Modification of Cu(111) Surface with Alkylphosphonic Acids in Aqueous and Ethanol Solution—An Experimental and Theoretical Study
Abstract
:1. Introduction
2. Methods and Computational Details
2.1. Chemicals and Electrodes
2.2. Electrochemical Measurements
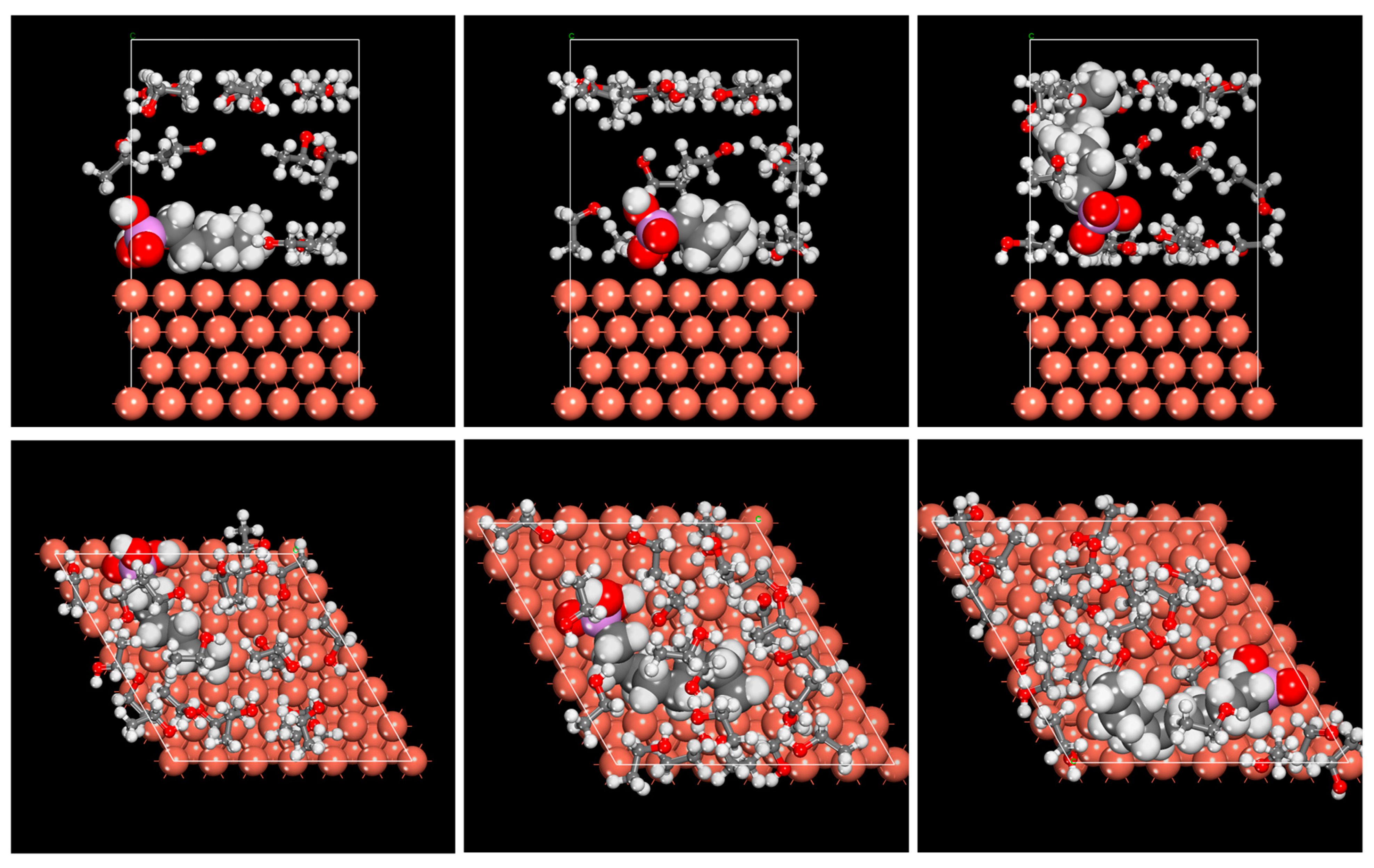
2.3. Monte Carlo/Molecular Dynamics
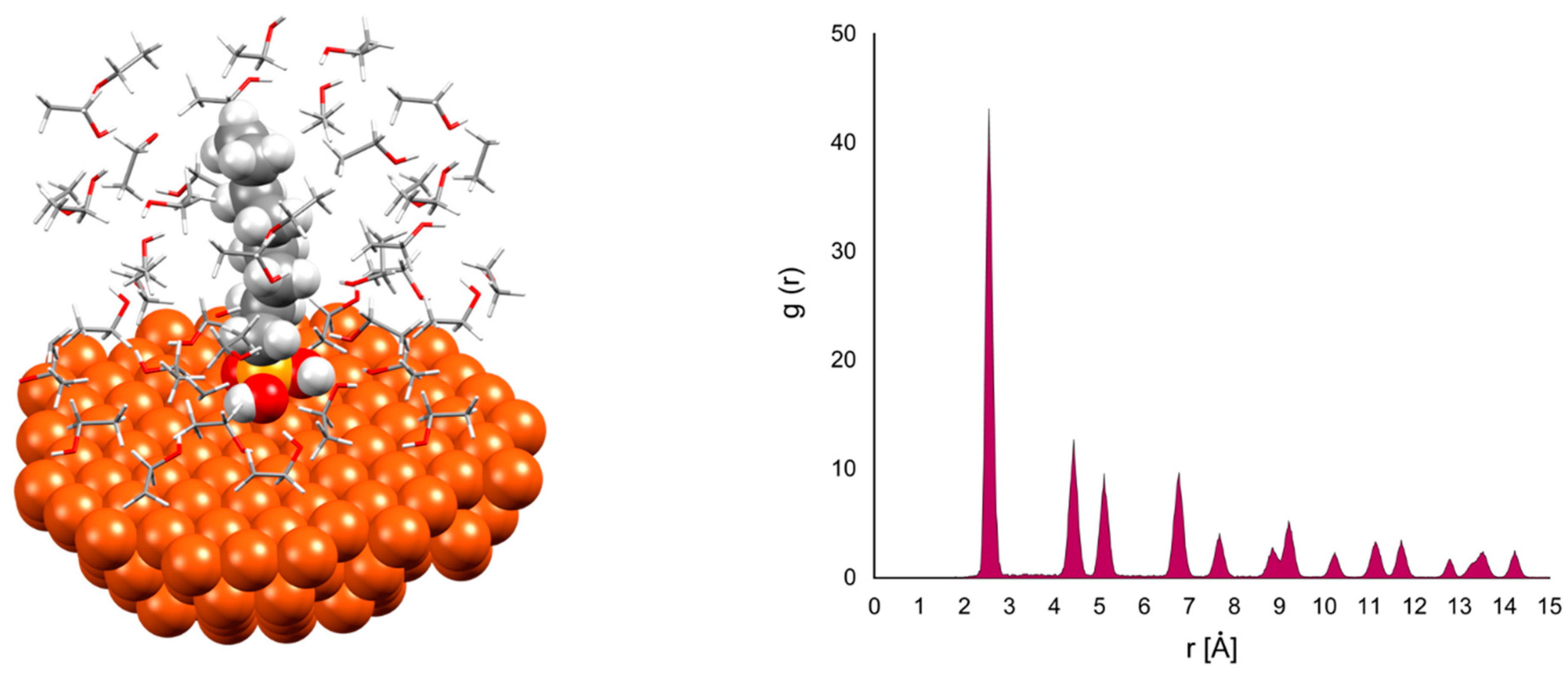
3. Results and Discussion
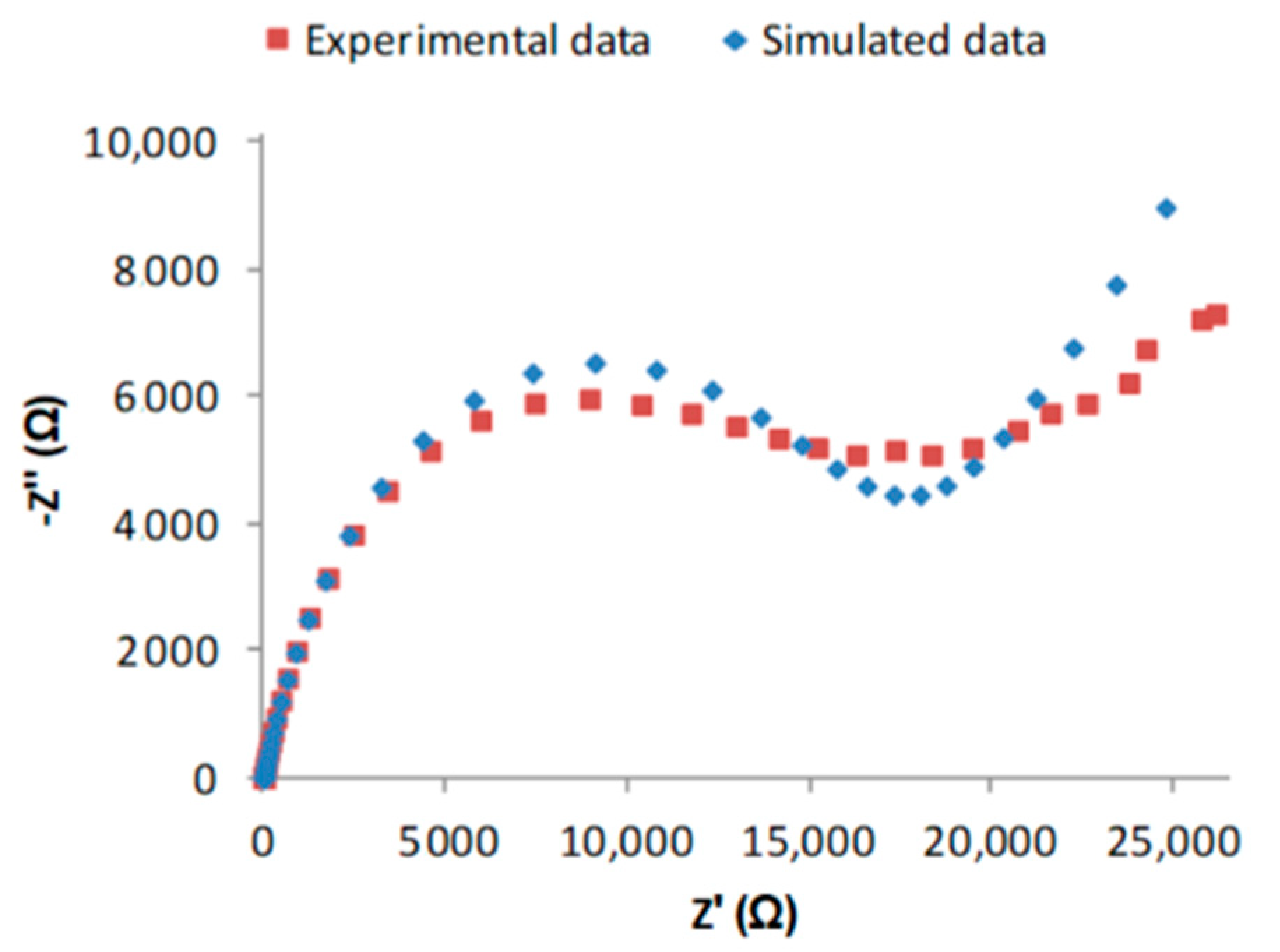
Nyquist Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Aramaki, K. Effect of organic inhibitors on corrosion of zinc in an aerated 0.5 M NaCl solution. Corros. Sci. 2001, 43, 1985–2000. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, Y.K.; Yoon, J.M.; Park, I.S. Discoloration resistance of electrolytic copper foil following 1,2,3-benzotriazole surface treatment with sodium molybdate. Coatings 2018, 8, 427. [Google Scholar] [CrossRef] [Green Version]
- Sabet, B.K.; Dehghanian, C.; Yari, S. Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate. Corros. Sci. 2017, 126, 272–285. [Google Scholar] [CrossRef]
- Heijerick, D.G.; Regoli, L.; Stubblefield, W. The chronic toxicity of molybdate to marine organisms. I. Generating reliable effects data. Sci. Total Environ. 2012, 430, 260–269. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Antón, J.G.; Guiñón, J.L.; Pérez Herranz, V. Comparison of inorganic inhibitors of copper, nickel and copper-nickels in aqueous lithium bromide solution. Electrochim. Acta 2004, 50, 957–966. [Google Scholar] [CrossRef]
- Karthik, B.B.; Selvakumar, P.; Thangavelu, C. Phosphonic acids used as corrosion inhibitors-A review. Asian J. Chem. 2012, 24, 3303–3308. [Google Scholar]
- Obot, I.B.; Onyeachu, I.B.; Wazzan, N.; Al-Amri, A.H. Theoretical and experimental investigation of two alkyl carboxylates as corrosion inhibitors for steel in acidic medium. J. Mol. Liq. 2019, 279, 190–207. [Google Scholar] [CrossRef]
- Mehmeti, V.; Podvorica, F.I. Experimental and theoretical studies on corrosion inhibition of niobium and tantalum surfaces by carboxylated graphene oxide. Materials 2018, 11, 893. [Google Scholar] [CrossRef] [Green Version]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Theoretical study of benzimidazole and its derivatives and their potential activity as corrosion inhibitors. Corros. Sci. 2010, 52, 657–660. [Google Scholar] [CrossRef]
- Luschtinetz, R.; Oliveira, A.F.; Frenzel, J.; Joswig, J.-O.; Seifert, G.; Duarte, H.A. Adsorption of phosphonic and ethylphosphonic acid on aluminum oxide surfaces. Surf. Sci. 2008, 602, 1347–1359. [Google Scholar] [CrossRef]
- Pellerite, M.J.; Dunbar, T.D.; Boardman, L.D.; Wood, E.J. Effects of Fluorination on Self-Assembled Monolayer Formation from Alkanephosphonic Acids on Aluminum: Kinetics and Structure. J. Phys. Chem. 2003, 107, 11726–11736. [Google Scholar] [CrossRef]
- Messerschmidt, C.; Schwartz, D.K. Growth mechanisms of octadecylphosphonic acid self-assembled monolayers on sapphire (corundum): Evidence for a quasi-equilibrium triple point. Langmuir 2001, 17, 462–467. [Google Scholar] [CrossRef]
- Bram, C.; Jung, C.; Stratmann, M. Self assembled molecular monolayers on oxidized inhomogeneous aluminum surfaces. Fresenius. J. Anal. Chem. 1997, 358, 108–111. [Google Scholar] [CrossRef]
- Auernheimer, J.; Kessler, H. Benzylprotected aromatic phosphonic acids for anchoring peptides on titanium. Bioorg. Med. Chem. Lett. 2006, 16, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Danahy, M.P.; Avaltroni, M.J.; Midwood, K.S.; Schwarzbauer, J.E.; Schwartz, J. Self-assembled monolayers of α,ω-diphosphonic acids on Ti enable complete or spatially controlled surface derivatization. Langmuir 2004, 20, 5333–5337. [Google Scholar] [CrossRef] [PubMed]
- Gawalt, E.S.; Avaltroni, M.J.; Danahy, M.P.; Silverman, B.M.; Hanson, E.L.; Midwood, K.S.; Schwarzbauer, J.E.; Schwartz, J. Bonding organics to Ti alloys: Facilitating human osteoblast attachment and spreading on surgical implant materials. Langmuir 2003, 19, 200–204. [Google Scholar] [CrossRef]
- Gawalt, E.S.; Avaltroni, M.J.; Koch, N.; Schwartz, J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir 2001, 17, 5736–5738. [Google Scholar] [CrossRef]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Van Alsten, J.G. Self-assembled monolayers on engineering metals: Structure, derivatization, and utility. Langmuir 1999, 15, 7605–7614. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F. Copper corrosion inhibition in O2-saturated H2SO4 solutions. Corros. Sci. 2010, 52, 1194–1204. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G.; Duran, M. Electrochemical synthesis and characterization of poly(m-phenylenediamine) films on copper for corrosion protection. Prog. Org. Coat. 2012, 73, 162–168. [Google Scholar] [CrossRef]
- Charlotte, M.S.; Mathieu, B.; Hélène, C.; Jaffrès, P.A. Phosphonic acid: Preparation and applications. Beilstein J. Org. Chem. 2017, 13, 2186–2213. [Google Scholar]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Nishikata, A.; Ichihara, Y.; Tsuru, T. Electrochemical impedance spectroscopy of metals covered with a thin electrolyte layer. Electrochim. Acta 1996, 41, 1057–1062. [Google Scholar] [CrossRef]
- McIntyre, J.M.; Pham, H.Q. Electrochemical impedance spectroscopy; a tool for organic coatings optimizations. Prog. Org. Coat. 1996, 27, 201–207. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: Boston, MA, USA, 2014; pp. 143–248. [Google Scholar]
- Dagdag, O.; Berisha, A.; Safi, Z.; Dagdag, S.; Berrani, M.; Jodeh, S.; Verma, C.; Ebenso, E.E.; Wazzan, N.; El Harfi, A. Highly durable macromolecular epoxy resin as anticorrosive coating material for carbon steel in 3% NaCl: Computational supported experimental studies. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Safi, Z.; Hamed, O.; Jodeh, S.; Verma, C.; Ebenso, E.E.; El Harfi, A. DGEBA-polyaminoamide as effective anti-corrosive material for 15CDV6 steel in NaCl medium: Computational and experimental studies. J. Appl. Polym. Sci. 2020, 137, 48402. [Google Scholar] [CrossRef]
- Hsissou, R.; Benzidia, B.; Rehioui, M.; Berradi, M.; Berisha, A.; Assouag, M.; Hajjaji, N.; Elharfi, A. Anticorrosive property of hexafunctional epoxy polymer HGTMDAE for E24 carbon steel corrosion in 1.0 M HCl: Gravimetric, electrochemical, surface morphology and molecular dynamic simulations. Polym. Bull. 2019, 77, 3577–3601. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Abbout, S.; Dagdag, O.; Benkhaya, S.; Berisha, A.; Erramli, H.; Elharfi, A. Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations. Inorg. Chem. Commun. 2020, 115, 107858. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Benhiba, F.; Serrar, H.; Hsissou, R.; Guenbour, A.; Bellaouchou, A.; Tabyaoui, M.; Boukhris, S.; Oudda, H.; Warad, I.; Zarrouk, A. Tetrahydropyrimido-Triazepine derivatives as anti-corrosion additives for acid corrosion: Chemical, electrochemical, surface and theoretical studies. Chem. Phys. Lett. 2020, 743, 137181. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Dagdag, O.; El Bouchti, M.; Nouneh, K.; Assouag, M.; Briche, S.; Zarrouk, A.; Elharfi, A. Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: Outlooks from experimental and computational investigations. J. Colloid Interface Sci. 2020, 574, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Wu, H.; Gao, L.X. Synergistic inhibition effect of l-phenylalanine and rare earth Ce(IV) ion on the corrosion of copper in hydrochloric acid solution. Mater. Chem. Phys. 2012, 133, 981–986. [Google Scholar] [CrossRef]
- Gong, Z.; Peng, S.; Huang, X.; Gao, L. Investigation the Corrosion Inhibition Effect of Itraconazole on Copper in H2SO4 at Different Temperatures: Combining Experimental and Theoretical Studies. Materials 2018, 11, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; de Lima-Neto, P. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182–192. [Google Scholar]
- Berisha, A. Interactions between the Aryldiazonium Cations and Graphene Oxide: A DFT Study. J. Chem. 2019, 2019, 5126071. [Google Scholar] [CrossRef]
- Berisha, A. The influence of the grafted aryl groups on the solvation properties of the graphyne and graphdiyne-a MD study. Open Chem. 2019, 17, 703–710. [Google Scholar] [CrossRef]
- Berisha, A.; Combellas, C.; Kanoufi, F.; Decorse, P.; Oturan, N.; Médard, J.; Seydou, M.; Maurel, F.; Pinson, J. Some Theoretical and Experimental Insights on the Mechanistic Routes Leading to the Spontaneous Grafting of Gold Surfaces by Diazonium Salts. Langmuir 2017, 33, 8730–8738. [Google Scholar] [CrossRef] [PubMed]
- Berisha, A.; Combellas, C.; Kanoufi, F.; Médard, J.; Decorse, P.; Mangeney, C.; Kherbouche, I.; Seydou, M.; Maurel, F.; Pinson, J. Alkyl-Modified Gold Surfaces: Characterization of the Au-C Bond. Langmuir 2018, 34, 11264–11271. [Google Scholar] [CrossRef]
- Berisha, A. First principles details into the grafting of aryl radicals onto the free-standing and borophene/Ag(1 1 1) surfaces. Chem. Phys. 2021, 544, 111124. [Google Scholar] [CrossRef]
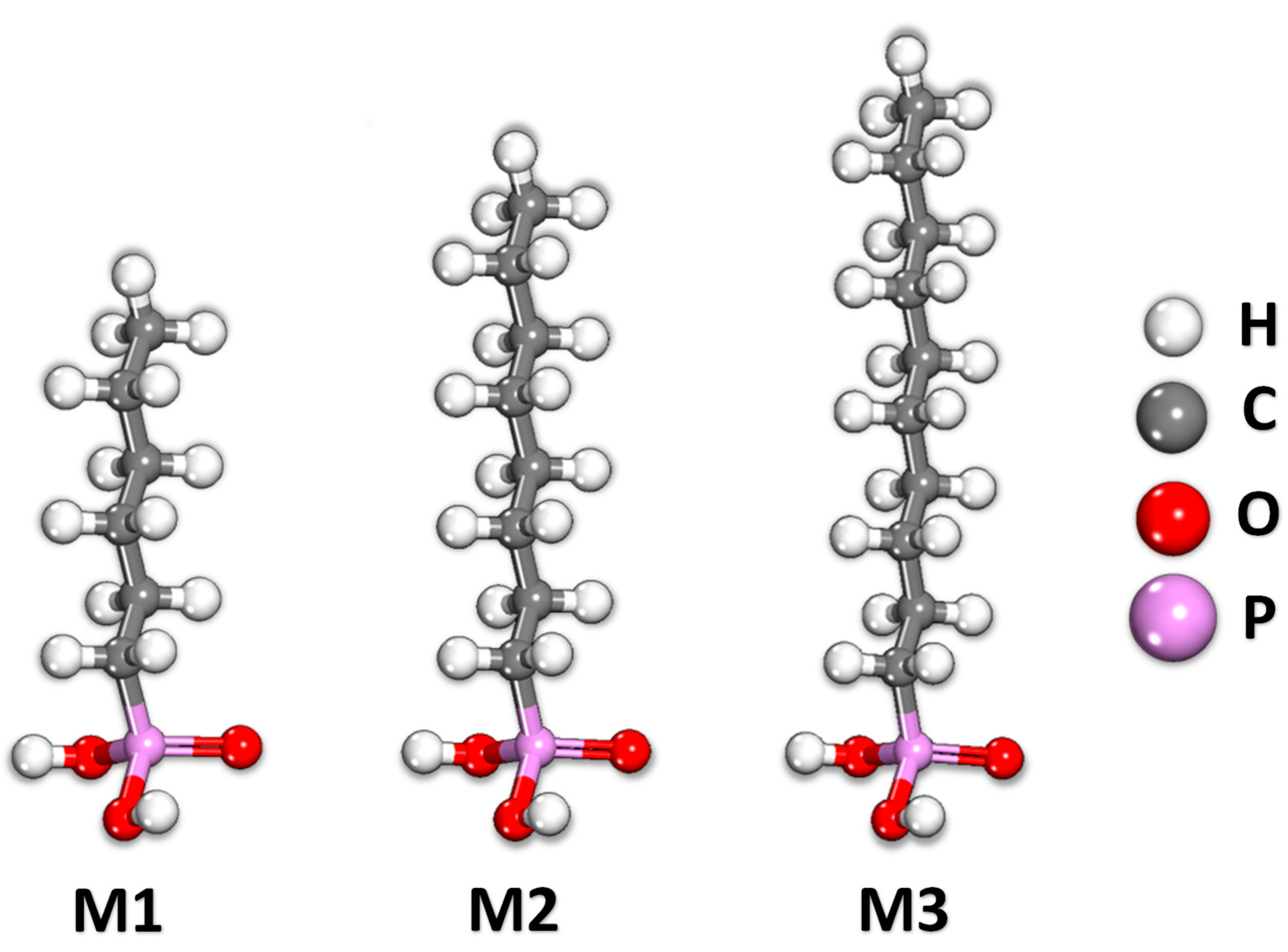

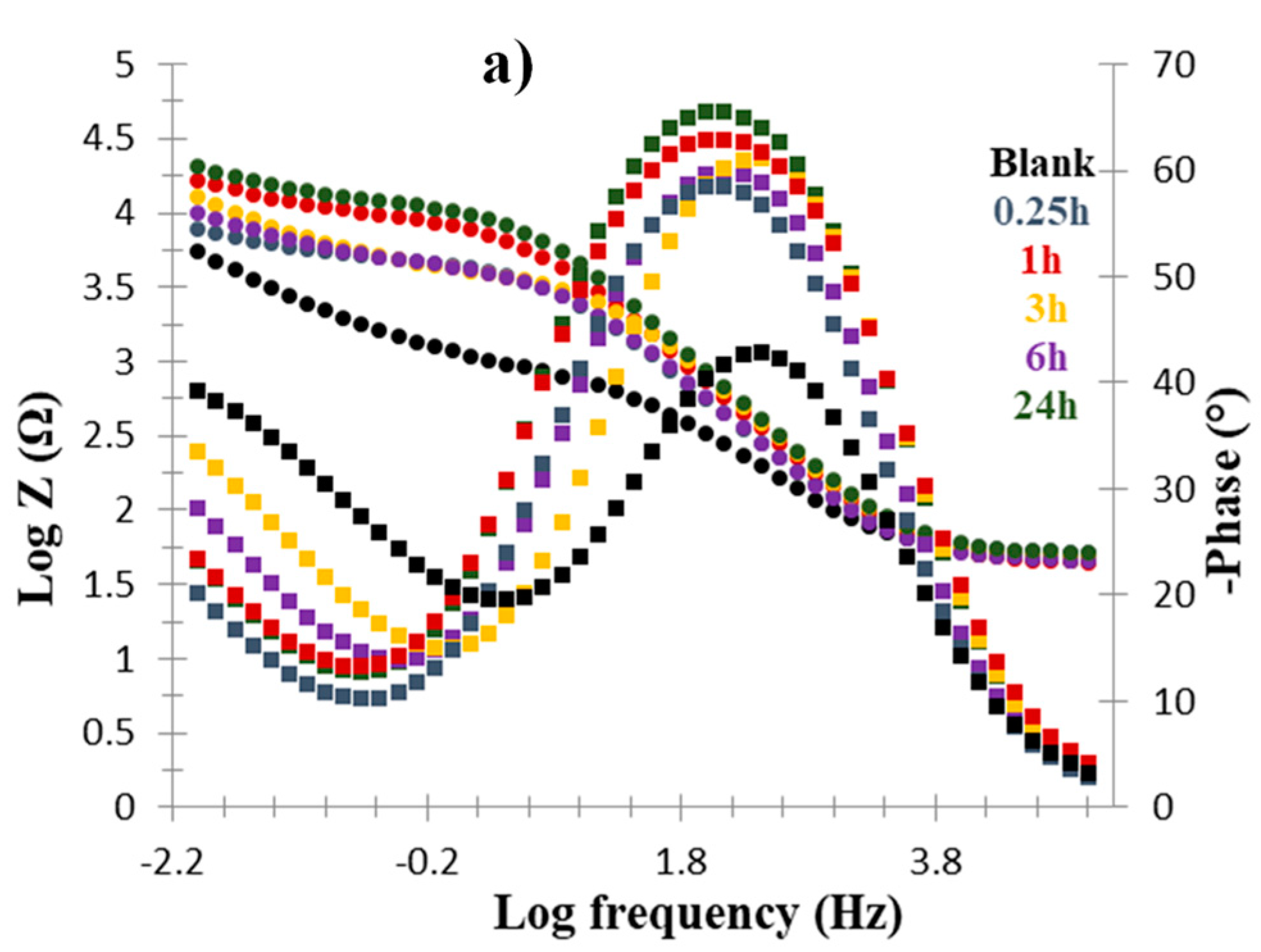
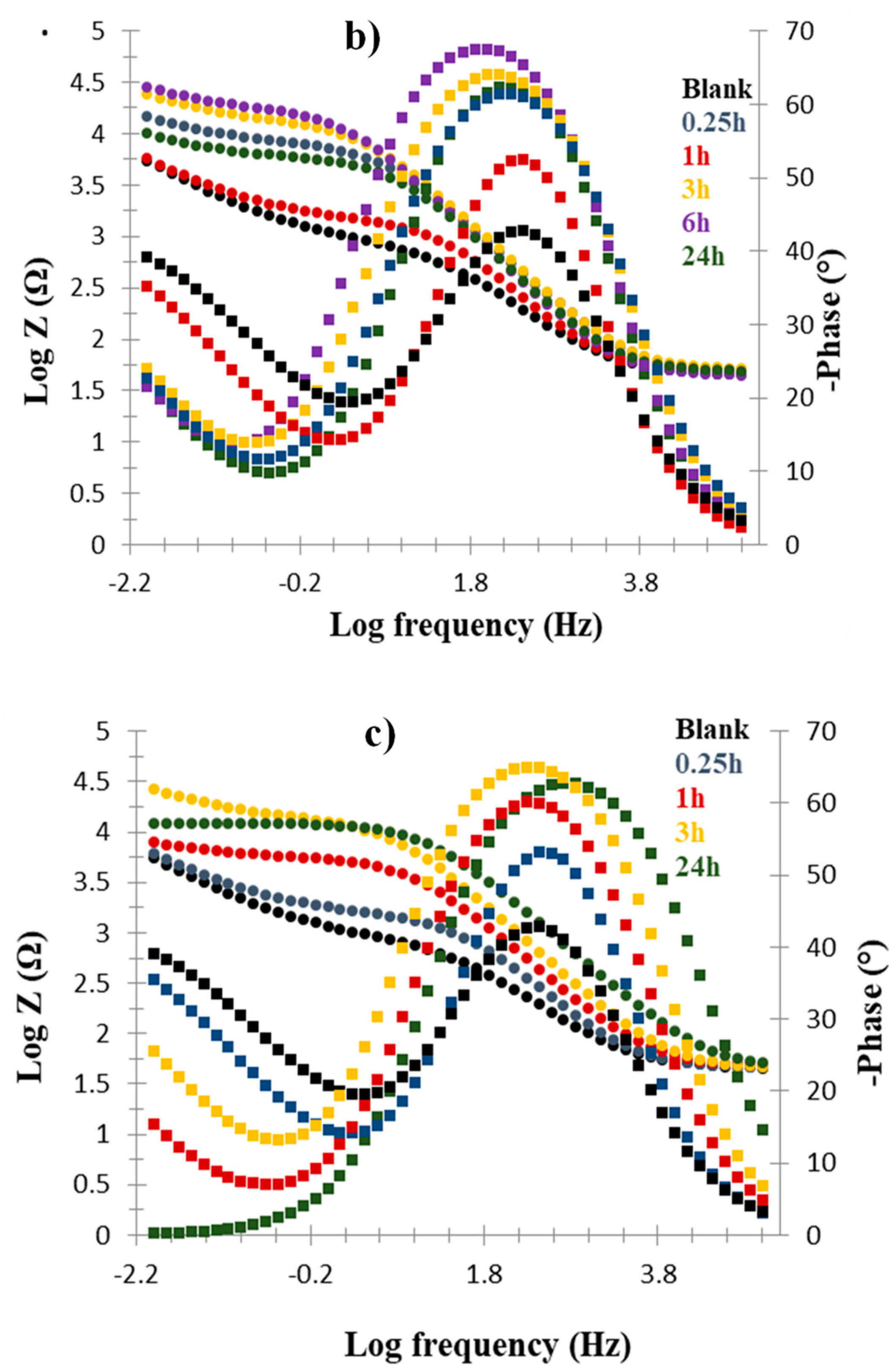
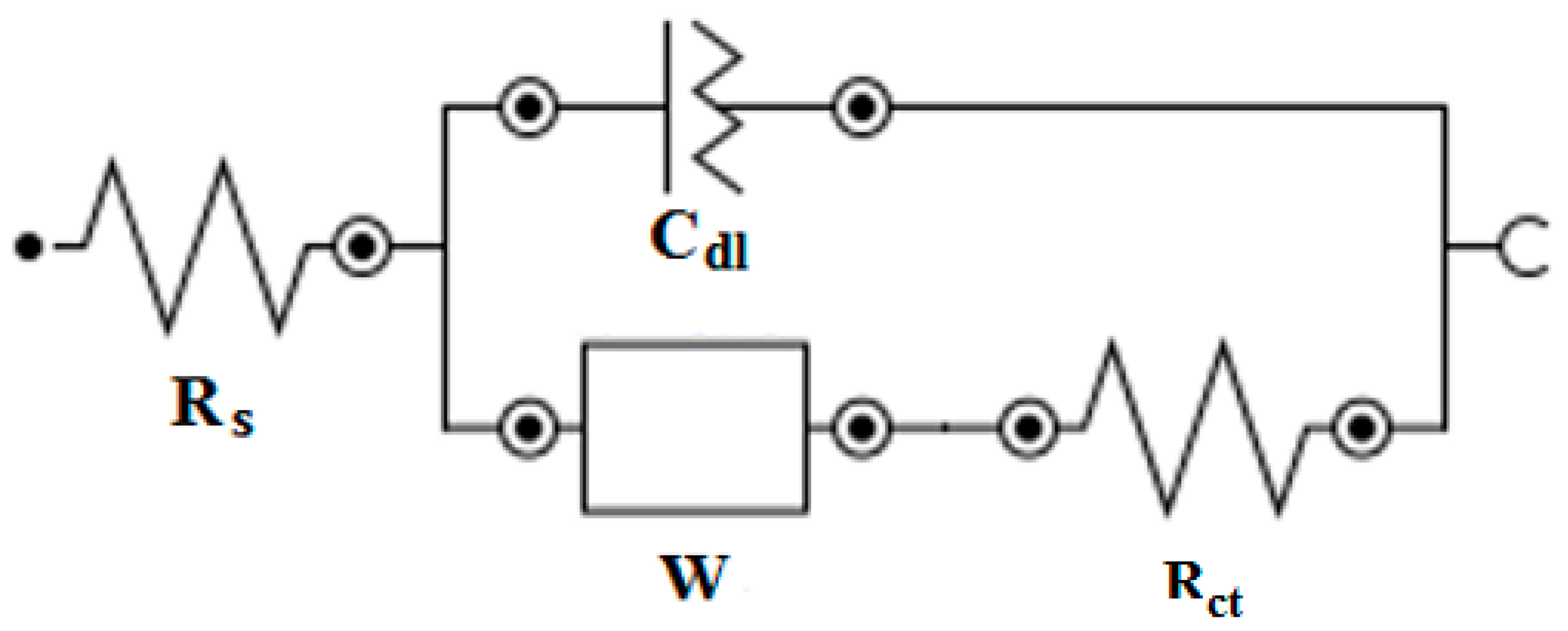

| Cu/Molecule | Rs (Ω) | Q (Ω) | N | Rct (Ω) | %IE |
|---|---|---|---|---|---|
| Blank | 44.32 | 2.95 × 105 | 0.70 | 835.64 | |
| Hexylphosphonic acid | 50.55 | 6.3 × 106 | 0.8 | 11071 | 92.4 |
| Octylphosphonic acid | 44 | 6.87 × 106 | 0.81 | 16655 | 95 |
| Decylphosphonic acid | 44.67 | 2.558 × 106 | 0.76 | 12320 | 93.2 |
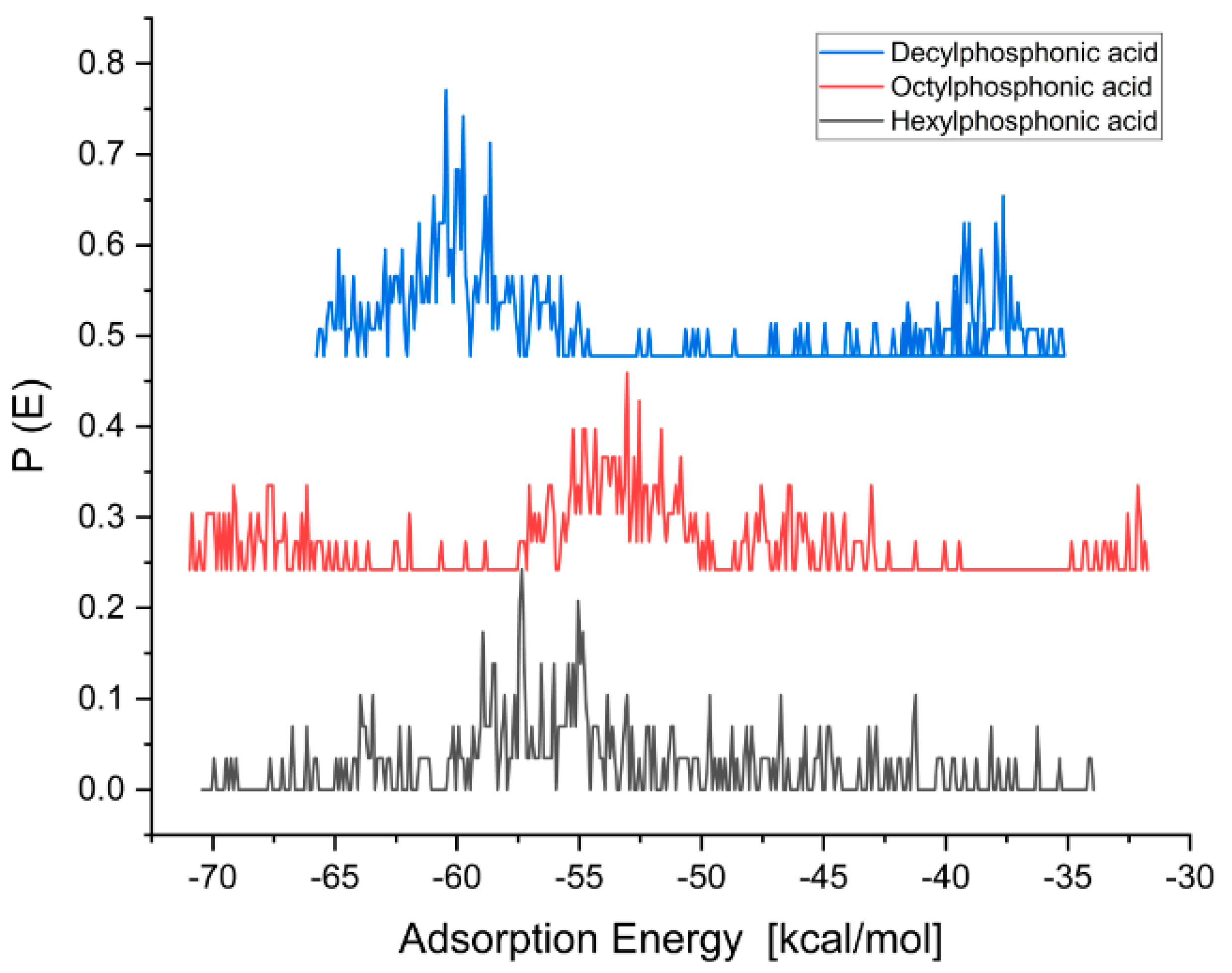
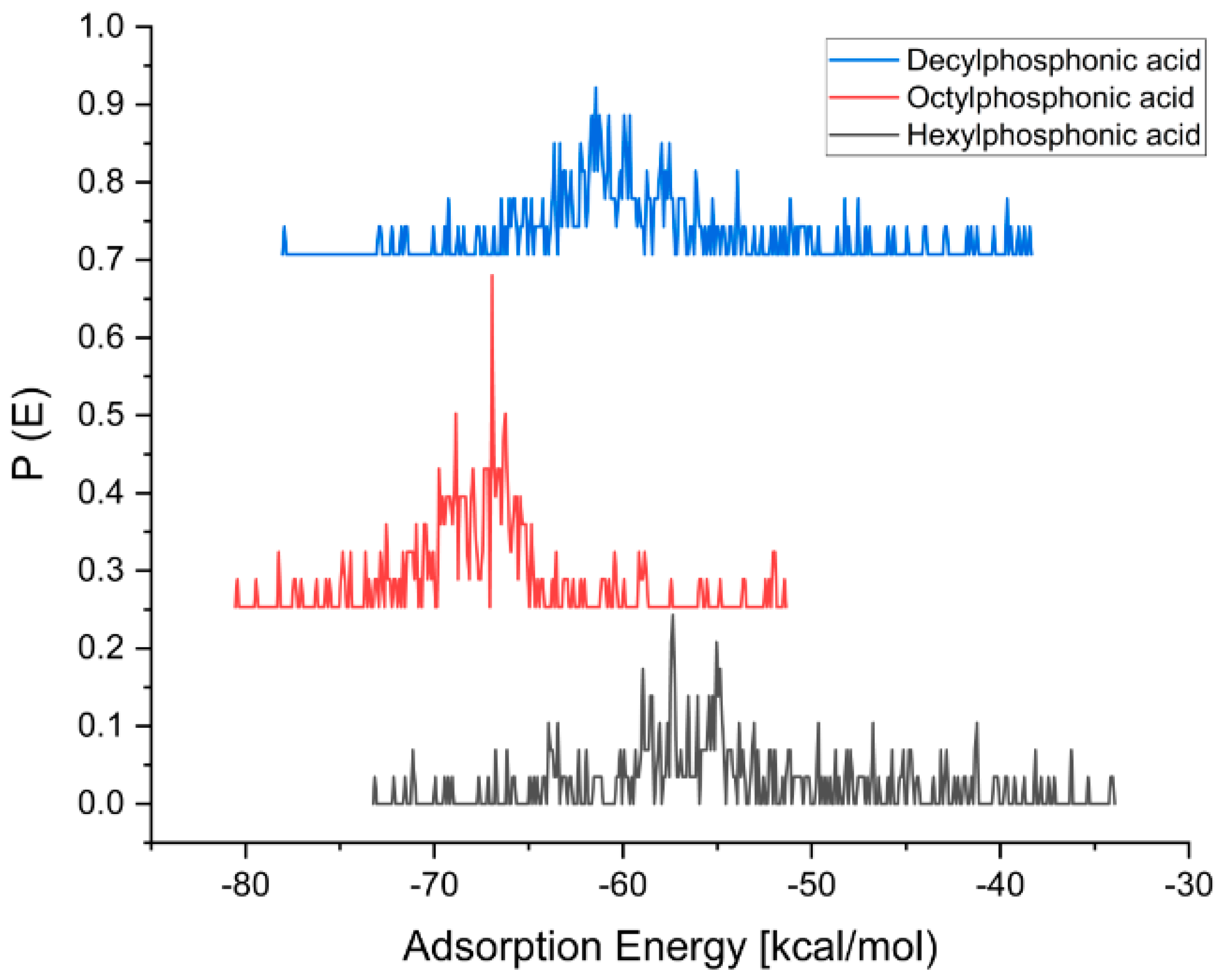
| Molecule | Adsorption Energy [kcal/mol] (Water) | Adsorption Energy [kcal/mol] (Ethanol) |
|---|---|---|
| Hexylphosphonic acid | −71 | −74 |
| Octylphosphonic acid | −72 | −82 |
| Decylphosphonic acid | −67 | −79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmeti, V.; Podvorica, F. Modification of Cu(111) Surface with Alkylphosphonic Acids in Aqueous and Ethanol Solution—An Experimental and Theoretical Study. Electrochem 2022, 3, 58-69. https://doi.org/10.3390/electrochem3010004
Mehmeti V, Podvorica F. Modification of Cu(111) Surface with Alkylphosphonic Acids in Aqueous and Ethanol Solution—An Experimental and Theoretical Study. Electrochem. 2022; 3(1):58-69. https://doi.org/10.3390/electrochem3010004
Chicago/Turabian StyleMehmeti, Valbonë, and Fetah Podvorica. 2022. "Modification of Cu(111) Surface with Alkylphosphonic Acids in Aqueous and Ethanol Solution—An Experimental and Theoretical Study" Electrochem 3, no. 1: 58-69. https://doi.org/10.3390/electrochem3010004
APA StyleMehmeti, V., & Podvorica, F. (2022). Modification of Cu(111) Surface with Alkylphosphonic Acids in Aqueous and Ethanol Solution—An Experimental and Theoretical Study. Electrochem, 3(1), 58-69. https://doi.org/10.3390/electrochem3010004







