A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes
Abstract
:1. Introduction
1.1. Dye Classification
1.2. Synthetic Dyes (Advantages and Disadvantages)
- They are not environmentally friendly as their synthesis involves extreme conditions such as high pH, high temperature, strong acids, and heavy metal catalysts.
- The most common substrate for dye production is petroleum, which is a non-renewable source of energy.
- Synthesis generates a large amount of effluent which contains toxic chemicals generated as side products.
1.3. Industrial Applications
1.4. Methods for Dye Detection
- (i)
- they decrease the capacitive current;
- (ii)
- they are best for organic and inorganic molecules, which are electroactive;
- (iii)
- they give lower detection limits as the preconcentration of the analyte is prevented;
- (iv)
- an easily renewable surface of the working electrodes is possible;
- (v)
- lightweight and small instruments are employed;
- (vi)
- they not only determine the concentration but also give the composition of different species;
- (vii)
- they can also be applied to solid analytes.
2. Voltammetric Detection of Dyes
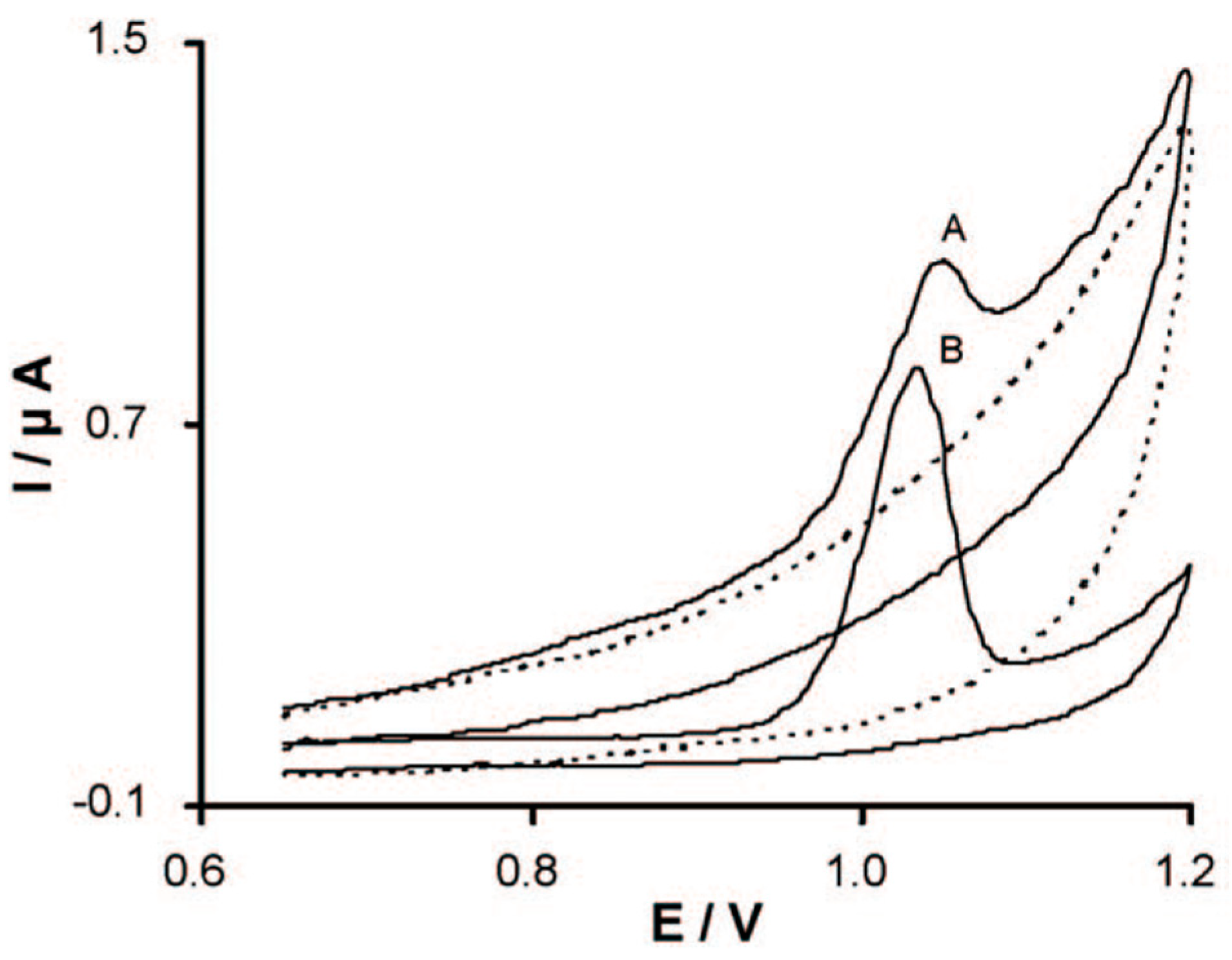
2.1. Detection of Sunset Yellow (SY)
2.2. Detection of Tartrazine (TR)
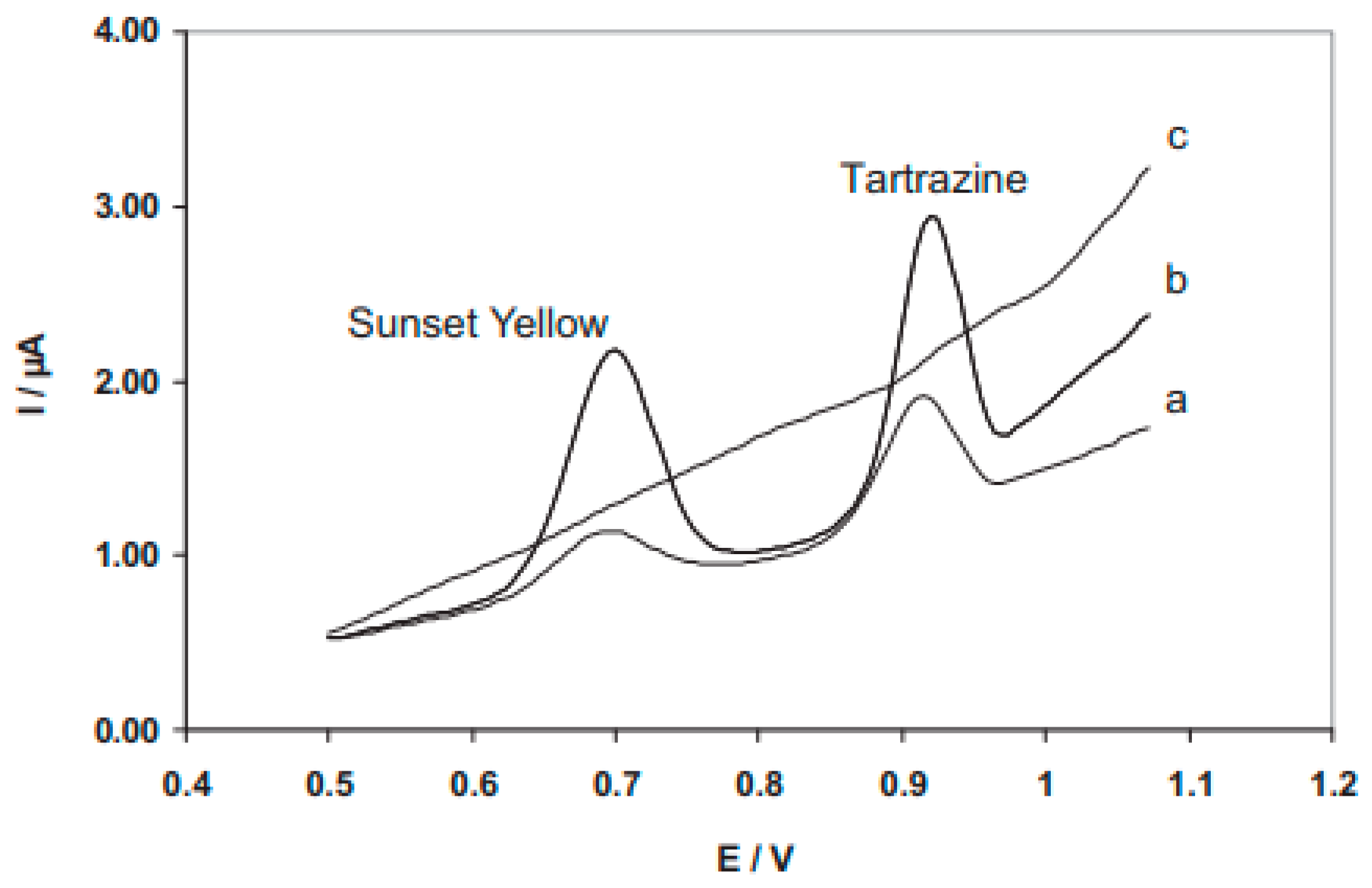
2.3. Simultaneous Detection of SY and TR
2.4. Detection of Amaranth (AM)
2.5. Detection of Allura Red (AR)
2.6. Detection of Carmoisine (CR)
2.7. Detection of Rhodamine B (RHB)
2.8. Detection of Erythrosine (ER)
2.9. Detection of Sudan
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Inter. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of Synthetic Dyes-A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.; Karmakar, S.; Arora, L.S.; Mishra, V. Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Moattari, R.M.; Mohammadi, T. Hybrid Adsorbents for Dye Removal from Wastewater. In Green Adsorbents to Remove Metals; Springer: New York, NY, USA, 2021; Volume 49, pp. 405–451. [Google Scholar]
- Sana, K.; Mohammad, A.; Abdul, M. Mutagenicity and genotoxicity evaluation of textile industry wastewater using bacterial and plant bioassays. Toxicol. Rep. 2019, 6, 193–201. [Google Scholar]
- Mondal, S. Methods of Dye Removal from Dye House Effluent—An Overview. Environ. Eng. Sci. 2008, 25, 383–396. [Google Scholar] [CrossRef]
- Goswami, L.; Chandrashekhar, M. India: Indian Food Safety Laws—Implications for Foreign Operators Importing Food Into India. Eur. Food Feed Law Rev. 2012, 7, 154–156. [Google Scholar]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 1077–3525. [Google Scholar] [CrossRef]
- Benkhaya, S.; Mrabet, S.; Harfi, A.E. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Šuleková, M.; Smrčová, M.; Hudák, A.; Hezelova, M.; Fedorová, M. Organic colouring agents in the pharmaceutical industry. Folia Vet. 2017, 61, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017, 9, 121–136. [Google Scholar]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Zima, J.; Barek, J.; Moreira, J.C.; Mejstřík, V.; Fogg, A.G. Electrochemical determination of trace amounts of environmentally important dyes. Fresenius’ J. Anal. Chem. 2001, 369, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, G.K.; Swamy, B.E.K.; Ramirez, H.N.G.; Ekanthappa, M.T.; Flores-Moreno, R. Quantum chemical and electrochemical studies of lysine modified carbon paste electrode surfaces for sensing dopamine. New J. Chem. 2018, 42, 4501–4506. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Casillas, N.; Flores-Moreno, R. Analytical Fukui and cyclic voltammetric studies on ferrocene modified carbon electrodes and effect of Triton X-100 by immobilization method. Electrochim. Acta 2017, 258, 1025–1034. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Chandrashekar, B.N.; Flores-Moreno, R. Theoretical and cyclic voltammetric studies on electrocatalysis of benzethonium chloride at carbon paste electrode for detection of dopamine in presence of ascorbic acid. J. Mol. Liq. 2017, 240, 395–401. [Google Scholar] [CrossRef]
- Marken, F.; Neudeck, A.; Bond, A.M. Cyclic Voltammetry. In Electroanalytical Methods; Scholz, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Chen, X.; Wu, K.; Sun, Y.; Song, X. Highly sensitive electrochemical sensor for sunset yellow based on the enhancement effect of alumina microfibers. Sens. Actuators B Chem. 2013, 185, 582–586. [Google Scholar] [CrossRef]
- Songyang, Y.; Yang, X.; Xie, S.; Hao, H.; Song, J. Highly-sensitive and rapid determination of sunset yellow using functionalized montmorillonite-modified electrode. Food Chem. 2014, 173, 640–644. [Google Scholar] [CrossRef]
- Sun, D.; Xu, C.; Long, J.; Ge, T. Determination of Sunset Yellow using a carbon paste electrode modified with a nanostructured resorcinol-formaldehyde resin. Microchim. Acta 2015, 182, 2601–2606. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, Y.; Xu, L.; Wu, G. Determination of the azo dye, sunset yellow, using carbon paste electrode modified with molecularly imprinted polymer. Indian J. Chem. Sect. A 2016, 55, 1458–1464. [Google Scholar]
- Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A Zinc Oxide Nanoflower-Based Electrochemical Sensor for Trace Detection of Sunset Yellow. Sensors 2017, 17, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Selective Voltammetric Determination of Tartrazine in the Presence of Red 10B by Nanogold-modified Carbon Paste Electrode. J. Chin. Chem. Soc. 2013, 60, 120–126. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z.; Zamani, M.; Dorraji, P.S.; Alamgholiloo, M. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2016, 25, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Chenthattil, R.; Manjunatha, J.G.; Tigari, G.; Nagarajappa, N.H. Fabrication of the Tartrazine Voltammetric Sensor based on Surfactant Modified Carbon Paste Electrode. Open Access J. Chem. 2018, 2, 21–26. [Google Scholar]
- Manjunatha, J.G. A novel voltammetric method for the enhanced detection of the food additive tartrazine using an electrochemical sensor. Heliyon 2018, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chenthattil, R.; Manjunatha, J.G. Development of Sodium Dodecyl Sulfate Based Electrochemical Sensor for Tartrazine Determination. Portugaliae Electrochim. Acta 2021, 39, 59–70. [Google Scholar]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of Sunset yellow and Tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef]
- Marquez-Mariño, K.; Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. Development of a Novel Electrochemical Sensor Based on a Carbon Paste Electrode Decorated with Nd2O3 for the Simultaneous Detection of Tartrazine and Sunset Yellow. Electroanalysis 2018, 30, 2760–2767. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; García-Beltrán, O.; Calderón, J.A.; Hurtado-Murillo, J.J.; Nagles, E.; Hurtado, J.J. Simultaneous determination of tartrazine, sunset yellow and allura red in foods using a new cobalt-decorated carbon paste electrode. J. Electroanal. Chem. 2019, 852, 113517. [Google Scholar] [CrossRef]
- Pandurangachar, M.; Niranjana, E.; Swamy, B.E.K.; Sherigara, B.S.; Pai, K.V. Electrochemical studies of amaranth at surfactant modified carbon paste electrode: A cyclic voltammetry. Int. J. Electrochem. Sci. 2008, 3, 588–596. [Google Scholar]
- Wang, M.L.; Zhang, J.; Ding, N.N.; Zhu, X.L.; Chen, Z.D. Electrochemical detection of amaranth in food based on the expanded graphite paste electrode. J. AOAC Int. 2013, 96, 625–629. [Google Scholar] [CrossRef]
- Sheikhshoaee, M.; Karimi-maleh, H.; Sheikhshoaie, I.; Ranjbar, M. Voltammetric amplified sensor employing RuO2 nano-road and room temperature ionic liquid for amaranth analysis in food samples. J. Mol. Liq. 2017, 229, 489–494. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahid, S.A. Simultaneous Determination of Amaranth and Nitrite in Foodstuffs via Electrochemical Sensor Based on Carbon Paste Electrode Modified with CuO/SWCNTs and Room Temperature Ionic Liquid. Food Anal. Methods 2017, 10, 3773–3780. [Google Scholar] [CrossRef]
- Akbari, S. A new voltammetric sensor according to graphene quantum dots/ionic liquid modified carbon paste electrode for amaranth sensitive determination. Int. J. Environ. Anal. Chem. 2020, 1–15. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, X.; Wang, W.; Chen, Z. Simultaneous determination of Ponceau-4R and Allura Red in soft drinks based on the ionic liquid modified expanded graphite paste electrode. Int. J. Environ. Anal. Chem. 2015, 95, 581–591. [Google Scholar] [CrossRef]
- Penagos-Llanos, J.; García, O.; Calderón, J.; Edgar, N.; Hurtado, J. Carbon Paste Composite with Co3O4 as a New Electrochemical Sensor for the Detection of Allura Red by Reduction. Electroanalysis 2019, 31, 695–703. [Google Scholar] [CrossRef]
- Pliuta, K.; Chebotarev, A.; Pliuta, A.; Snigur, D. Voltammetric Determination of Allura Red AC onto Carbone-Paste Electrode Modified by Silica with Embedded Cetylpyridinium Chloride. Electroanalysis 2020, 33, 987–992. [Google Scholar] [CrossRef]
- Rozhin, D.; Shabani-Nooshabadi, M. NiFe2O4-rGO/ionic liquid modified carbon paste electrode: An amplified electrochemical sensitive sensor for determination of Sunset Yellow in the presence of Tartrazine and Allura Red. Food Chem. 2021, 339, 127841/1–9. [Google Scholar]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahidi, S.A. An electrochemical-amplified-platform based on the nanostructure voltammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food Meas. Charact. 2018, 12, 634–640. [Google Scholar] [CrossRef]
- Nezhad, H.M.; Seyed-Ahmad, S.; Bijad, M. Fabrication of a Nanostructure Voltammetric Sensor for Carmoisine Analysis as a Food Dye Additive. Anal. Bioanal. Electrochem. 2018, 10, 220–229. [Google Scholar]
- Chebotarev, A.; Pluta, K.; Snigur, D. Determination of Carmoisine onto Carbon-Paste Electrode Modified by Silica Impregnated with Cetylpyridinium Chloride. Chem. Select. 2020, 5, 3688–3693. [Google Scholar] [CrossRef]
- Noviandri, I.; Raminas, A. The Effect of Tartrazine in Voltammetric Determination of Rhodamine B with Molecularly Imprinted Poly (3-Aminophenol) Modified Carbon Electrode. Funct. Mater. Technol. 2019, 811, 107–112. [Google Scholar]
- Golestaneh, M.; Ghoreishi, S.M. Sensitive Determination of Rhodamine B in Real Samples at the Surface of a Multi-walled Carbon Nanotubes Paste Electrode. Anal. Bioanal. Electrochem. 2020, 12, 81–92. [Google Scholar]
- Setiyanto, H.; Ferizal, F.; Rahayu, V.S.R.S.; Zulfikar, M.A. Carbon paste electrode modified Poly-Glutamic Acid (PGA) with molecularly imprinted for detection of Rhodamine B. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1088, 012113. [Google Scholar] [CrossRef]
- Golestaneh, M.; Ghoreishi, S.M. Electrochemical determination of erythrosine in real sample using a carbon paste electrode modified with multi-walled carbon nanotube. In Proceedings of the Iranian Biennial Electrochemistry Seminar, Guilan, Iran, 9–11 September 2014. [Google Scholar]
- Nayak, D.S.; Shetti, N.P. A novel sensor for a food dye erythrosine at glucose modified electrode. Sens. Actuators B Chem. 2016, 230, 140–148. [Google Scholar] [CrossRef]
- Arvand, M.; Pourhabib, A.; Asadi, M. Template-based synthesis of uniform bimetallic nickel-tin oxide hollow nanospheres as a new sensing platform for detection of erythrosine in food products. Sens. Actuators B Chem. 2018, 255, 1716–1725. [Google Scholar] [CrossRef]
- Lin, H.; Li, G.; Wu, K. Electrochemical determination of Sudan I using montmorillonite calcium modified carbon paste electrode. Food Chem. 2007, 107, 531–536. [Google Scholar] [CrossRef]
- Elyasi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples. Food Chem. 2013, 141, 4311–4317. [Google Scholar] [CrossRef]
- Raoof, J.B.; Teymoori, N.; Khalilzadeh, M. ZnO Nanoparticle Ionic Liquids Carbon Paste Electrode as a Voltammetric Sensor for Determination of Sudan I in the Presence of Vitamin B6 in Food Samples. Food Anal. Methods 2014, 8, 885–892. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Moazampour, M.; Yoosefian, M.; Sanati, A.L.; Tahernejad-Javazmi, F.; Mahani, M. An Electrochemical Nanosensor for Simultaneous Voltammetric Determination of Ascorbic Acid and Sudan I in Food Samples. Food Anal. Methods 2014, 7, 2169–2176. [Google Scholar] [CrossRef]
- Heydari, M.; Ghoreishi, S.M.; Khoobi, A. Novel electrochemical procedure for sensitive determination of Sudan II based on nanostructured modified electrode and multivariate optimization. Measurement 2019, 142, 105–112. [Google Scholar] [CrossRef]
- Heydari, M.; Ghoreishi, S.M.; Khoobi, A. Response surface modeling of electrochemical data for sensitive determination of Sudan III in food products at the surface of a nanocomposite modified electrode. Food Anal. Methods 2019, 12, 1781–1790. [Google Scholar] [CrossRef]
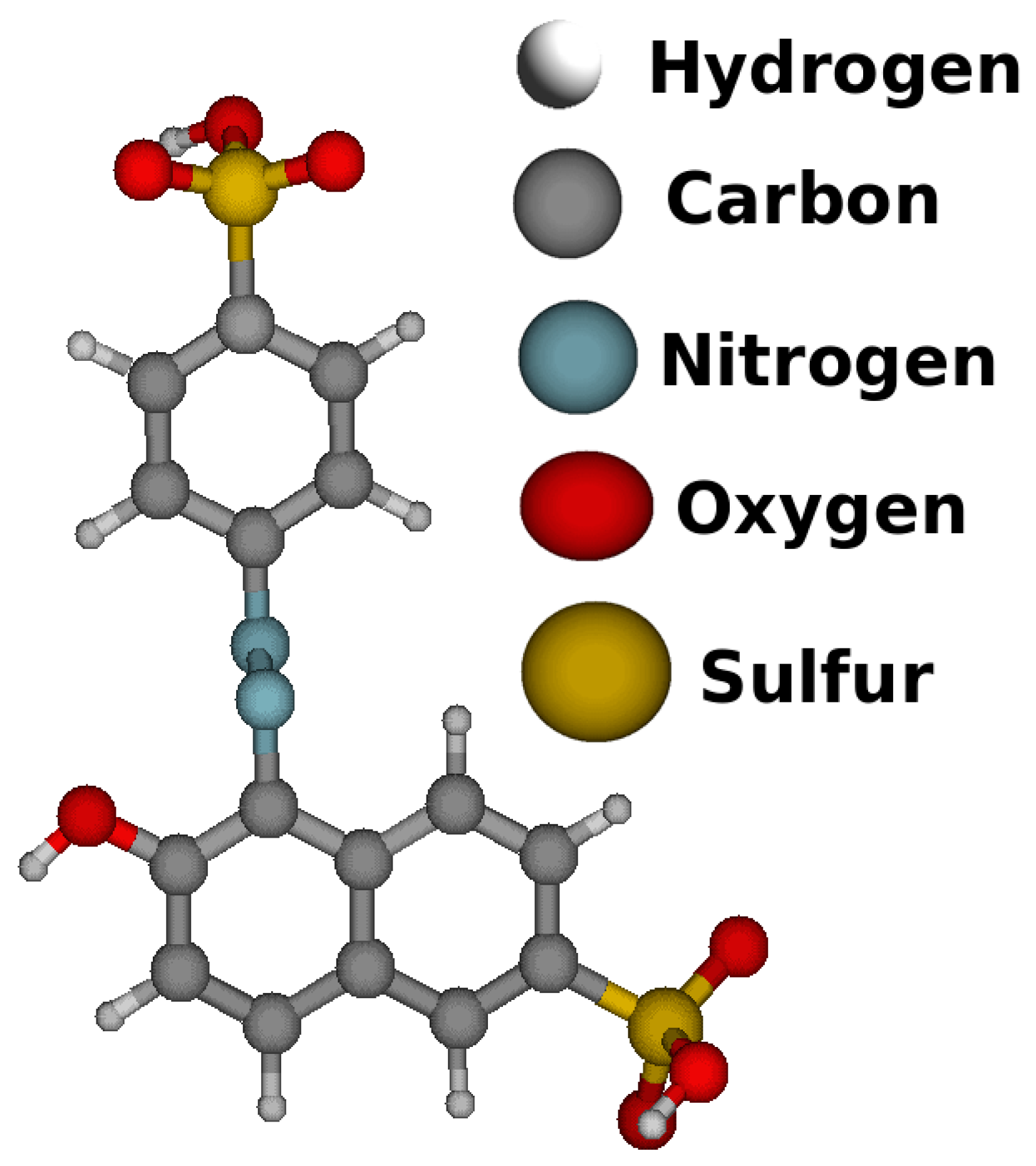
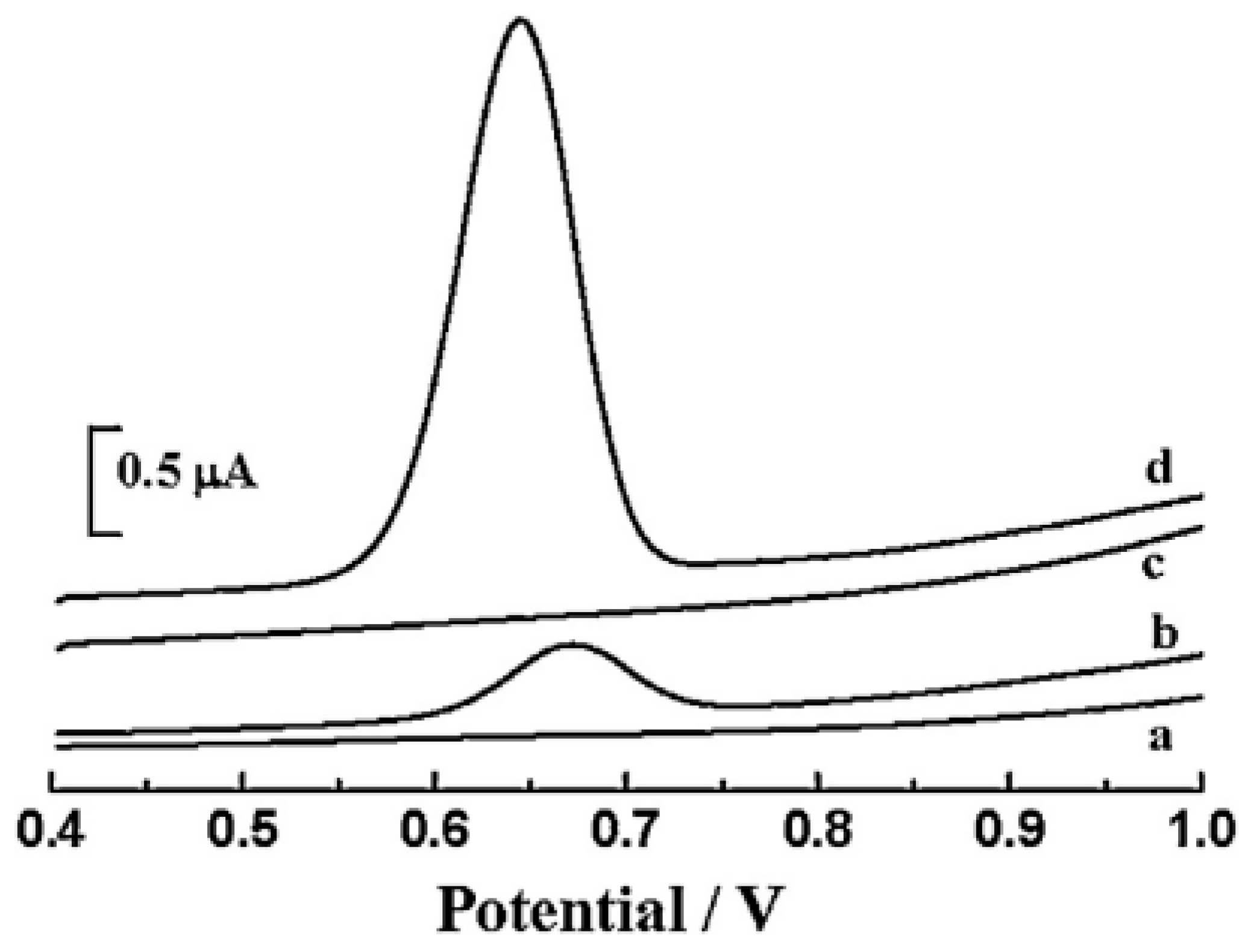
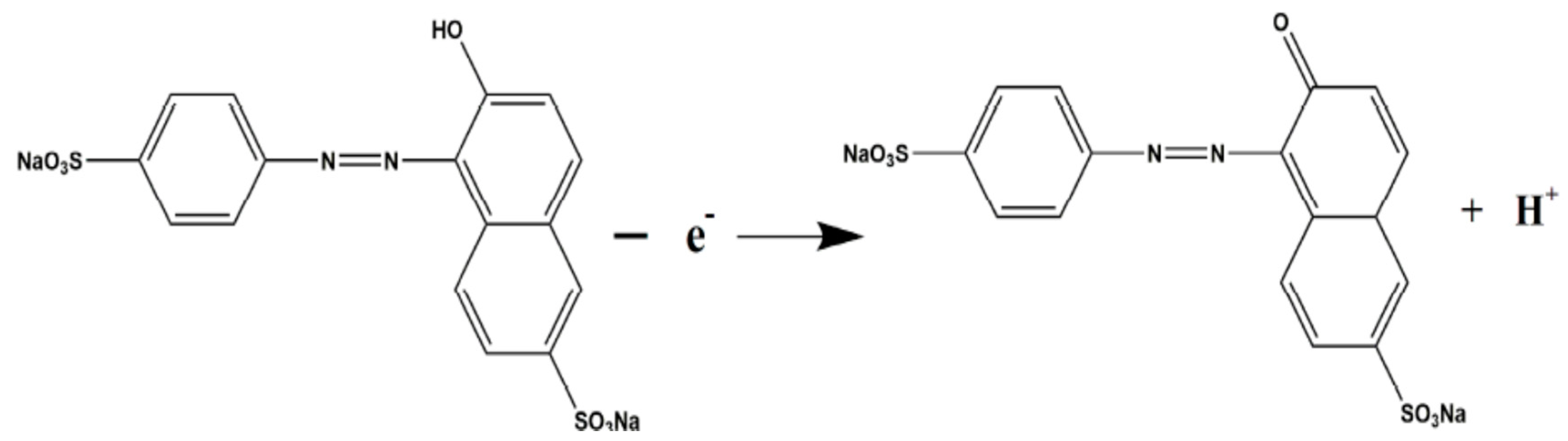
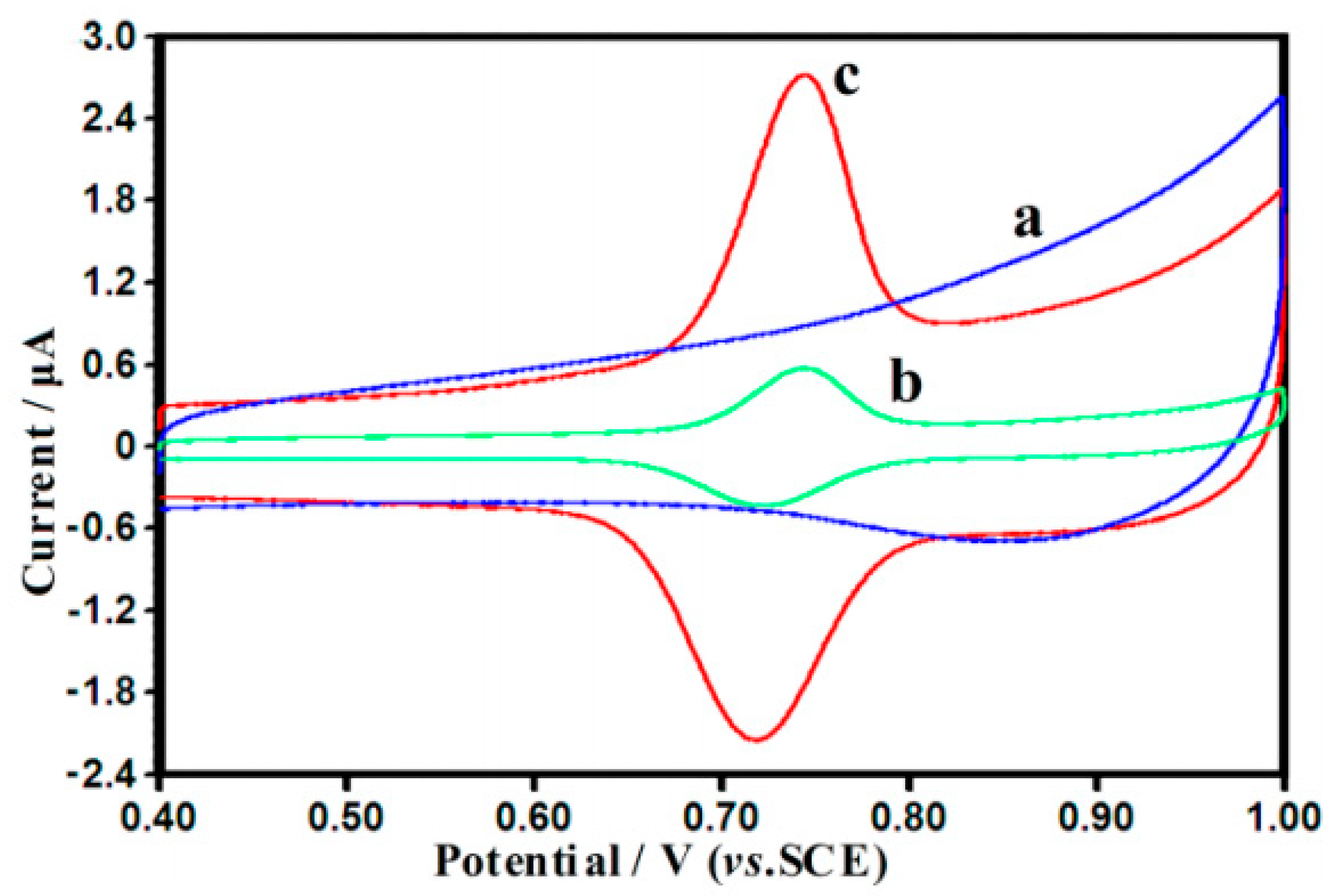

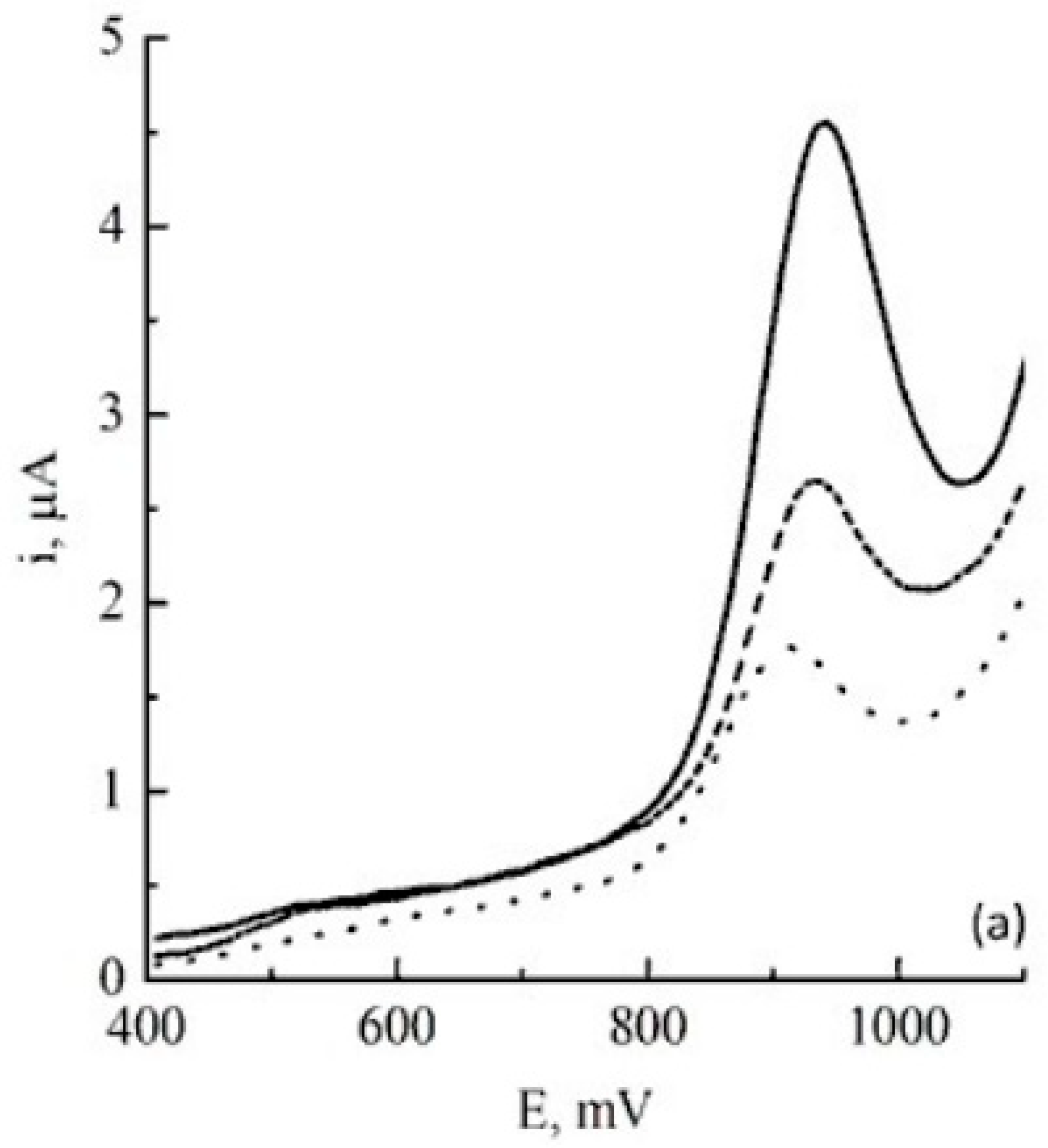


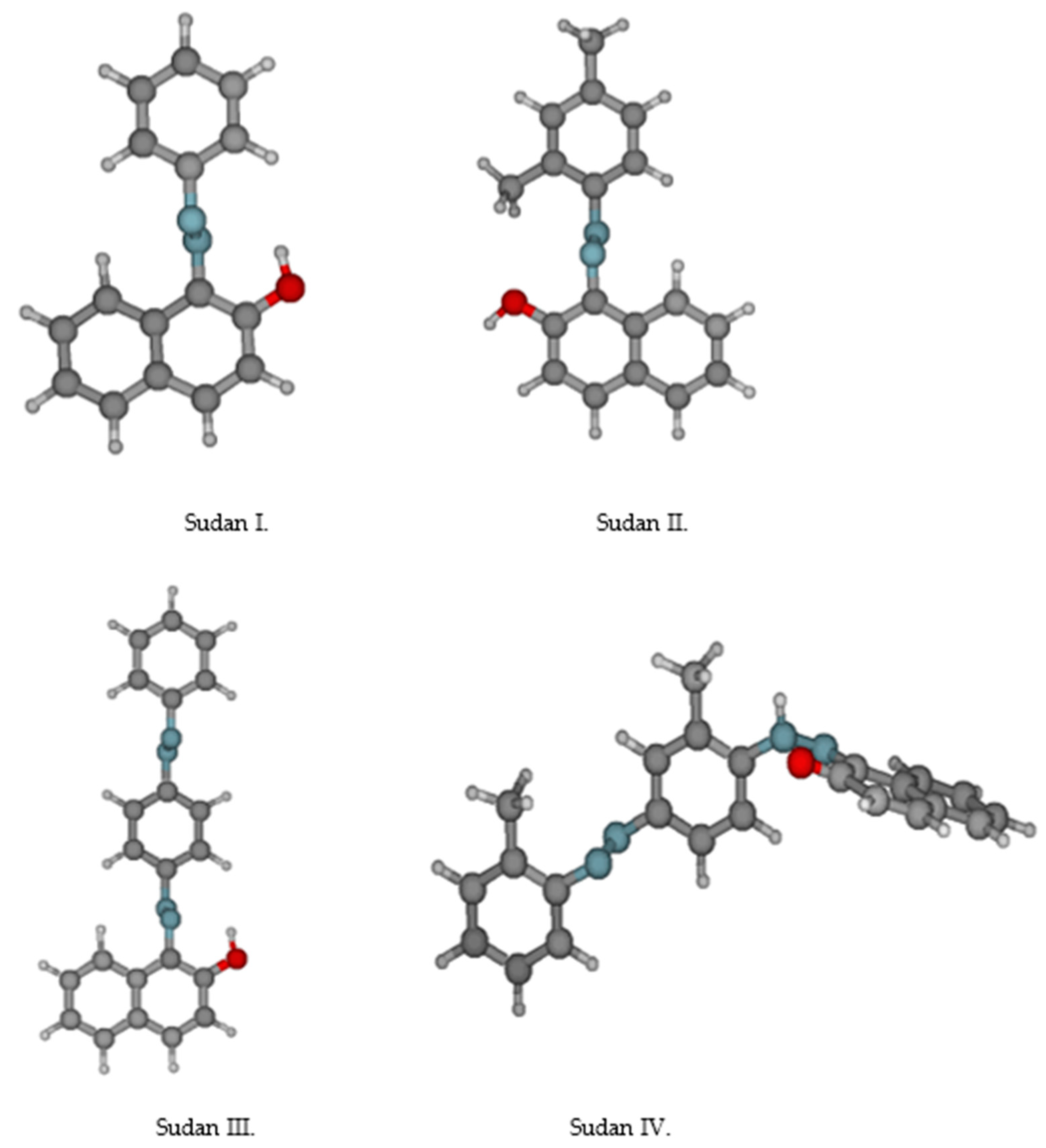
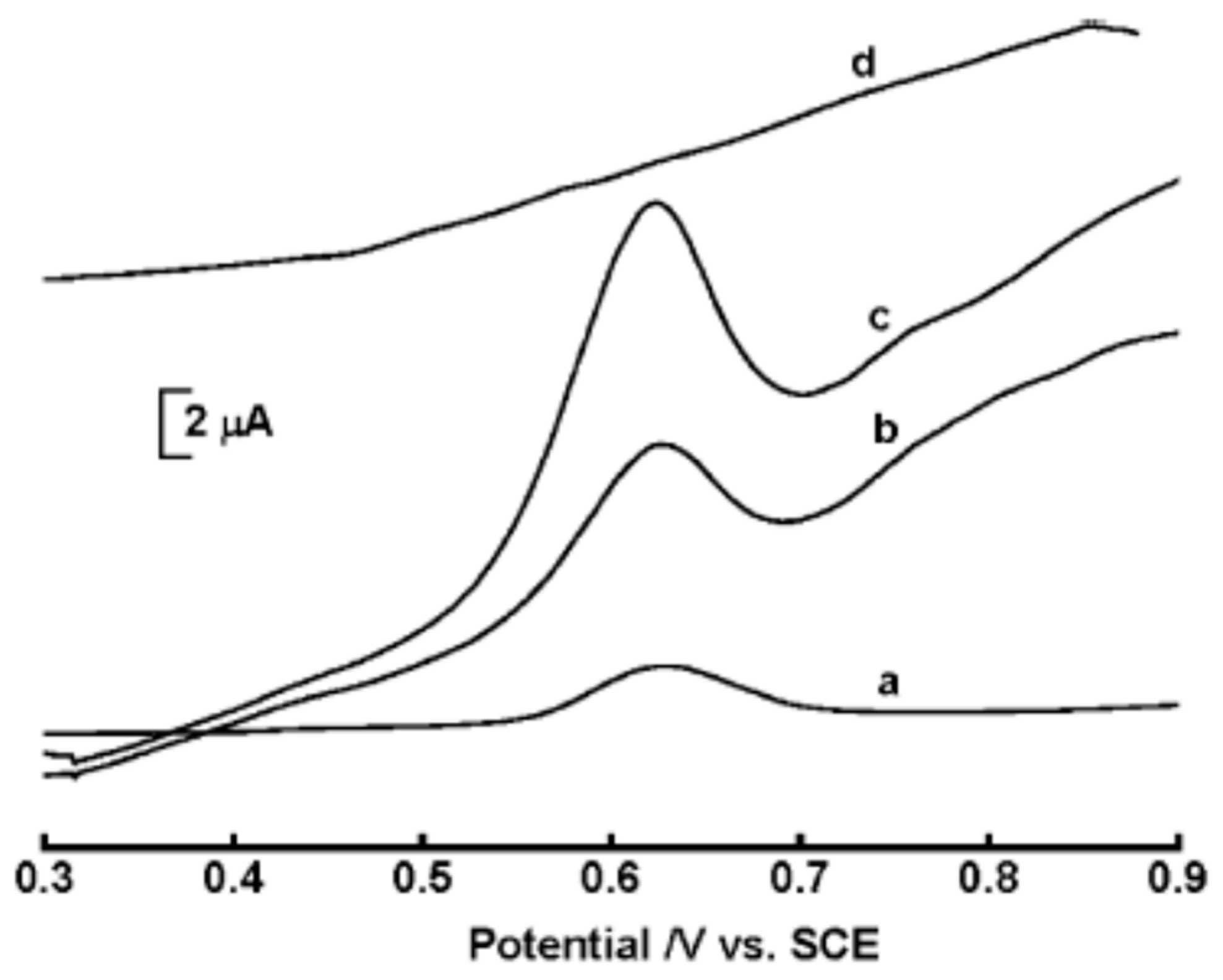
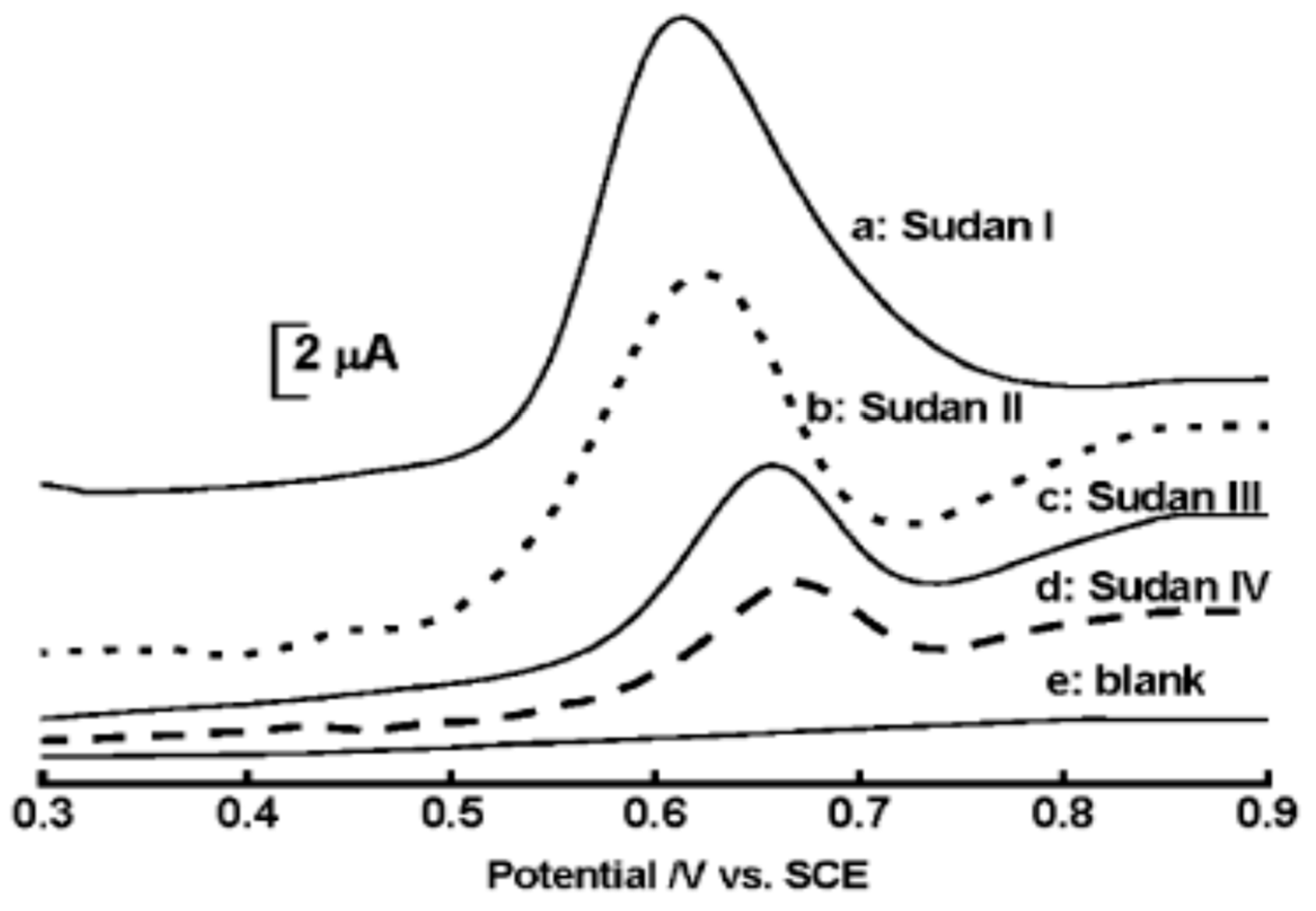
| Color | Wavelength Å | Dyes |
|---|---|---|
| Red | 6400–7000 | Carmoisine, Erythrosine, Allura Red |
| Yellow | 5500–5900 | Tartrazine, Sunset Yellow |
| Blue | 4500–5100 | Brilliant Blue, Indigo Carmine |
| Green | 5100–5500 | Fast Green |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | Alumina nanofibers | CPE | 0.16 nM | Soft drink samples | [20] |
| 2. | MMT-Ca | CPE | 0.71 nM | Different drink samples | [21] |
| 3. | Resorcinol-formaldehyde resin | CPE | 0.09 nM | Wastewater and drink samples | [22] |
| 4. | MWCNT/MIP | CPE | 26.67 nM | Soft drink samples | [23] |
| 5. | ZnONano flower | CPE | 0.10 µg/L | Soft drink samples | [24] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | Nano-Au | CPE | 0.017 µM | Soft drink samples | [25] |
| 2. | Poly (BSA)+ZnO NPs | CPE | 80 nM | soft drink samples & orange powder | [26] |
| 3. | TX-100 | CPE | 1.114 µM | Not analyzed | [27] |
| 4. | Poly (Glycine) | CPE | 0.283 µM | Candy & soft drink | [28] |
| 5. | SDDS | CPE | 5.2 µM | Tiger lemon yellow powder | [29] |
| S.No. | Modifier | Dye | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|---|
| 1. | Au NPs | SY TR | CPE | 30 nM 2 nM | Soft drink samples | [30] |
| 2. | Nd2O3 | SY TR | CPE | 0.09 µM 0.02 µM | Soft drink samples & orange powder | [31] |
| 3. | Cobalt complex | SY TR | CPE | 0.9 µM 0.3 µM | Flavored gelatine powder | [32] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | CTAB | CPE | Not detected | Not analyzed | [33] |
| 2. | Graphite paste | CPE | 0.005 µM | Wine samples | [34] |
| 3. | RuO2/DPIR | CPE | 3 nM | Soft drinks, apple and orange juices | [35] |
| 4. | Cu2O/SWCNT | CPE | 0.001 µM | Sausages, apple and orange juices | [36] |
| 5. | Graphene/IL | CPE | 30 nM | Tap water, well water, river water, apple juice, and orange juice | [37] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | Cobalt decorated | CPE | 0.08 µM | Flavored gelatine powder | [32] |
| 2. | IL | CPE | 0.085 µg/L | Soft drink samples | [38] |
| 3. | Cobalt composite | CPE | 0.05 µM | Spiked unflavored gelatins | [39] |
| 4. | SG/CPC | CPE | 0.005 µM | Jelly candies | [40] |
| 5. | NiFe2O4/rGO/IL | CPE | 0.03 µM | Orange juice powder, hair shampoo | [41] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | NiO/CNT/3BIBr | CPE | 20 nM | Dried fruit and soft drink samples | [42] |
| 2. | Cd/CNT/EMITB | CPE | 40 nM | Orange and lemon juices | [43] |
| 3. | Silica | CPE | 0.01 µM | Non-alcoholic soft drinks | [44] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | MIP(PAP) | CPE | 1.17 pM | Sekoteng and crackers | [45] |
| 2. | MWCNT | CPE | 20 nM | Hair color and water samples | [46] |
| 3. | MIP(PGA) | CPE | 8.91 µM | Not analyzed | [47] |
| S.No. | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|
| 1. | MWCNT | CPE | 5 nM | Drinks samples and cosmetic products | [48] |
| 2. | Glucose | CPE | 21.6 nM | Fruit juices and pharmaceutical products | [49] |
| 3. | Ni-Sn oxide NSs | CPE | 2.1 nM | Powered gelatins, candies, and smarties | [50] |
| S.No. | Dye | Modifier | Working Electrode | Detection Limit | Real Sample Used | Reference |
|---|---|---|---|---|---|---|
| 1. | Sudan I | MMT-Ca | CPE | 80.6 nM | Chili powder, chili sauce, and ketchup | [51] |
| Pt/CNT | 0.003 µM | Chili sauce, chili powder, tomato sauce, and strawberry sauce | [52] | |||
| ZnO NPs/IL | 0.008 µM | Chili sauce, chili powder, tomato sauce, and strawberry sauce | [53] | |||
| NiO NPs/DEDED | 0.2 µM | Spiked urine, chili sauce, and tomato sauce | [54] | |||
| 2. | Sudan II | ZnO NPs | CPE | 0.0017 µM | Chili sauce and ketchup | [55] |
| 3. | Sudan III | ZnO NPs | CPE | 2.56 nm | Chili sauce and ketchup | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, I.; Kumar, P.; Sharma, S.; Kudur Jayaprakash, G. A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes. Electrochem 2021, 2, 274-294. https://doi.org/10.3390/electrochem2020020
Soni I, Kumar P, Sharma S, Kudur Jayaprakash G. A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes. Electrochem. 2021; 2(2):274-294. https://doi.org/10.3390/electrochem2020020
Chicago/Turabian StyleSoni, Isha, Pankaj Kumar, Shruti Sharma, and Gururaj Kudur Jayaprakash. 2021. "A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes" Electrochem 2, no. 2: 274-294. https://doi.org/10.3390/electrochem2020020
APA StyleSoni, I., Kumar, P., Sharma, S., & Kudur Jayaprakash, G. (2021). A Short Review on Electrochemical Sensing of Commercial Dyes in Real Samples Using Carbon Paste Electrodes. Electrochem, 2(2), 274-294. https://doi.org/10.3390/electrochem2020020








