An Experimental and Theoretical Investigation of the Efficacy of Pantoprazole as a Corrosion Inhibitor for Mild Steel in an Acidic Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrument, Material, Electrode Preparation, and the Corrosive Solution
2.2. DFT Calculations
2.3. Monte Carlo (MC) and Molecular Dynamic (MD) Simulation
3. Results
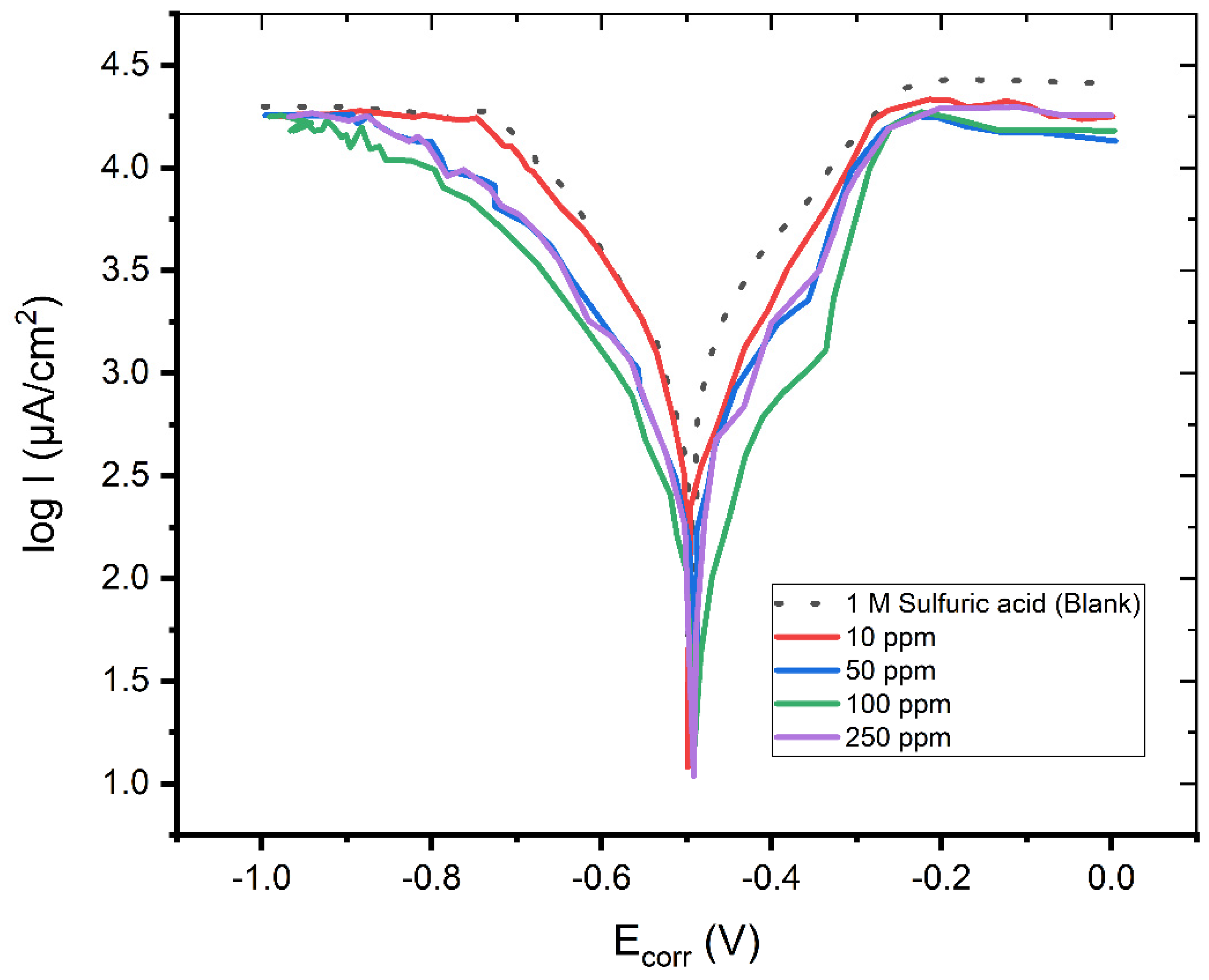
3.1. Polarization Measurements
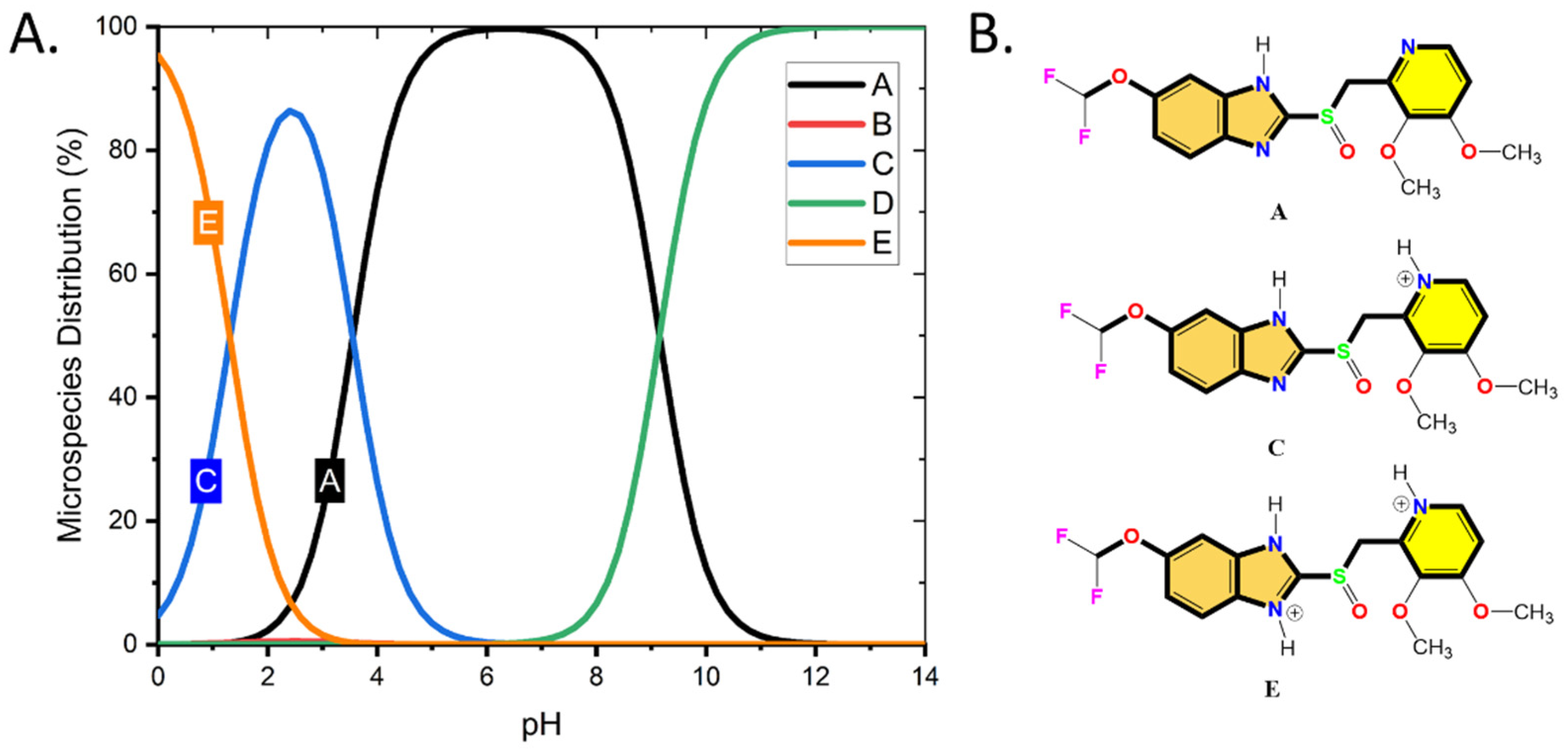
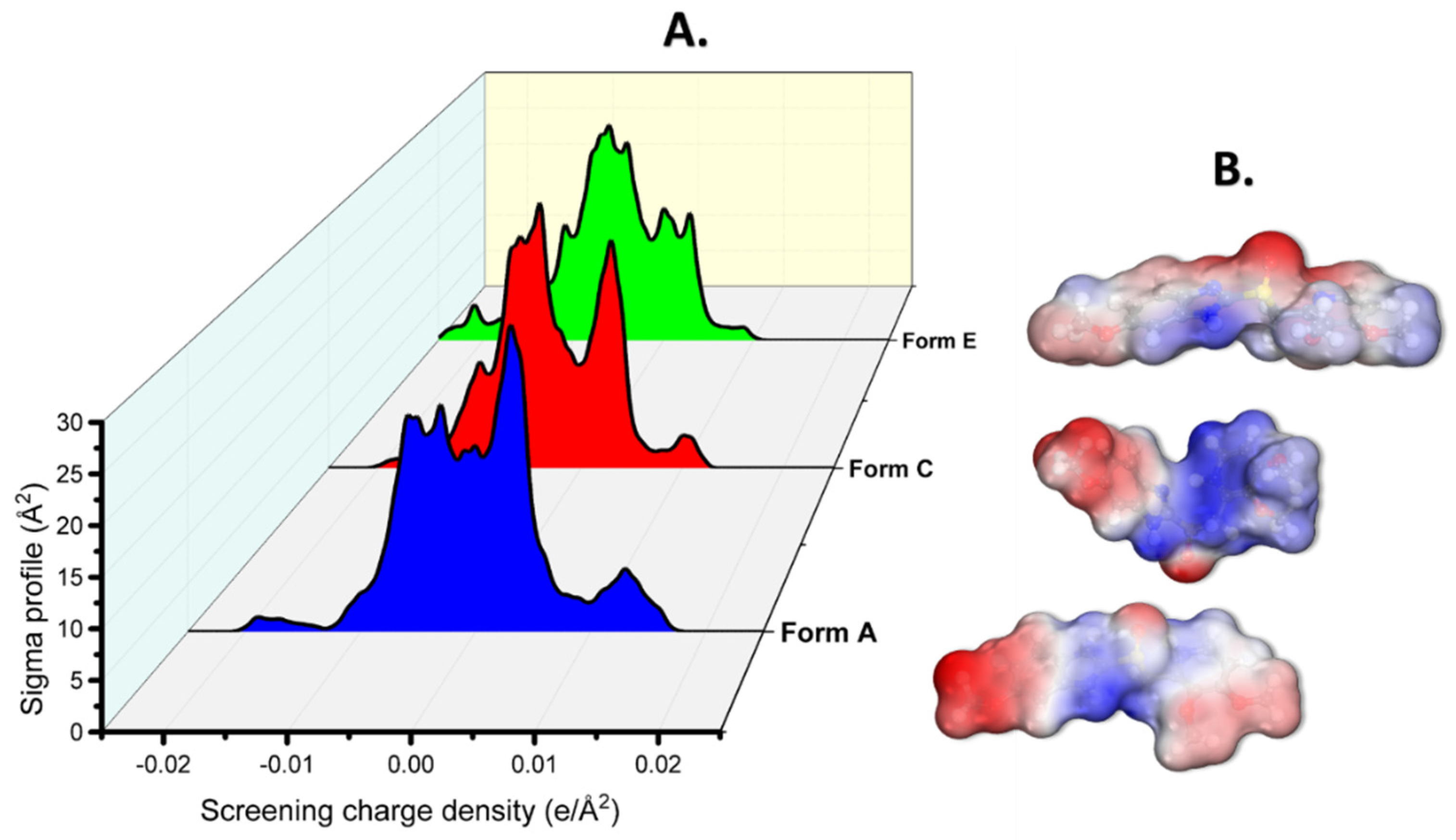
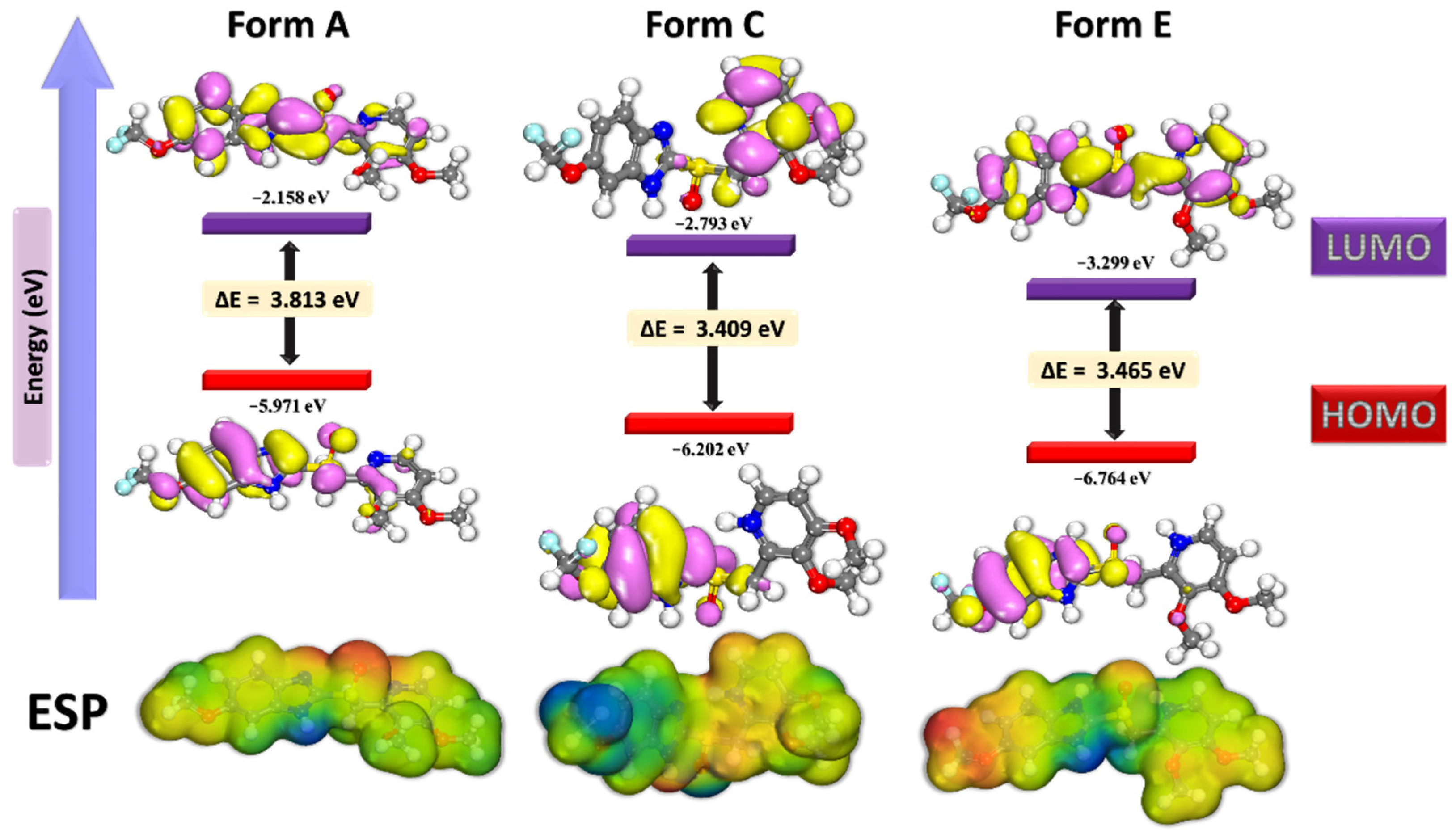
3.2. DFT, MC, and MD Results
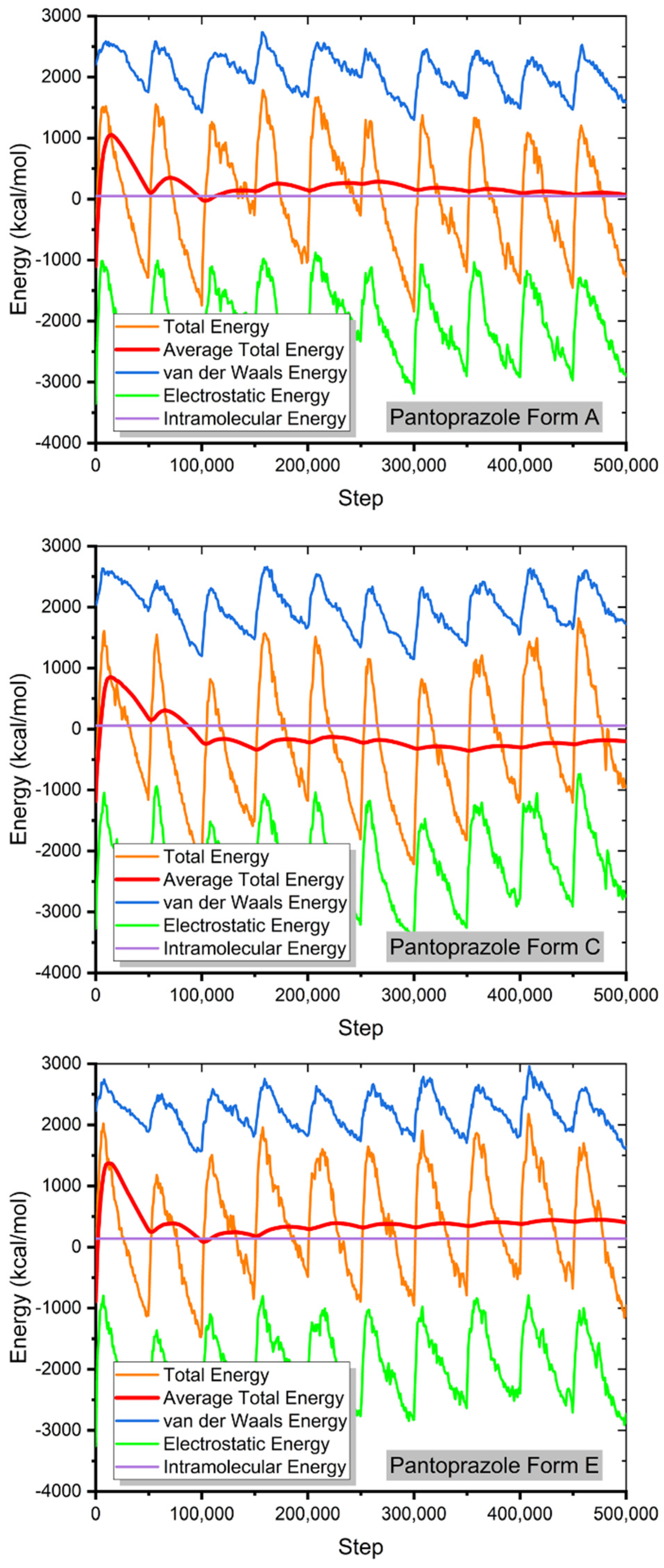
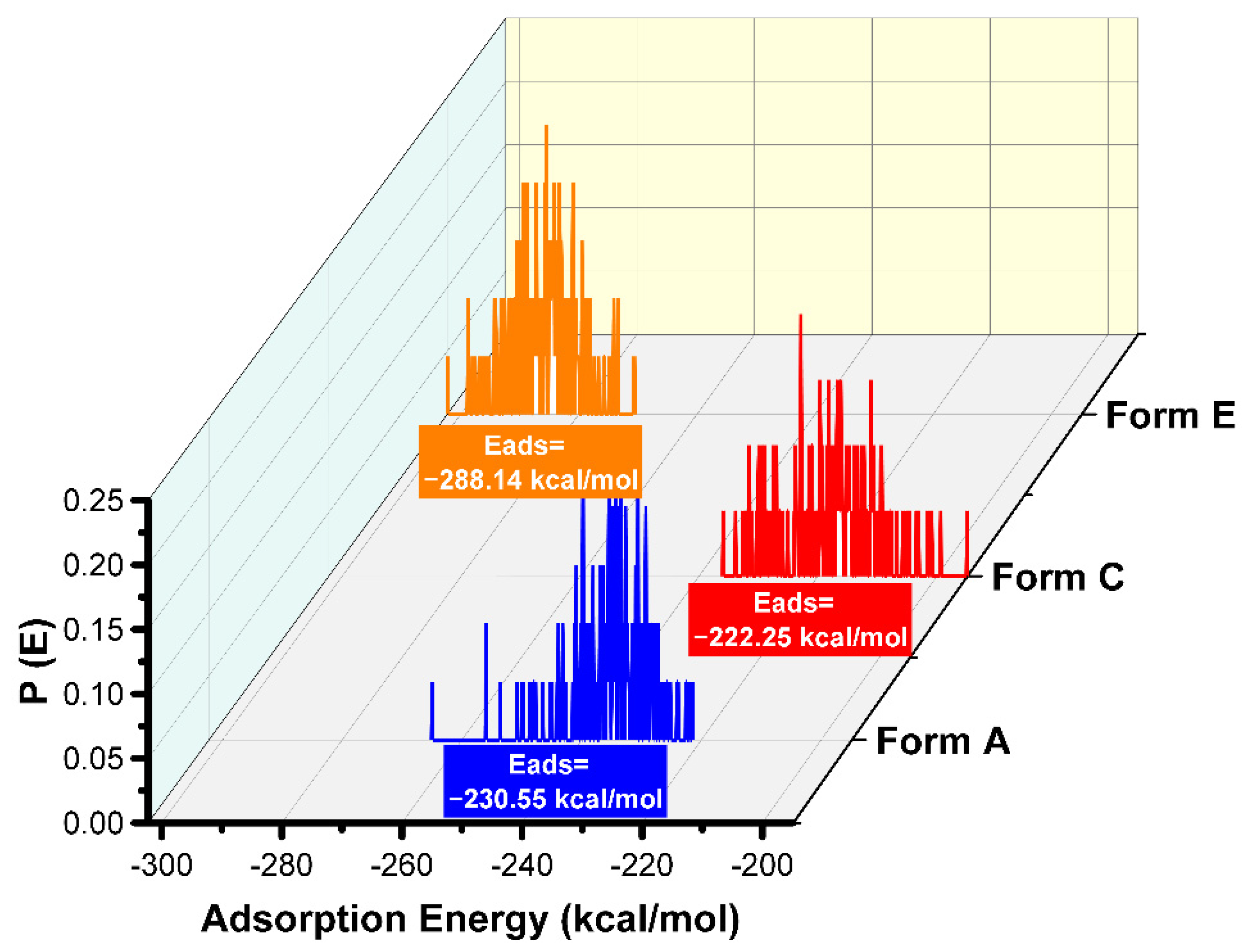
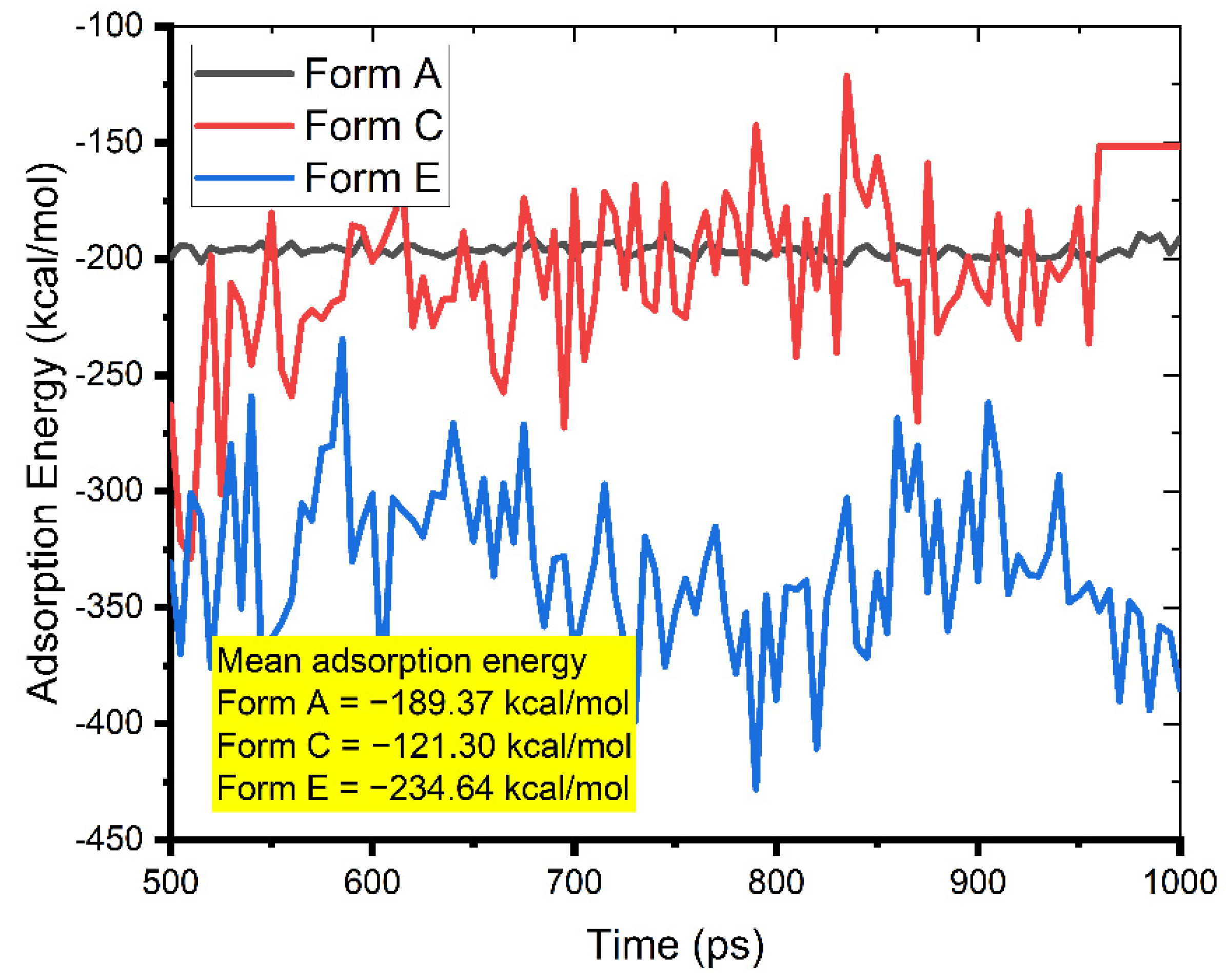
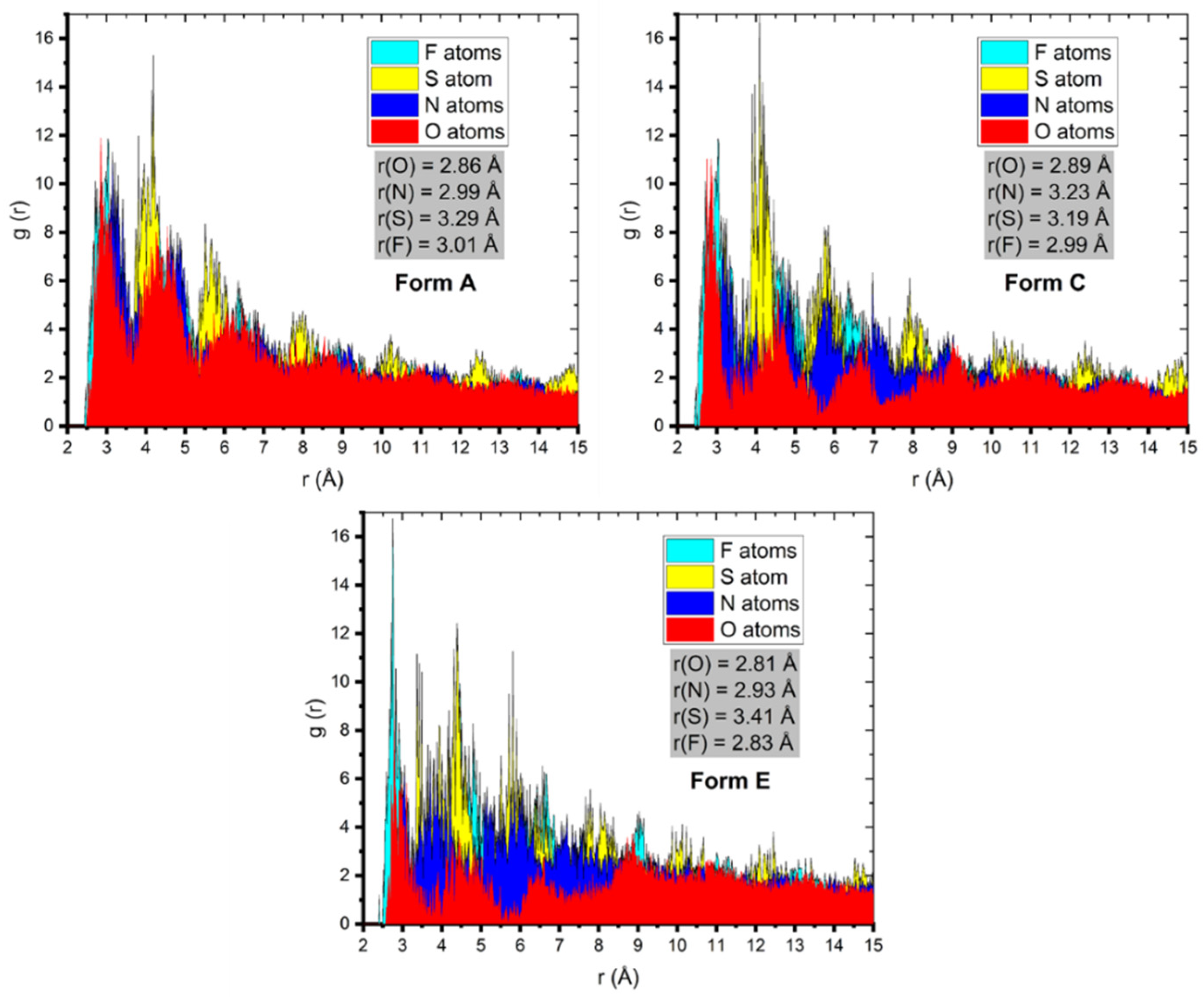
3.3. MC and MD Results
3.4. The Study of the Film Density and the Self-Diffusion Coefficients of Pantoprazole
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, N.; Fitoz, A.; Ergun, Ü.; Emregül, K.C. A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corros. Sci. 2016, 111, 110–120. [Google Scholar] [CrossRef]
- Rbaa, M.; Ouakki, M.; Galai, M.; Berisha, A.; Lakhrissi, B.; Jama, C.; Warad, I.; Zarrouk, A. Simple preparation and characterization of novel 8-Hydroxyquinoline derivatives as effective acid corrosion inhibitor for mild steel: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125094. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Chaouiki, A.; Shubhalaxmi; Jodeh, S.; Bhat, K.S. Pyrazoline derivatives as possible corrosion inhibitors for mild steel in acidic media: A combined experimental and theoretical approach. Cogent Eng. 2018, 5, 1441585. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Jafar Mazumder, M.A.; Ali, S.A.; Aljeaban, N.A.; Alharbi, B.G.; Al-Saadi, A.A.; Obot, I.B. Impact of Degree of Hydrophilicity of Pyridinium Bromide Derivatives on HCl Pickling of X-60 Mild Steel: Experimental and Theoretical Evaluations. Coatings 2020, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Berisha, A.; Podvorica, F.; Mehmeti, V.; Syla, F.; Vataj, D. Theoretical and experimental studies of the corrosion behavior of some thiazole derivatives toward mild steel in sulfuric acid media. Maced. J. Chem. Chem. Eng. 2015, 34, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Echihi, S.; Benkhaya, S.; Hilali, M.; Berisha, A.; Briche, S.; Zarrouk, A.; Nouneh, K.; Elharfi, A. New epoxy composite polymers as a potential anticorrosive coating for carbon steel in 3.5% NaCl solution: Experimental and computational approaches. Chem. Data Collect. 2021, 31, 100619. [Google Scholar] [CrossRef]
- Rbaa, M.; Dohare, P.; Berisha, A.; Dagdag, O.; Lakhrissi, L.; Galai, M.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. New Epoxy sugar based glucose derivatives as eco friendly corrosion inhibitors for the carbon steel in 1.0 M HCl: Experimental and theoretical investigations. J. Alloys Compd. 2020, 833, 154949. [Google Scholar] [CrossRef]
- Mehmeti, V.V.; Berisha, A.R. Corrosion study of mild steel in aqueous sulfuric acid solution using 4-methyl-4h-1,2,4-triazole-3-thiol and 2-mercaptonicotinic acid-an experimental and theoretical study. Front. Chem. 2017, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Andzelm, J.; King-Smith, R.D.; Fitzgerald, G. Geometry optimization of solids using delocalized internal coordinates. Chem. Phys. Lett. 2001, 335, 321–326. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Berisha, A. Interactions between the aryldiazonium cations and graphene oxide: A DFT study. J. Chem. 2019, 2019, 5126071. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Inada, Y.; Orita, H. Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: Evidence of small basis set superposition error compared to Gaussian basis sets. J. Comput. Chem. 2008, 29, 225–232. [Google Scholar] [CrossRef]
- Delley, B. Ground-state enthalpies: Evaluation of electronic structure approaches with emphasis on the density functional method. J. Phys. Chem. A 2006, 110, 13632–13639. [Google Scholar] [CrossRef] [PubMed]
- Berisha, A. First principles details into the grafting of aryl radicals onto the free-standing and borophene/Ag(1 1 1) surfaces. Chem. Phys. 2021, 544, 111124. [Google Scholar] [CrossRef]
- Thaçi, V.; Hoti, R.; Berisha, A.; Bogdanov, J. Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study. Open Chem. 2020, 18, 1412–1420. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Abbout, S.; Dagdag, O.; Benkhaya, S.; Berisha, A.; Erramli, H.; Elharfi, A. Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations. Inorg. Chem. Commun. 2020, 115, 107858. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182–192. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Safi, Z.; Hamed, O.; Jodeh, S.; Verma, C.; Ebenso, E.E.; El Harfi, A. DGEBA-polyaminoamide as effective anti-corrosive material for 15CDV6 steel in NaCl medium: Computational and experimental studies. J. Appl. Polym. Sci. 2020, 137, 48402. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Berisha, A.; Safi, Z.; Verma, C.; Ebenso, E.E.; Touhami, M.E.; El Gouri, M. Fabrication of polymer based epoxy resin as effective anti-corrosive coating for steel: Computational modeling reinforced experimental studies. Surf. Interfaces 2020, 18, 100454. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Berisha, A.; Kerroum, Y.; Jama, C.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. An experimental-coupled empirical investigation on the corrosion inhibitory action of 7-alkyl-8-Hydroxyquinolines on C35E steel in HCl electrolyte. J. Mol. Liq. 2020, 317, 113973. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-Based Epoxy Cured with a Polyaminoamide as an Anticorrosive Coating for Aluminum 2024-T3 Surface: Experimental Studies Supported by Computational Modeling. J. Bio-Tribo-Corros. 2019, 5, 58. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Safi, Z.; Berisha, A.; Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Wazzan, N.; Jodeh, S.; et al. Epoxy resins and their zinc composites as novel anti-corrosive materials for copper in 3% sodium chloride solution: Experimental and computational studies. J. Mol. Liq. 2020, 315, 113757. [Google Scholar] [CrossRef]
- Jessima, S.H.M.; Berisha, A.; Srikandan, S.S.; Subhashini, S. Preparation, characterization, and evaluation of corrosion inhibition efficiency of sodium lauryl sulfate modified chitosan for mild steel in the acid pickling process. J. Mol. Liq. 2020, 320, 114382. [Google Scholar] [CrossRef]
- Hsissou, R.; Benzidia, B.; Rehioui, M.; Berradi, M.; Berisha, A.; Assouag, M.; Hajjaji, N.; Elharfi, A. Anticorrosive property of hexafunctional epoxy polymer HGTMDAE for E24 carbon steel corrosion in 1.0 M HCl: Gravimetric, electrochemical, surface morphology and molecular dynamic simulations. Polym. Bull. 2020, 77, 3577–3601. [Google Scholar] [CrossRef]
- El Arrouji, S.; Karrouchi, K.; Berisha, A.; Alaoui, K.I.; Warad, I.; Rais, Z.; Radi, S.; Taleb, M.; Zarrouk, A. New pyrazole derivatives as effective corrosion inhibitors on steel-electrolyte interface in 1 M HCl: Electrochemical, surface morphological (SEM) and computational analysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125325. [Google Scholar] [CrossRef]
- Abbout, S.; Zouarhi, M.; Chebabe, D.; Damej, M.; Berisha, A.; Hajjaji, N. Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 2020, 6, e03574. [Google Scholar] [CrossRef]
- Jarray, A.; Gerbaud, V.; Hemati, M. Polymer-plasticizer compatibility during coating formulation: A multi-scale investigation. Prog. Org. Coat. 2016, 101, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1338. [Google Scholar] [CrossRef]
- Berisha, A. Experimental, Monte Carlo and Molecular Dynamic Study on Corrosion Inhibition of Mild Steel by Pyridine Derivatives in Aqueous Perchloric Acid. Electrochem 2020, 1, 188–199. [Google Scholar] [CrossRef]
- Berisha, A. The influence of the grafted aryl groups on the solvation properties of the graphyne and graphdiyne—A MD study. Open Chem. 2019, 17, 703–710. [Google Scholar] [CrossRef]
- Ongari, D.; Boyd, P.G.; Kadioglu, O.; Mace, A.K.; Keskin, S.; Smit, B. Evaluating Charge Equilibration Methods to Generate Electrostatic Fields in Nanoporous Materials. J. Chem. Theory Comput. 2019, 15, 382–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsissou, R.; Abbout, S.; Berisha, A.; Berradi, M.; Assouag, M.; Hajjaji, N.; Elharfi, A. Experimental, DFT and molecular dynamics simulation on the inhibition performance of the DGDCBA epoxy polymer against the corrosion of the E24 carbon steel in 1.0 M HCl solution. J. Mol. Struct. 2019, 1182, 340–351. [Google Scholar] [CrossRef]
- Mehmeti, V.; Podvorica, F.I. Experimental and Theoretical Studies on Corrosion Inhibition of Niobium and Tantalum Surfaces by Carboxylated Graphene Oxide. Materials 2018, 11, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Thaçi, V.; Belafhaili, A.; Benmessaoud, M.; Labjar, N.; El Hajjaji, S. Contribution to the corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by a new epoxy resin PGEPPP. Int. J. Corros. Scale Inhib. 2021, 10, 399–418. [Google Scholar]
- Dagdag, O.; Berisha, A.; Safi, Z.; Dagdag, S.; Berrani, M.; Jodeh, S.; Verma, C.; Ebenso, E.E.; Wazzan, N.; El Harfi, A. Highly durable macromolecular epoxy resin as anticorrosive coating material for carbon steel in 3% NaCl: Computational supported experimental studies. J. Appl. Polym. Sci. 2020, 137, 49003. [Google Scholar] [CrossRef]
- Berisha, A. Ab inito exploration of nanocars as potential corrosion inhibitors. Comput. Theor. Chem. 2021, 1201, 113258. [Google Scholar] [CrossRef]
- Uppalapati, P.K.; Berisha, A.; Velmurugan, K.; Nandhakumar, R.; Khosla, A.; Liang, T. Salen type additives as corrosion mitigtor for Ni–W alloys: Detailed electronic/atomic-scale computational illustration. Int. J. Quantum Chem. 2020, 12, e26600. [Google Scholar] [CrossRef]
- Jessima, S.H.M.; Subhashini, S.; Berisha, A.; Oral, A.; Srikandan, S.S. Corrosion mitigation performance of disodium EDTA functionalized chitosan biomacromolecule—Experimental and theoretical approach. Int. J. Biol. Macromol. 2021, 178, 477–491. [Google Scholar] [CrossRef]
- Dagdag, O.; Berisha, A.; Mehmeti, V.; Haldhar, R.; Berdimurodov, E.; Hamed, O.; Jodeh, S.; Lgaz, H.; El-Sayed, M.S.; Elbenso, E.E. Epoxy coating as effective anti-corrosive polymeric material for aluminum alloys: Formulation, electrochemical and computational approaches: Computational and experimental studies. J. Mol. Liq. 2021, 117886. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; El Gana, L.; Safi, Z.S.; Guo, L.; Berisha, A.; Verma, C.; Ebenso, E.E.; Wazzan, N.; El Gouri, M. Designing of phosphorous based highly functional dendrimeric macromolecular resin as an effective coating material for carbon steel in NaCl: Computational and experimental studies. J. Appl. Polym. Sci. 2021, 138, 49673. [Google Scholar] [CrossRef]
- Oukhrib, R.; Abdellaoui, Y.; Berisha, A.; Abou Oualid, H.; Halili, J.; Jusufi, K.; El Had, M.A.; Bourzi, H.; El Issami, S.; Asmary, F.A.; et al. DFT, Monte Carlo and molecular dynamics simulations for the prediction of corrosion inhibition efficiency of novel pyrazolylnucleosides on Cu(111) surface in acidic media. Sci. Rep. 2021, 11, 3771. [Google Scholar] [CrossRef] [PubMed]
- Alahiane, M.; Oukhrib, R.; Berisha, A.; Albrimi, Y.A.; Akbour, A.A.E.; Hamed, O.; Oualid, H.A.S.; Bourzi, H.; Assabbane, A.; Nahlé, A.; et al. Electrochemical, thermodynamic and molecular dynamics studies of some benzoic acid derivatives on the corrosion inhibition of 316 stainless steel in HCl solutions. J. Mol. Liq. 2021, 328, 115413. [Google Scholar] [CrossRef]
- Yu, L.J.; Zhang, J.; Qiao, G.M.; Yan, Y.G.; Ti, Y.; Zhang, Y. Effect of alkyl chain length on inhibition performance of imidazoline derivatives investigated by molecular dynamics simulation. Mater. Corros. 2013, 64, 225–230. [Google Scholar] [CrossRef]


| Fe | C | P | Mn | Si | Cr | S | Mo | Ni |
|---|---|---|---|---|---|---|---|---|
| 99.54 | 0.1252 | 0.0316 | 0.1836 | 0.0561 | 0.0124 | 0.0282 | 0.0125 | 0.0015 |
| C (ppm) | Ecorr (V) | Icorr (μA/cm2) | bc (mV/dec) | ba (mV/dec) | IE (%) |
|---|---|---|---|---|---|
| - | −0.502 | 0.4891 | −153.1 | 84.4 | - |
| 10 | −0.480 | 0.2908 | −155.2 | 92.1 | 48.34 |
| 50 | −0.491 | 0.1933 | −166.1 | 96.4 | 72.12 |
| 100 | −0.495 | 0.1689 | −166.9 | 98.1 | 78.27 |
| 250 | −0.472 | 0.1014 | −172.2 | 99.4 | 94.53 |
| Theoretical Parameters | Pantoprazole Form A | Pantoprazole Form C | Pantoprazole Form E |
|---|---|---|---|
| HOMO | −5.9710 | −6.2020 | −6.7640 |
| LUMO | −2.1580 | −2.7930 | −3.2990 |
| ∆E (HOMO-LUMO) | 3.813 | 3.409 | 3.465 |
| Ionization energy (I) | 5.9710 | 6.2020 | 6.7640 |
| Electron affinity A) | 2.1580 | 2.7930 | 3.2990 |
| Electronegativity (Χ) | 4.0645 | 4.4975 | 5.0315 |
| Global hardness (η) | 1.9065 | 1.7045 | 1.7325 |
| Chemical potential (π) | −4.0645 | −4.4975 | −5.0315 |
| Global softness (σ) | 0.5245 | 0.5867 | 0.5772 |
| Global electrophilicity (ω) | 4.3326 | 5.9336 | 7.3062 |
| Electrodonating (ω−) power | 6.6032 | 8.3954 | 10.0385 |
| Electroaccepting(ω+) power | 2.5387 | 3.8979 | 5.0070 |
| Net electrophilicity (∆ω±) | 2.3872 | 3.7788 | 4.9074 |
| Fraction of transferred electrons (∆N) | −0.2189 | −0.3718 | −0.5199 |
| Energy from Inhib to Metals (∆N) | 0.0913 | 0.2356 | 0.4683 |
| ∆E back-donation | −0.4766 | −0.4261 | −0.4331 |
| Pantoprazole | a = b = c (Å) | α = β = γ (°) | Density (g/cm3) | Number of Molecules | Number of Particles |
|---|---|---|---|---|---|
| Form A | 31.814 | 90 | 1.407 | 50 | 1 |
| Form C | 31.842 | 90 | 1.382 | 50 | 1 |
| Form E | 31.869 | 90 | 1.425 | 50 | 1 |
| Pantoprazole | Form A | Form C | Form E |
|---|---|---|---|
| (Self-diffusion coefficient) (cm2/s) | 2.67 × 10−7 | 1.16 × 10−8 | 8.33 × 10−9 |
| Free volume (Å3) | 2814.56 | 3288.26 | 2276.13 |
| Occupied volume (Å3) | 20,010.63 | 19,868.82 | 20,180.50 |
| FFV | 0.123309 | 0.141998 | 0.101357 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berisha, A. An Experimental and Theoretical Investigation of the Efficacy of Pantoprazole as a Corrosion Inhibitor for Mild Steel in an Acidic Medium. Electrochem 2022, 3, 28-41. https://doi.org/10.3390/electrochem3010002
Berisha A. An Experimental and Theoretical Investigation of the Efficacy of Pantoprazole as a Corrosion Inhibitor for Mild Steel in an Acidic Medium. Electrochem. 2022; 3(1):28-41. https://doi.org/10.3390/electrochem3010002
Chicago/Turabian StyleBerisha, Avni. 2022. "An Experimental and Theoretical Investigation of the Efficacy of Pantoprazole as a Corrosion Inhibitor for Mild Steel in an Acidic Medium" Electrochem 3, no. 1: 28-41. https://doi.org/10.3390/electrochem3010002
APA StyleBerisha, A. (2022). An Experimental and Theoretical Investigation of the Efficacy of Pantoprazole as a Corrosion Inhibitor for Mild Steel in an Acidic Medium. Electrochem, 3(1), 28-41. https://doi.org/10.3390/electrochem3010002






