, Y. The Hydrophilicity of Carbon for the Performance Enhancement of Direct Ascorbic Acid Fuel Cells. Int. J. Hydrogen Energy 2018, 43, 21908–21917. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamazaki, S.; Siroma, Z.; Ioroi, T.; Yasuda, K. Direct Oxidation of L-Ascorbic Acid on a Carbon Black Electrode in Acidic Media and Polymer Electrolyte Fuel Cells. Electrochem. Commun. 2006, 8, 720–724. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamazaki, S.; Siroma, Z.; Ioroi, T.; Yasuda, K. L-Ascorbic Acid as an Alternative Fuel for Direct Oxidation Fuel Cells. J. Power Sources 2007, 167, 32–38. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Ioroi, T.; Yasuda, K. Rapid Evaluation of the Electrooxidation of Fuel Compounds with a Multiple-Electrode Setup for Direct Polymer Electrolyte Fuel Cells. J. Power Sources 2007, 164, 457–463. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yasuda, K.; Ioroi, T.; Siroma, Z.; Miyazaki, Y.; Kobayashi, T. Direct Polymer Electrolyte Fuel Cells Using L-Ascorbic Acid as a Fuel. Electrochem. Solid-State Lett. 2003, 6, A257. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic Acid: Chemistry, Biology and the Treatment of Cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Mogi, H.; Fukushi, Y.; Koide, S.; Sano, R.; Sasaki, T.; Nishioka, Y. A Flexible Ascorbic Acid Fuel Cell with a Microchannel Fabricated Using MEMS Techniques. J. Phys. Conf. Ser. 2013, 476, 012065. [Google Scholar] [CrossRef]
- Falk, M.; Andoralov, V.; Blum, Z.; Sotres, J.; Suyatin, D.B.; Ruzgas, T.; Arnebrant, T.; Shleev, S. Biofuel Cell as a Power Source for Electronic Contact Lenses. Biosens. Bioelectron. 2012, 37, 38–45. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Z.; Ying, Y.; Chan, P.C.H. Development of a Miniature Silicon Wafer Fuel Cell Using L-Ascorbic Acid as Fuel. J. Zhejiang Univ. A 2008, 9, 955–960. [Google Scholar] [CrossRef]
- Kaneto, K.; Nishikawa, M.; Uto, S. Characteristics of Ascorbic Acid Fuel Cells Using SWCNT and PEDOTPSS Composite Anodes. Chem. Lett. 2019, 48, 1533–1536. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Zalineeva, A.; Serov, A.; Padilla, M.; Martinez, U.; Artyushkova, K.; Baranton, S.; Coutanceau, C.; Atanassov, P.B. Self-Supported PdxBi Catalysts for the Electrooxidation of Glycerol in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 3937–3945. [Google Scholar] [CrossRef]
- Muneeb, O.; Do, E.; Tran, T.; Boyd, D.; Huynh, M.; Ghosn, G.; Haan, J.L. A Direct Ascorbate Fuel Cell with an Anion Exchange Membrane. J. Power Sources 2017, 351, 74–78. [Google Scholar] [CrossRef]
- An, L.; Zhao, T.S.; Chen, R.; Wu, Q.X. A Novel Direct Ethanol Fuel Cell with High Power Density. J. Power Sources 2011, 196, 6219–6222. [Google Scholar] [CrossRef]
- Shang, Z.; Wycisk, R.; Pintauro, P. Electrospun Composite Proton-Exchange and Anion-Exchange Membranes for Fuel Cells. Energies 2021, 14, 6709. [Google Scholar] [CrossRef]
- Pan, Z.; Zhuang, H.; Bi, Y.; An, L. A Direct Ethylene Glycol Fuel Cell Stack as Air-Independent Power Sources for Underwater and Outer Space Applications. J. Power Sources 2019, 437, 226944. [Google Scholar] [CrossRef]
- Keramati, A.; Hendrix, K.; Nguyen, D.; Gonzales, F.; Waters, K.; Fry-Petit, A.; Haan, J.L. A Non-Precious Metal Ascorbate Fuel Cell. Int. J. Energy Res. 2021, 45, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; He, Y.; Liu, Y.; Jin, L. Performance of Direct Formate-Peroxide Fuel Cells. J. Power Sources 2015, 287, 75–80. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Sun, X.; He, Y. A Sodium-Ion-Conducting Direct Formate Fuel Cell: Generating Electricity and Producing Base. Angew. Chem. Int. Ed. 2017, 56, 5734–5737. [Google Scholar] [CrossRef]
- Mondal, S.K.; Raman, R.K.; Shukla, A.K.; Munichandraiah, N. Electrooxidation of Ascorbic Acid on Polyaniline and Its Implications to Fuel Cells. J. Power Sources 2005, 145, 16–20. [Google Scholar] [CrossRef]

| Anode (Catalyst Loading) | Anode Fuel | Flow (mL/min) | Cathode (Catalyst Loading) | Cathode Fuel | Flow (mL/min) | Membrane | Temp. (°C) | Peak Power Density (mW/cm2) | Current Density (mA/cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Acid-treated Vulcan XC-72 (2 mg/cm2) | 1 M AA and 1 M NaOH | 2 | Acid-treated Vulcan XC-72 (2 mg/cm2) | 30% H2O2 and 1 M H2SO4 | 5 | NaOH treated Nafion 115 CEM | 60 | 158 | ~475 | [41] |
| PdCu/C (2 mg/cm2) | 1 M AA and 1 M KOH | 1 | Pt black (4 mg/cm2) | O2 | 100 | Tokuyama A201 AEM | 60 | 89 | ~560 | [37] |
| Pd catalyst (4 mg/cm2) | 1 M AA and 1 M KOH | 5 | Pt black (2 mg/cm2) | O2 | 100 | Tokuyama A201 AEM | 60 | 73 | 497 | [55] |
| Cu/C (5 mg/cm2) | 1 M AA and 2 M NaOH | 1 | Carbon (5 mg/cm2) | 3% H2O2 and 1 M H2SO4 | 2 | NaOH treated Nafion 115 CEM | 60 | 51 | ~240 | [59] |
| Acid treated carbon (1 mg/cm2) | 1 mM AA + 0.5 M H2SO4 | 15 | Pt/C (1 mg/cm2) | O2 | 200 | Nafion 211 | 80 | 31 | ~180 | [43] |
| Oxidized Carbon (SGL 35AA) (no metal loading) | 1 M AA | 5 | Pt on Carbon (SGL 35BC GDL) (3 mg/cm2) | O2 | 200 | Nafion 115 CEM | 60 | 18 | ~90 | [38] |
| Vulcan XC72 on PTFE sheet (3 mg/cm2) | 0.5 M AA | 4 | Pt black on PTFE sheet (3 mg/cm2) | O2 | 100 | Nafion 117 CEM | 25 | 16 | ~115 | [45] |
| Vulcan XC72 (0.3 mg/cm2) | 0. 5 M AA + 0.5 M H2SO4 | 4 | Pt–PTFE black (3 mg/cm2) | O2 | 100 | Nafion-117 CEM | 25 | 15 | ~85 | [44] |
| aSWCNT@PEDOT*PSS (1 mg/cm2) | 0.5 M AA | 2 | Pt black (3 mg/cm2) | O2 | 100 | Nafion 117 CEM | 25 | 11.3 | ~60 | [52] |
| Poly aniline on TGPH 090 (35 mg/cm2) | 1 M AA in 0.5 M H2SO4 | - | Pt on TGPH 090 (no information) | O2 | - | Nafion 117 CEM | 70 | 4.3 | 15 | [62] |
| Acid treated Carbon on Toray-060 (1 mg/cm2) | 0.5 M AA | 4 | Pt/C on (SGL, 10BC) (1 mg/cm2) | O2 | 100 | Nafion 115 CEM | 36.5 | ~0.053 | ~0.66 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M. Environment-Friendly Ascorbic Acid Fuel Cell. Electrochem 2023, 4, 31-41. https://doi.org/10.3390/electrochem4010003
Hasan MM. Environment-Friendly Ascorbic Acid Fuel Cell. Electrochem. 2023; 4(1):31-41. https://doi.org/10.3390/electrochem4010003
Chicago/Turabian StyleHasan, Md. Mahmudul. 2023. "Environment-Friendly Ascorbic Acid Fuel Cell" Electrochem 4, no. 1: 31-41. https://doi.org/10.3390/electrochem4010003
APA StyleHasan, M. M. (2023). Environment-Friendly Ascorbic Acid Fuel Cell. Electrochem, 4(1), 31-41. https://doi.org/10.3390/electrochem4010003






