Molybdenum-Suboxide Thin Films as Anode Layers in Planar Lithium Microbatteries
Abstract
1. Introduction
2. Materials and Methods
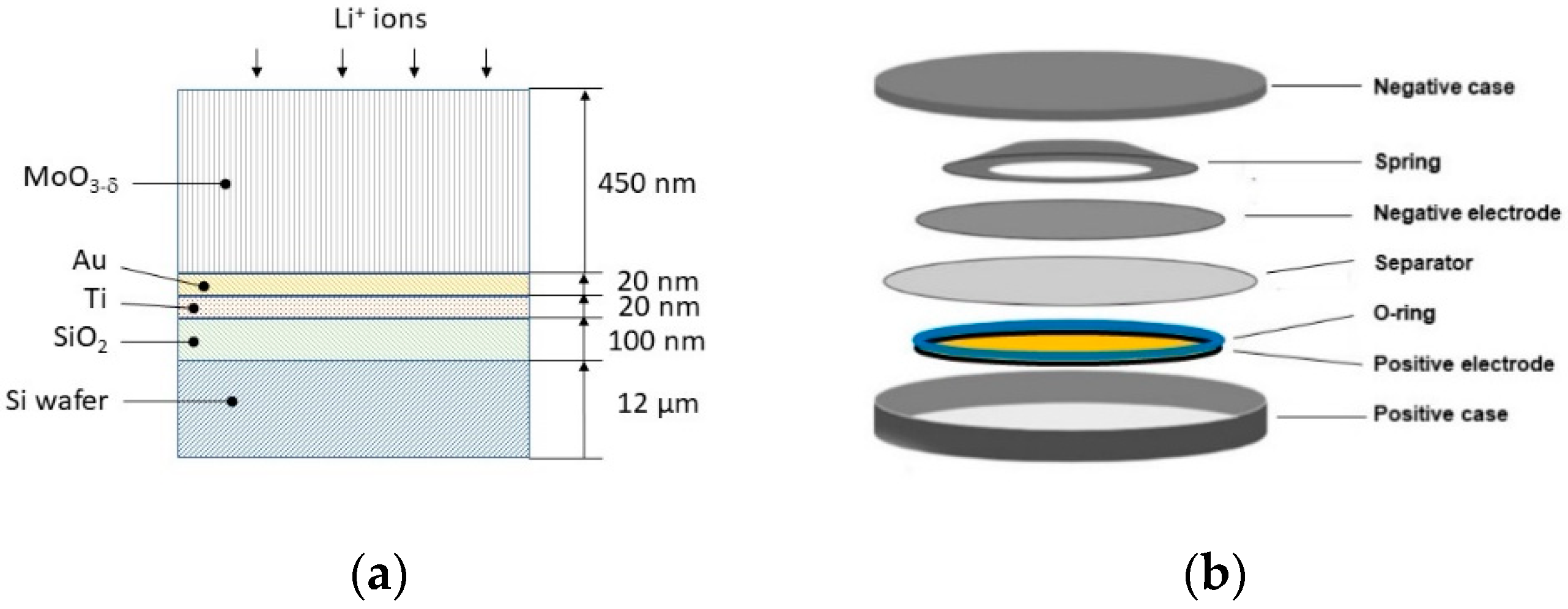
2.1. Preparation of Au/Ti/SiO2/flexible-Si Substrates
2.2. MoOx Thin Films Fabrication
2.3. Film Characterization
3. Results
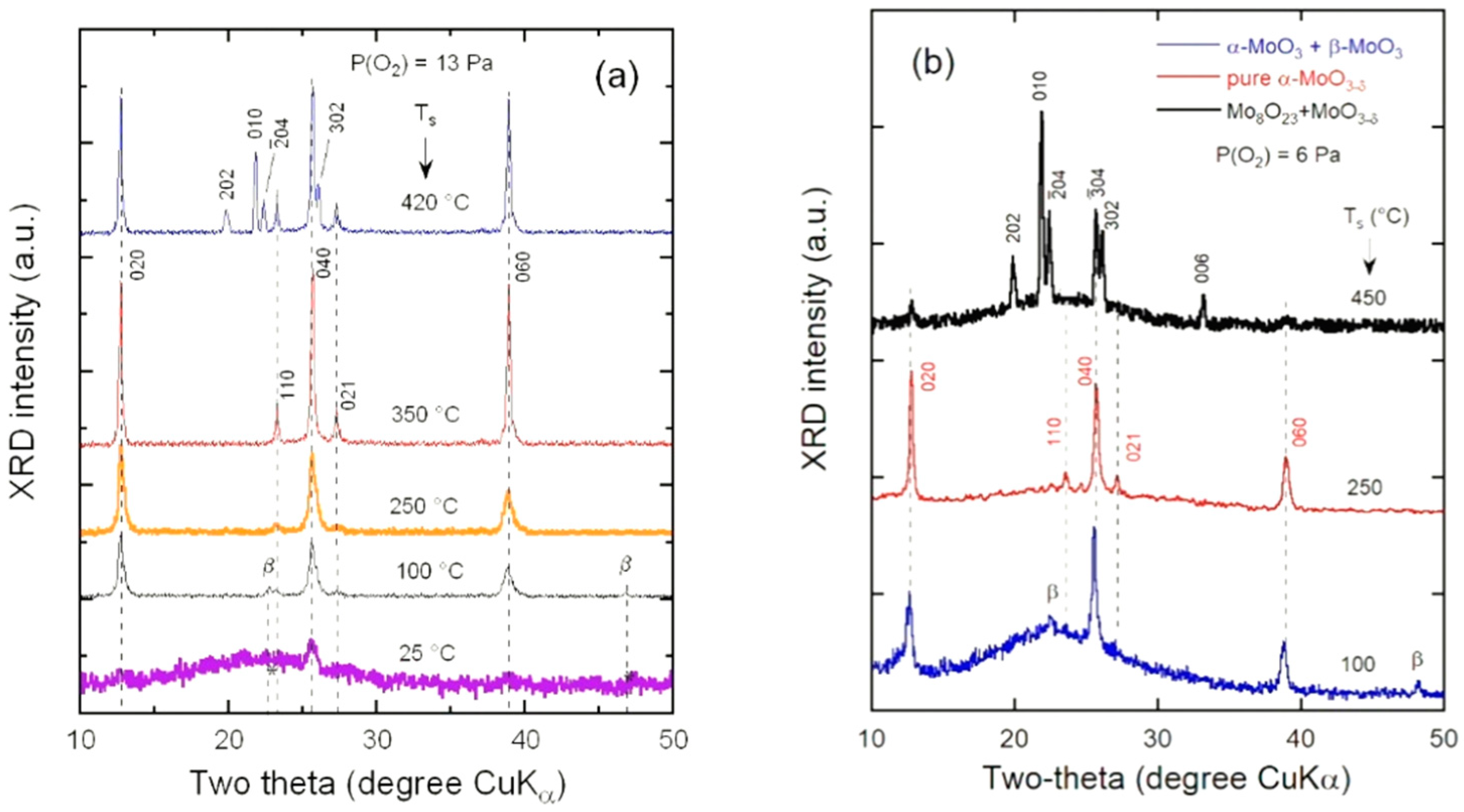
3.1. Structural Properties
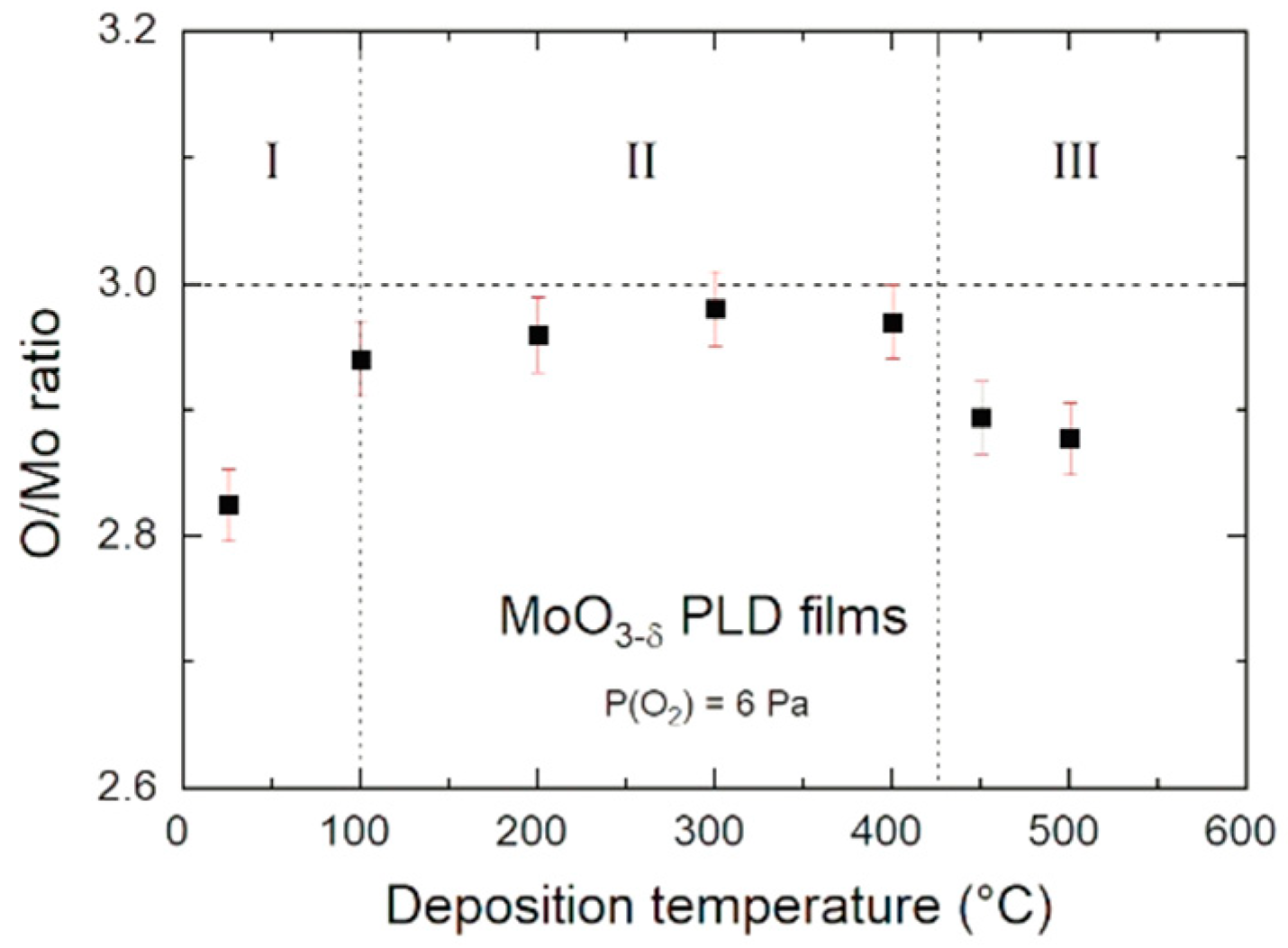
3.2. Composition
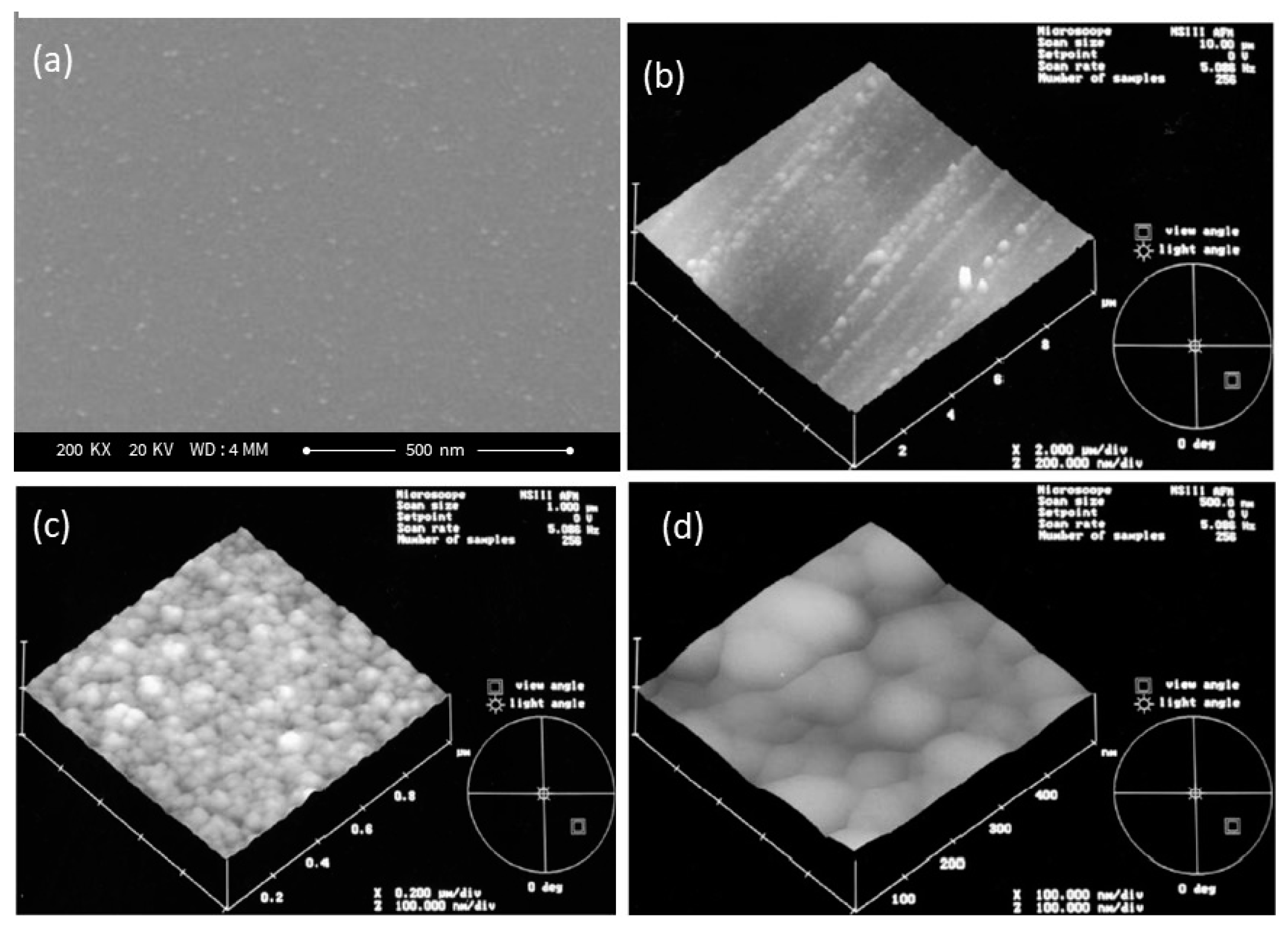
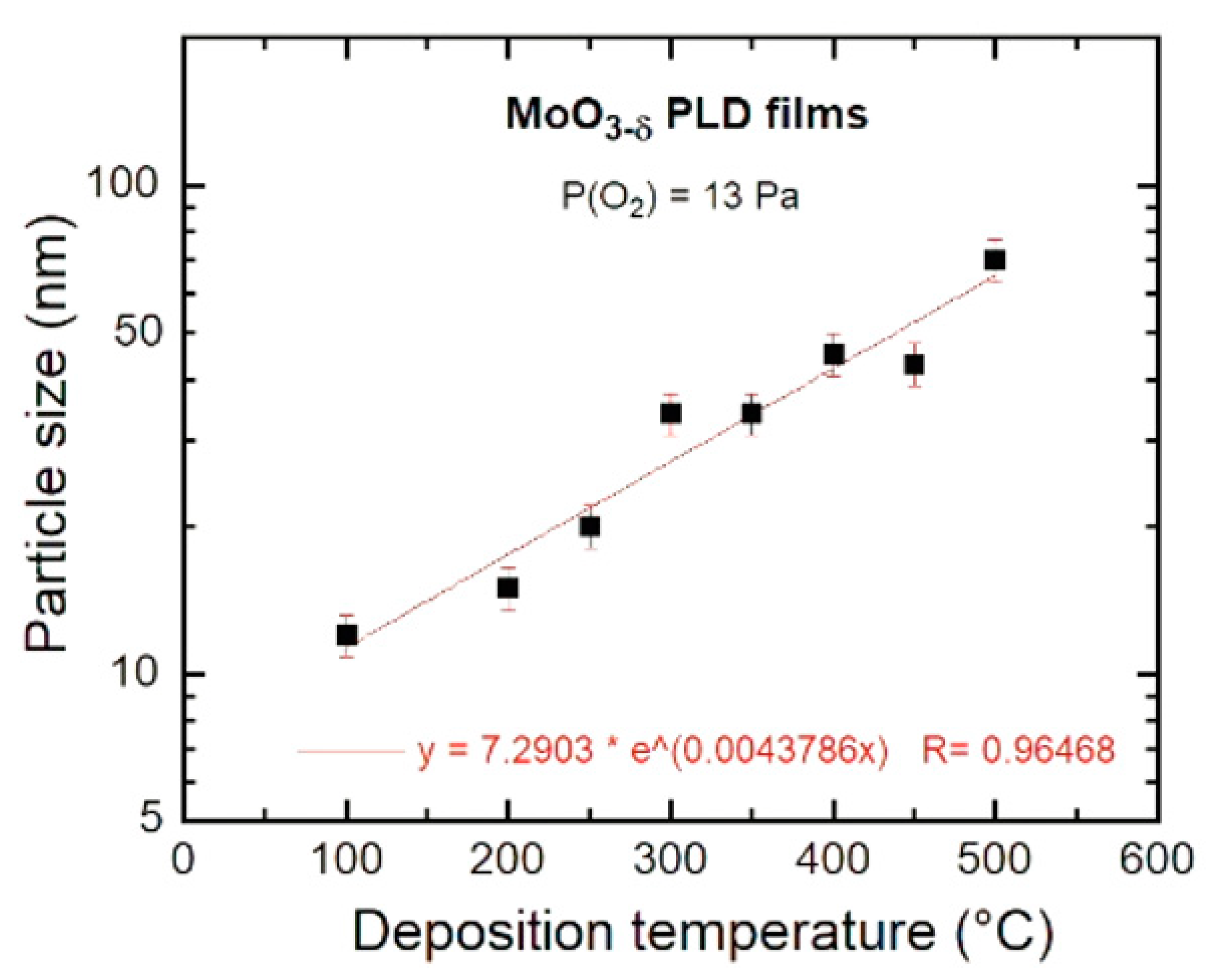
3.3. Morphology
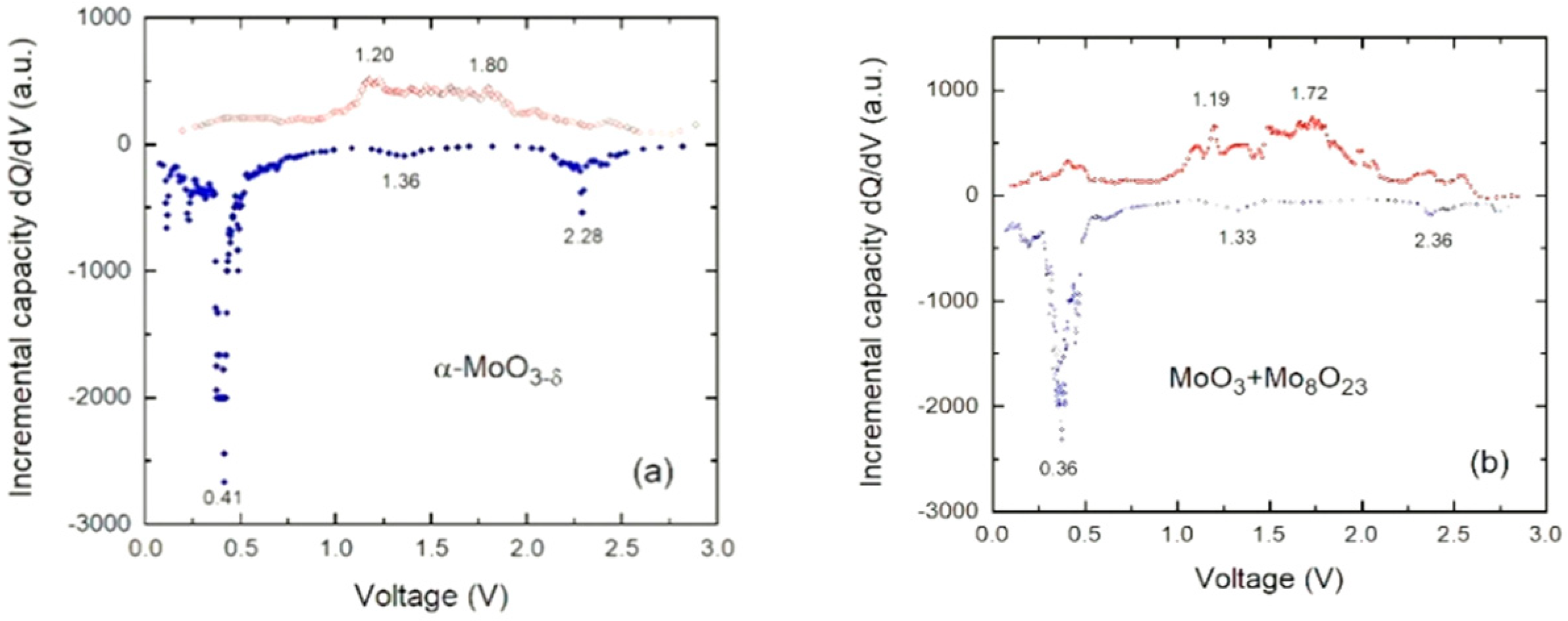
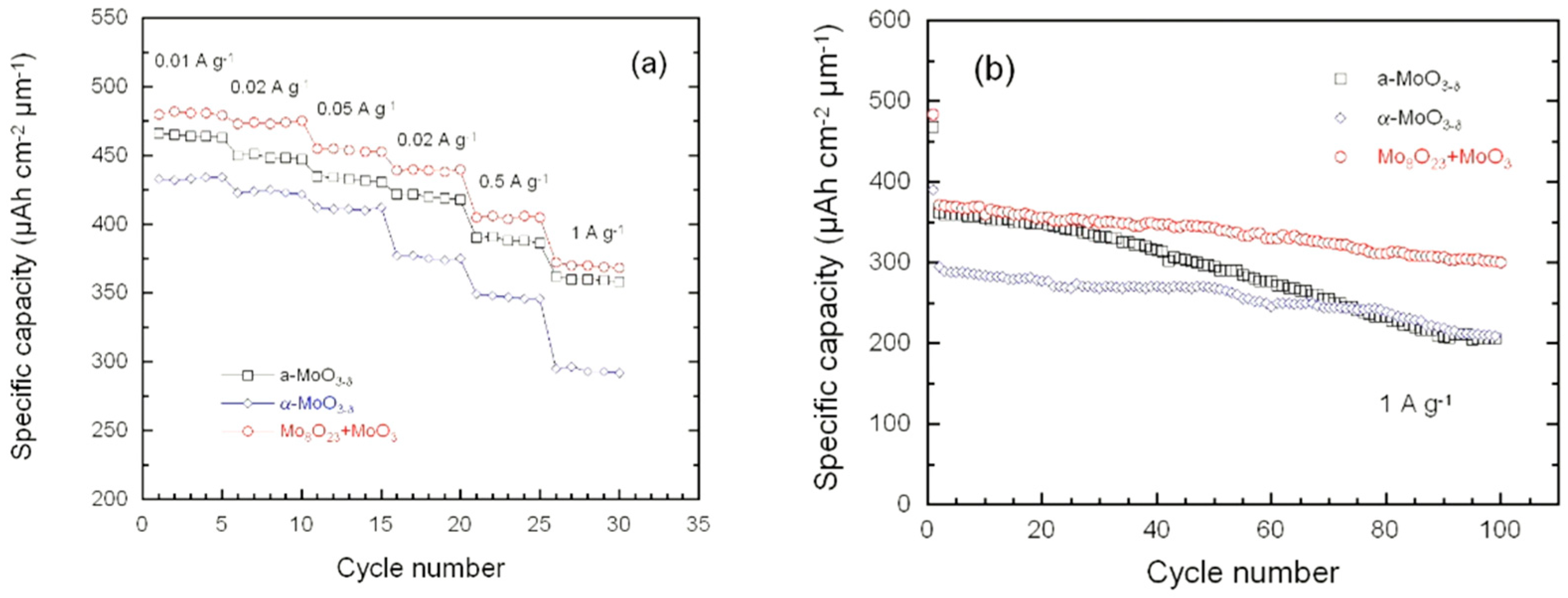
3.4. Electrochemical Properties
4. Discussion
4.1. Growth Conditions
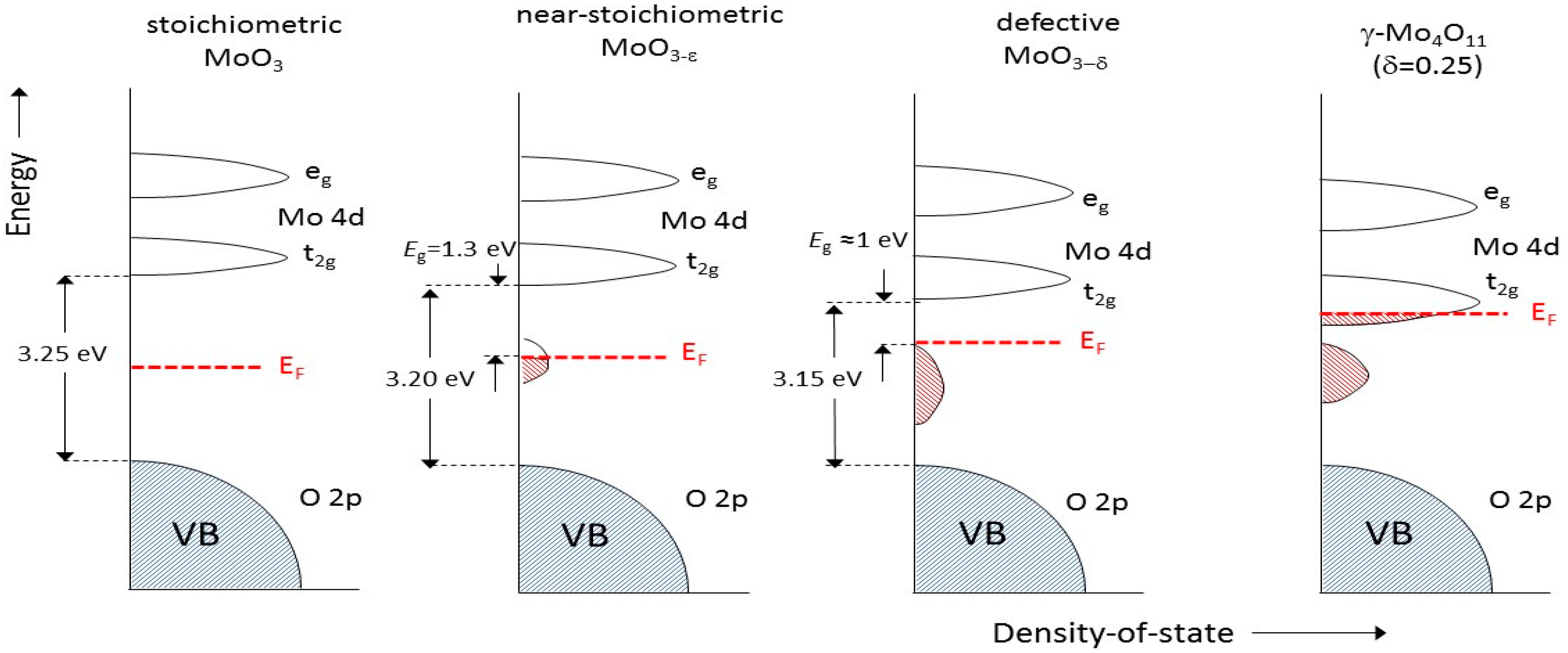
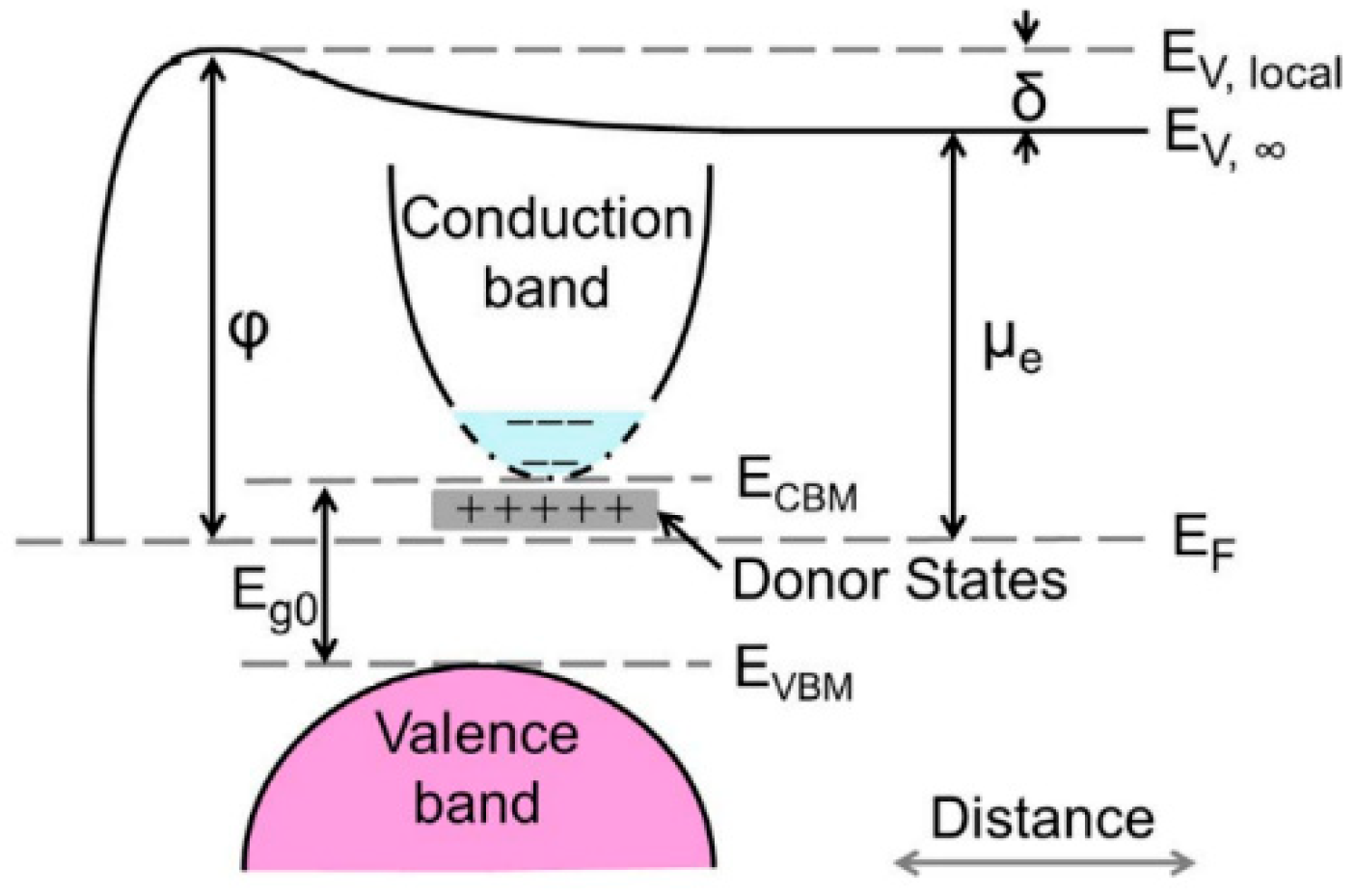
4.2. Electronic Properties
4.3. Electrochemistry
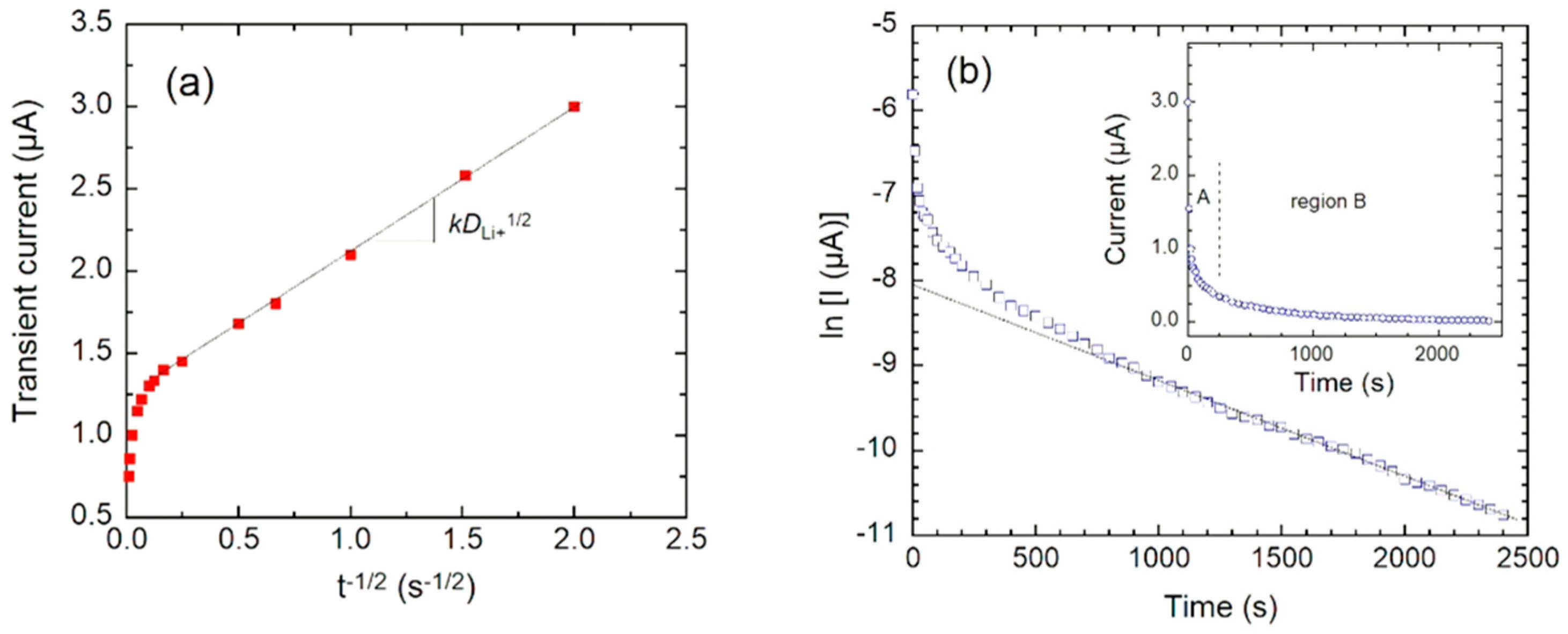
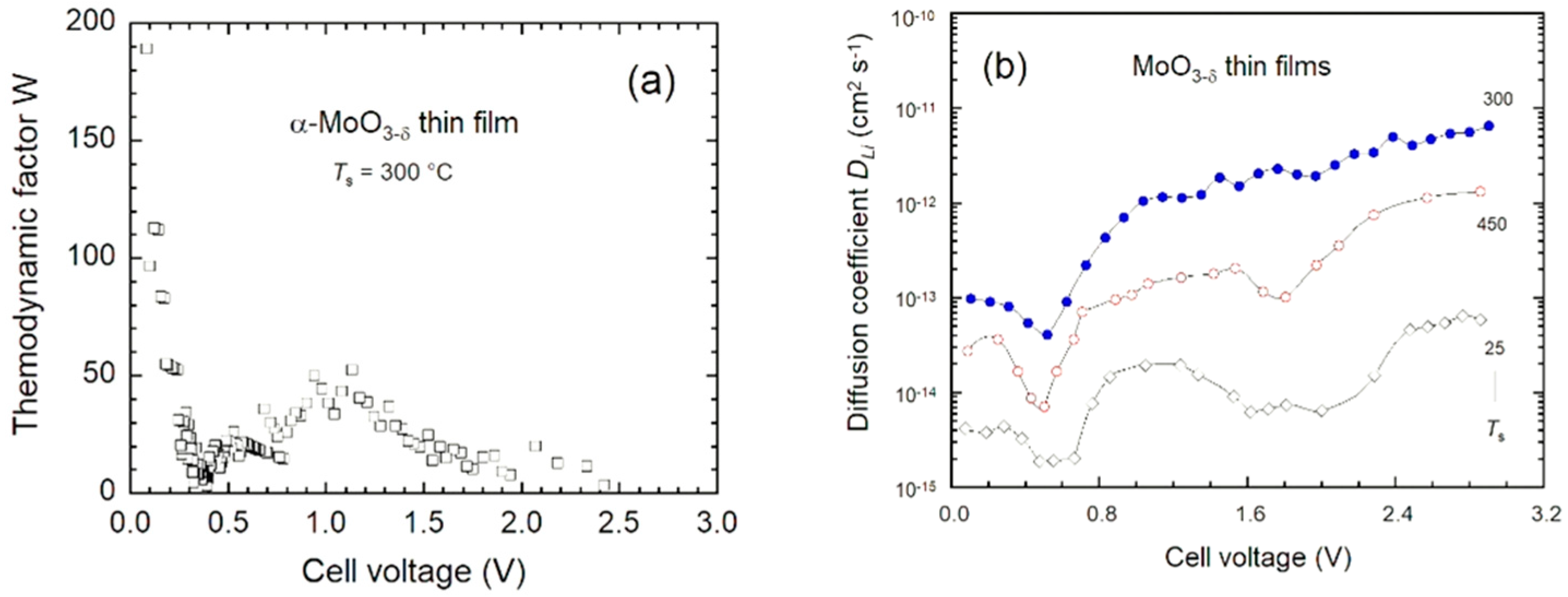
4.4. Li+-ion Kinetics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Julien, C. Solid State Batteries. In Handbook of Solid-State Electrochemistry; Gellings, P.J., Bouwmeester, H.J.M., Eds.; CRC Press: Roca Raton, FL, USA, 1997; Chapter 11; pp. 371–406. [Google Scholar]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016; pp. 120–124. [Google Scholar]
- Ramana, C.V.; Atuchin, V.V.; Groult, H.; Julien, C.M. Electrochemical properties of sputter-deposited MoO3 films in lithium microbatteries. J. Vac. Sci. Technol. A 2012, 30, 04D105. [Google Scholar] [CrossRef]
- Ramana, C.V.; Julien, C.M. Chemical and electrochemical properties of molybdenum oxide thin films prepared by reactive pulsed-laser assisted deposition. Chem. Phys. Lett. 2006, 428, 114–118. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Shobana, R.; Ravi, G.; Ganesh, V.; Yuvakkumar, R. Preparation and electrochemical characterization of Mo9O26 nanopowders for supercapacitors applications. Nano-Struct. Nano-Objects 2019, 19, 100340. [Google Scholar] [CrossRef]
- Sunu, S.S.; Prabhu, E.; Jayaraman, V.; Gnanasekar, K.I.; Gnanasekaran, T. Gas sensing properties of PLD made MoO3 film. Sens. Actuators B 2003, 94, 189–196. [Google Scholar] [CrossRef]
- Dillon, A.C.; Mahan, A.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; Lee, S.-H. Metal oxide nano-particles for improved electrochromic and lithium-ion battery technologies. Thin Solid Films 2008, 516, 794–797. [Google Scholar] [CrossRef]
- Simchi, H.; McCandless, B.E.; Meng, T.; Boyle, J.H.; Shafarman, W.N. Characterization of reactively sputtered molybdenum oxide films for solar cell application. J. Appl. Phys. 2013, 114, 13503. [Google Scholar] [CrossRef]
- Bullock, J.; Cuevas, A.; Allen, T.; Battaglia, C. Molybdenum oxide MoOx: A versatile hole contact for silicon solar cells. Appl. Phys. Lett. 2014, 105, 232109. [Google Scholar] [CrossRef]
- Jacieniak, J.J.; Seifter, J.; Jo, J.; Mates, T.; Heegeer, A.J. A solution-processed MoOx anode interlayer for use within organic photovoltaic devices. Adv. Funct. Mater. 2012, 22, 2594–2605. [Google Scholar] [CrossRef]
- Gesheva, K.A.; Ivanova, T.M.; Bodurov, G.K. APCVD transition metal oxides—Functional layers in smart windows. J. Phys. Conf. Ser. 2014, 559, 012002. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Palilis, L.C.; Georgiadou, D.G.; Kennou, S.; Kostis, I.; Davazoglou, D.; Argitis, P. Barrierless hole injection through sub-bandgap occupied states in organic light emitting diodes using substoichiometric MoOx anode interfacial layer. Appl. Phys. Lett. 2012, 100, 013311. [Google Scholar] [CrossRef]
- Hanson, E.D.; Lajaunie, L.; Hao, S.; Myers, B.D.; Shi, F.; Murthy, A.A.; Wolverton, C.; Arenal, R.; Dravid, V.P. Systematic study of oxygen vacancy tunable transport properties of few-layer MoO3−x enabled by vapor-based synthesis. Adv. Func. Mater. 2017, 27, 1605380. [Google Scholar] [CrossRef]
- Kihlborg, L. The crystal structure of Mo18O52 and the existence of homologous series of structures based on MoO3. Ark. Kemi 1963, 21, 443–460. [Google Scholar]
- Magnéli, A. The crystal structures of Mo9O26 (beta’-molybdenum oxide) and Mo8O23 (beta-molybdenum oxide). Acta Chem. Scand. 1948, 2, 501–517. [Google Scholar] [CrossRef]
- Kihlborg, L. Studies on molybdenum oxides. Acta Chem. Scand. 1959, 13, 954–962. [Google Scholar] [CrossRef]
- Kihlborg, L. The crystal structure of Mo17O47. Acta Chem. Scand. 1960, 14, 1612–1622. [Google Scholar] [CrossRef]
- Åsbrink, S.; Kihlborg, L. A study of the crystal symmetry and structure of orthorhombic Mo4O11 by least-squares techniques. Acta Chem. Scand. 1964, 18, 1571–1573. [Google Scholar] [CrossRef]
- Sato, M.; Onoda, O.; Matsuda, Y. Structural transitions in MonO3n-1 (n = 9 and 10). J. Phys. C Solid State Phys. 1987, 20, 4763–4771. [Google Scholar] [CrossRef]
- Inzani, K.; Grande, T.; Vullum-Bruer, F.; Selbach, S.M. A van der Waals density functional study of MoO3 and its oxygen vacancies. J. Phys. Chem. C 2016, 120, 8959–8968. [Google Scholar] [CrossRef]
- Tahini, H.A.; Tan, X.; Lou, S.N.; Scott, J.; Amal, R.; Ng, Y.H.; Smith, S.C. Mobile polaronic states in α-MoO3: An ab initio investigation of the role of oxygen vacancies and alkali ions. ACS Appl. Mater. Interfaces 2016, 8, 10911–10917. [Google Scholar] [CrossRef]
- Inzani, K.; Nematollahi, M.; Vullum-Bruer, F.; Grande, T.; Reenaasb, T.W.; Selbach, S.M. Electronic properties of reduced molybdenum oxides. Phys. Chem. Chem. Phys. 2017, 19, 9232–9245. [Google Scholar] [CrossRef]
- Mattinen, M.; King, P.J.; Khriachtchev, L.; Heikkilä, M.J.; Fleming, B.; Rushworth, S.; Mizohata, K.; Meinander, K.; Räisänen, J.; Ritala, M.; et al. Atomic layer deposition of crystalline molybdenum oxide thin films and phase control by post-deposition annealing. Mater. Today Chem. 2018, 9, 17–27. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Douvas, A.M.; Georgiadou, D.G.; Palilis, L.C.; Kennou, S.; Sygellou, L.; Soultati, A.; Kostis, I.; Papadimitropoulos, G.; Davazoglou, D. The influence of hydrogenation and oxygen vacancies on molybdenum oxides work function and gap states for application in organic optoelectronics. J. Am. Chem. Soc. 2012, 134, 16178–16187. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Hou, C.; Fan, Y.; Zhai, Y.; Li, H.; Dang, F.; Chou, S. Oxygen vacancies promoting the electrocatalytic performance of CeO2 nanorods as cathode materials for Li–O2 batteries. J. Mater. Chem. A 2019, 7, 6552–6561. [Google Scholar] [CrossRef]
- Kröger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Kowalsky, W.; Kahn, A. Role of the deep-lying electronic states of MoO3 in the enhancement of hole injection in organic thin films. Appl. Phys. Lett. 2009, 95, 123301. [Google Scholar] [CrossRef]
- Shin, J.Y.; Joo, J.H.; Samuelis, D.; Maier, J. Oxygen-deficient TiO2−δ nanoparticles via hydrogen reduction for high rate capability lithium batteries. Chem. Mater. 2012, 24, 543–551. [Google Scholar] [CrossRef]
- Kim, H.; Cook, J.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2016, 16, 454–460. [Google Scholar] [CrossRef]
- Akin, U.; Safal, H. Thickness dependence of dispersion parameters of the MoOx thin films prepared using the vaccum evaporation technique. J. Alloys and Compd 2015, 647, 146–151. [Google Scholar] [CrossRef]
- Borah, D.J.; Mostako, A.T.T.; Saikia, P.K.; Dutta, P. Effect of thickness and post deposition annealing temperature on the structural and optical properties of thermally evaporated molybdenum oxide films. Mater. Sci. Semicond. Process. 2019, 93, 111–122. [Google Scholar] [CrossRef]
- Julien, C.; Khelfa, A.; Hussain, O.M.; Nazri, G.A. Synthesis and characterization of flash-evaporated MoO3 thin films. J. Cryst. Growth 1995, 156, 235–244. [Google Scholar] [CrossRef]
- Sun, S.; Sun, Y.; Wen, J.; Zhang, B.; Liao, X.; Yin, G.; Huang, Z.; Pu, X. MoO3−x-deposited TiO2 nanotubes for stable and high-capacitance supercapacitor electrodes. RSC Adv. 2018, 8, 21823–21828. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Kappertz, O.; Ngaruiya, J.M.; Pedersen, T.P.L.; Drese, R.; Wuttig, M. Correlation between structure, stress and optical properties in direct current sputtered molybdenum oxide films. Thin Solid Films 2003, 429, 135–143. [Google Scholar] [CrossRef]
- Wang, D.; Yang, R.; Wu, L.; Shen, K.; Wang, D. Band alignment of CdTe with MoOx oxide and fabrication of high efficiency CdTe solar cells. Solar Energy 2018, 162, 637–645. [Google Scholar] [CrossRef]
- Greiner, M.T.; Helander, M.G.; Wang, Z.B.; Tang, W.M.; Qiu, J.; Lub, Z.H. A metallic molybdenum suboxide buffer layer for organic electronic devices. Appl. Phys. Lett. 2010, 96, 213302. [Google Scholar] [CrossRef]
- Bouzidi, A.; Benramdane, N.; Tabet-Derraz, H.; Mathieu, C.; Khelifa, B.; Desfeux, R. Effect of substrate temperature on the structural and optical properties of MoO3 thin films prepared by spray pyrolysis technique. Mater. Sci. Eng. B 2003, 97, 5–8. [Google Scholar] [CrossRef]
- Sivakumar, R.; Gopalakrishnan, R.; Jayachandran, M.; Sanjeeviraja, C. Characterization on electron beam evaporated MoO3 thin films by the influence of substrate temperature. Curr. Appl. Phys. 2007, 7, 51–59. [Google Scholar] [CrossRef]
- Sivakumar, R.; Gopinath, C.S.; Jayachandran, M.; Sanjeeviraja, C. An electrochromic device (ECD) cell characterization on electron beam evaporated MoO3 films by intercalating/deintercalating the H+ ions. Curr. Appl. Phys. 2007, 7, 76–86. [Google Scholar] [CrossRef]
- Macco, B.; Vos, M.; Thissen, N.F.W.; Bol, A.A.; Kessels, W.M.M. Low-temperature atomic layer deposition of MoOx for silicon heterojunction solar cells. Phys. Status Solidi: Rapid Res. Lett. 2015, 9, 393–396. [Google Scholar]
- Vos, M.F.J.; Macco, B.; Thissen, N.F.W.; Bol, A.A.; Kessels, W.M.M. Atomic layer deposition of molybdenum oxide from (NtBu)2(NMe2)2Mo and O2 plasma. J. Vac. Sci. Technol. A 2016, 34, 01A103. [Google Scholar] [CrossRef]
- Haro-Poniatowski, E.; Jouanne, M.; Morhange, J.F.; Julien, C.; Diamant, R.; Fernandez-Guasti, M.; Fuentes, G.A.; Alonso, J.C. Micro-Raman characterization of WO3 and MoO3 thin films obtained by pulsed laser irradiation. Appl. Surf. Sci. 1998, 127–129, 674–678. [Google Scholar] [CrossRef]
- Iriyama, Y.; Abe, T.; Inaba, M.; Ogumi, Z. Transmission electron microscopy (TEM) analysis of two-phase reaction in electrochemical lithium insertion within α-MoO3. Solid State Ionics 2000, 135, 95–100. [Google Scholar] [CrossRef]
- Hussain, O.M.; Srinivasa-Rao, K.; Madhuri, K.V.; Ramana, C.V.; Naidu, B.S.; Pai, S.; John, J.; Pinto, R. Growth and characteristics of reactive pulsed laser deposited molybdenum trioxide thin films. Appl. Phys. A 2002, 75, 417–422. [Google Scholar] [CrossRef]
- Camacho-Lopez, M.A.; Escobar-Alarcon, L.; Haro-Poniatowski, E. Structural transformations in MoOx thin films grown by pulsed laser deposition. Appl. Phys. A 2004, 78, 59–65. [Google Scholar] [CrossRef]
- Bhosle, V.; Tiwari, A.; Narayan, J. Epitaxial growth and properties of MoOx (2 < x < 2.75) films. J. Appl. Phys. 2005, 97, 083539. [Google Scholar]
- Torres, J.; Alfonso, J.E.; Lopez-Carreno, L.D. XPS and X-ray diffraction characterization of MoO3 thin films prepared by laser evaporation. Phys. Status Solidi C 2005, 2, 3726–3729. [Google Scholar] [CrossRef]
- Puppala, H.K.; Pelton, A.T.; Mayanovic, R.A. A comparative characterization study of molybdenum oxide thin films grown using femtosecond and nanosecond pulsed laser deposition. MRS Adv. 2016, 1, 2585–2590. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z. Growth of [010] oriented α-MoO3 nanorods by pulsed electron beam deposition. Appl. Phys. Lett. 2011, 99, 223104. [Google Scholar] [CrossRef]
- Robinson-Azariah, J.C.; Ponmudi-Selvan, T.; Rajasekar, M.S.; Sheebha, I.; Vidhya, B.; Rajesh, S. Pulsed laser deposited molybdenum oxides (MoO3 and MoO2) thin films for nanoelectronics device application. In Proceedings of the 4th Int. Conf. Devices, Circuits and Systems (ICDCS’18), Karunya Nagar, Coimbatore, India, 17–18 March 2018; pp. 42–47. [Google Scholar]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A. Pulsed laser deposition of molybdenum oxide thin films. Appl. Phys. A 2010, 98, 609–615. [Google Scholar] [CrossRef]
- Aoki, T.; Matsushita, T.; Mishiro, K.; Suzuki, A.; Okuda, M. Optical recording characteristics of molybdenum oxide films prepared by pulsed laser deposition method. Thin Solid Films 2008, 517, 1482–1486. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Li, X.; Zhou, J.; Guo, W. Chemical vapor deposition of ultra-thin molybdenum dioxide nanosheets. Mater. Lett. 2016, 174, 188–191. [Google Scholar] [CrossRef]
- Vorobeva, N.S.; Lipatov, A.; Muratov, D.S.; Sinitskii, A. Chemical vapor deposition and characterization of two-dimensional molybdenum dioxide (MoO2) nanoplatelets. Nanotechnology 2018, 29, 505707. [Google Scholar] [CrossRef]
- Zhang, X.; You, F.; Zheng, Q.; Zhang, Z.; Cai, P.; Xue, X.; Xiong, J.; Zhang, J. Solution-processed MoOx hole injection layer towards efficient organic light-emitting diode. Org. Electron. 2016, 39, 43–49. [Google Scholar] [CrossRef]
- Santhosh, S.; Mathankumar, M.; Chandraekaran, S.S.; Nanda-Kumar, A.K.; Murugan, P.; Subramanian, B. Effect of ablation rate on the microstructure and electrochromic properties of pulsed-laser-deposited molybdenum oxide thin films. Langmir 2017, 33, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Pulsed laser deposited films for microbatteries. Coatings 2019, 9, 386. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A.; Al-Shukri, A.M. Optical constants and thermocoloration of pulsed laser deposited molybdenum oxide thin films. Opt. Commun. 2010, 283, 2857–2862. [Google Scholar] [CrossRef]
- Kern, W. The evolution of silicon wafer cleaning technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, G.; Jin, J.; Zhang, L.; Wen, Z.; Yang, J. Self-catalyzed decomposition of discharge products on the oxygen vacancy sites of MoO3 nanosheets for low-overpotential Li-O2 batteries. Nano Energy 2017, 36, 186–196. [Google Scholar] [CrossRef]
- Guerfi, A.; Paynter, R.W.; Dao, L.H. Characterization and stability of electrochromic MoO3 thin films prepared by electrodeposition. J. Electrochem. Soc. 1995, 142, 3457–3464. [Google Scholar] [CrossRef]
- Navas, I.; Vinodkumar, R.; Lethy, K.J.; Detty, A.P.; Ganesan, V.; Sathe, V.; Mahadevan Pillai, V.P. Growth and characterization of molybdenum oxide nanorods by RF magnetron sputtering and subsequent annealing. J. Phys. D Appl. Phys. 2009, 42, 175305. [Google Scholar] [CrossRef]
- Hari-Krishna, K.; Hussain, O.M.; Guillen, C. Photo- and electrochromic properties of activated reactive evaporated MoO3 thin films grown on flexible substrates. Res. Lett. Nanotechnol. 2008, 2008, 217510. [Google Scholar]
- Rahman, F.; Ahmed, T.; Walia, S.; Mayes, E.; Sriram, S.; Bhaskaran, M.; Balendhran, S. Reversible resistive switching behavior in CVD grown, large area MoOx. Nanoscale 2018, 10, 19711–19719. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Hu, S.; Zhuang, J.; Wang, X. MoO3−x-based hybrids with tunable localized surface plasmon resonances: Chemical oxidation driving transformation from ultrathin nanosheets to nanotubes. Chem. Eur. J. 2012, 18, 15283–15287. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Huang, W.; Song, C.; Yan, M.; Guo, C.; Liu, S. Non-stoichiometric MoO3−x quantum dots as a light-harvesting material for interfacial water evaporation. Chem. Commun. 2017, 53, 6744–6747. [Google Scholar] [CrossRef] [PubMed]
- Eda, K. Raman spectra of hydrogen molybdenum bronze, H0.30MoO3. J. Solid State Chem. 1992, 98, 350–357. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Chen, X.; Hao, J.; Jin, Y.; Cui, Q.; Zou, G. Pressure-induced structural transitions in MoO3−xH2O (x = 1/2, 2) molybdenum trioxide hydrates: A Raman study. J. Phys. Chem. B 2009, 113, 16479–16482. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wing, R.M. properties of metal-to-oxygen multiple bonds, especially molybdenum-to-oxygen bonds. Inorg. Chem. 1965, 4, 867–873. [Google Scholar] [CrossRef]
- Diskus, M.; Nilsen, O.; Fjellvag, H.; Diplas, S.; Beato, P.; Harvey, C.; Lantman, E.S.; Weckhuysen, B.M. Combination of characterization techniques for atomic layer deposition MoO3 coatings: From the amorphous to the orthorhombic α-MoO3 crystalline phase. J. Vac. Sci. Technol. A 2012, 30, 01A107. [Google Scholar] [CrossRef]
- Ni, J.; Wang, G.; Yang, J.; Gao, D.; Chen, J.; Gao, L.; Li, Y. Carbon nanotube-wired and oxygen-deficient MoO3 nanobelts with enhanced lithium-storage capability. J. Power Sources 2014, 247, 90–94. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, Y.; Tang, X.D. Evidence of oxygen vacancy and possible intermediate gap state in layered α-MoO3 single-crystal nanobelts. Physica B 2016, 481, 192–196. [Google Scholar] [CrossRef]
- Lee, S.H.; Seong, M.J.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of electrochromic a-MoO3 thin films. Solid State Ionics 2002, 147, 129–133. [Google Scholar] [CrossRef]
- Cuando-Espitia, N.; Redenius, J.; Mensink, K.; Camacho-Lopez, M.; Camacho-Lopez, S.; Aguilar, G. Influence of oxygen pressure on the fs laser-induced oxidation of molybdenum thin films. Opt. Mater. Express 2018, 8, 581–596. [Google Scholar] [CrossRef]
- Eda, K. Infrared spectra of hydrogen molybdenum bronze, H0.34MoO3. J. Solid State Chem. 1989, 83, 292–303. [Google Scholar] [CrossRef]
- Mizuno, N.; Katamura, K.; Yoneda, Y.; Misono, M. Catalysis by heteropoly compounds: V. The reduction mechanism of H3PMo12O40. J. Catal. 1983, 83, 384–392. [Google Scholar]
- Dong, W.; Mansour, A.N.; Dunn, B. Structural and electrochemical properties of amorphous and crystalline molybdenum oxide aerogels. Solid State Ionics 2001, 144, 31–40. [Google Scholar] [CrossRef]
- Sun, Y.; Takacs, C.J.; Cowan, S.R.; Seo, J.H.; Gong, X.; Roy, A.; Heeger, A.J. Efficient, air-stable bulk heterojunction polymer solar cells using MoOx as the anode interfacial layer. Adv. Mater. 2011, 23, 2226–2230. [Google Scholar] [CrossRef]
- Choi, J.-G.; Thompson, L.T. XPS study of as-prepared and reduced molybdenum oxides. Appl. Surf. Sci. 1996, 93, 143–149. [Google Scholar] [CrossRef]
- Gacitua, M.; Boutaleb, Y.; Cattin, L.; Abe, S.Y.; Lare, Y.; Soto, G.; Louarn, G.; Morsli, M.; Rehamnia, R.; Del valle, A.; et al. Electrochemical preparation of MoO3 buffer layer deposited onto the anode in organic solar cells. Phys. Status Solidi A 2010, 207, 1905–1911. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, H.; Shen, W. A facile and controllable electrochemically fabricated nonstoichiometric MoOx film for novel opto-electronic devices. J. Micromech. Microeng. 2019, 29, 065012. [Google Scholar] [CrossRef]
- Chang, L.L.Y.; Phillips, B. Phase relations in refractory metal–oxygen systems. J. Am. Ceram. Soc. 1969, 52, 527–533. [Google Scholar] [CrossRef]
- Tong, J.; Wan, Y.; Cyi, J.; Lim, S.; Song, N.; Lennon, A. Solution-processed molybdenum oxide for hole-selective contacts on crystalline silicon solar cells. Appl. Surf. Sci. 2017, 423, 139–146. [Google Scholar] [CrossRef]
- Novotny, P.; Lamb, H.H. Nanostructured MoOx films deposited on c-plane sapphire. J. Vac. Sci. Technol. A 2019, 37, 051504. [Google Scholar] [CrossRef]
- Fernandes-Cauduro, A.L.; Dos Reis, R.; Chen, G.; Schmid, A.K.; Méthivier, C.; Rubahn, H.G.; Bossard-Giannesini, L.; Cruguel, H.; Witkowski, N.; Madsen, M. Crystalline molybdenum oxide thin-films for application as interfacial layers in optoelectronic devices. ACS Appl. Mater. Interfaces 2017, 9, 7717–7724. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Gao, M.; Wan, Y.; Li, Y.; Song, W.; Ma, Z. Effect of post-annealing on the properties of thermally evaporated molybdenum oxide films: Interdependence of work function and oxygen to molybdenum ratio. Mater. Sci. Semicond. Proc. 2018, 75, 166–172. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Liu, M.; Dong, G.; Liu, F.; Wang, W.; Yu, D. Molybdenum oxide hole selective transport layer by hot wire oxidation-sublimation deposition for silicon heterojunction solar cells. Available online: https://arxiv.org/ftp/arxiv/papers/1902/1902.09127.pdf (accessed on 25 February 2019).
- Mehmood, H.; Bektas, G.; Yildiz, I.; Tauqeer, T.; Nasser, H.; Turan, R. Electrical, optical and surface characterization of reactive RF magnetron sputtered molybdenum oxide films for solar cell applications. Mater. Sci. Semicond. Proc. 2019, 101, 46–56. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Zhan, X.; Gao, D.; Xiao, Q.; Lei, G. High capacity three-dimensional ordered microporous CoFe2O4 as anode material for lithium ion batteries. Electrochim. Acta 2010, 55, 4594–4598. [Google Scholar] [CrossRef]
- Yonekura, D.; Iwama, E.; Ota, N.; Muramatsu, M.; Saito, M.; Orikasa, Y.; Naoi, W.; Naoi, K. Progress of the conversion reaction of Mn3O4 particles as a function of the depth of discharge. Phys. Chem. Chem. Phys. 2014, 16, 6027–6032. [Google Scholar] [CrossRef]
- Su, Q.; Wang, S.; Du, G.; Xu, B.; Ma, S.; Shang, L. Microstructure evolution and conversion mechanism of Mn3O4 under electrochemical cyclings. Phys. Chem. C 2018, 122, 2475–2480. [Google Scholar] [CrossRef]
- Huang, S.Z.; Cai, Y.; Jin, J.; Liu, J.; Li, Y.; Yu, Y.; Wang, H.E.; Chen, L.H.; Su, B.L. Hierarchical mesoporous urchin-like Mn3O4/ carbon microspheres with highly enhanced lithium battery performance by in-situ carbonization of new lamellar manganese alkoxide (Mn-DEG). Nano Energy 2015, 12, 833–844. [Google Scholar] [CrossRef]
- Dickens, P.G.; Reynolds, G.J. Transport and equilibrium properties of some oxide insertion compounds. Solid State Ionics 1981, 5, 331–334. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lee, S.K.; Ahn, D.J.; Dillon, A.C.; Lee, S.H. Electrochemical reactivity of ball-milled MoO3−y as anode materials for lithium-ion batteries. J. Power Sources 2009, 188, 286–291. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Gendron, F.; Julien, C.M.; Qilu, R. Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abdel-Ghany, A.E.; Scheuermann, M.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Doped nanoscale NMC333 as cathode materials for Li-ion batteries. Materials 2019, 12, 2899. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Hashem, A.M.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Electrochemical properties of α-MoO3 as cathode materials for Li batteries. J. Power Sources 2012, 219, 126–132. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R.A. Determination of the kinetic parameters of mixed-conducting electrodes and application to the system Li3Sb. J. Electrochem. Soc. 1977, 124, 1569–1578. [Google Scholar] [CrossRef]
- Wen, C.J.; Boukamp, B.A.; Huggins, R.A.; Weppner, W. Thermodynamic and mass transport properties of LiAl. J. Electrochem. Soc. 1979, 126, 2258–2266. [Google Scholar] [CrossRef]
- Jang, Y.I.; Neudecker, B.J.; Dudney, N.J. Lithium diffusion in LixCoO2 (0.45 <x <0.7 intercalation cathodes. Electrochem. Solid-State Lett. 2001, 4, A74–A77. [Google Scholar]
- Tang, S.B.; Lai, M.O.; Lu, L. Li-ion diffusion in highly (003) oriented LiCoO2 thin film cathode prepared by pulsed laser deposition. J. Alloys Compd 2008, 449, 300–303. [Google Scholar] [CrossRef]
- Honders, A.; der Kinderen, J.M.; van Heeren, A.H.; de Wit, J.H.W.; Broers, G.H.J. Bounded diffusion in solid solution electrode powder compacts. Part II. The simultaneous measurement of the chemical diffusion coefficient and the thermodynamic factor in LixTiS2 and LixCoO2. Solid State Ionics 1985, 15, 265–276. [Google Scholar] [CrossRef]
- Levi, M.D.; Aurbach, D. Frumkin intercalation isotherm—A tool for the description of lithium insertion into host materials: Areview. Electrochim. Acta 1999, 45, 167–185. [Google Scholar] [CrossRef]
- Montella, C. Discussion of the potential step method for the determination of the diffusion coefficients of guest species in host materials Part I. Influence of charge transfer kinetics and ohmic potential drop. J. Electroanal. Chem. 2002, 518, 61–83. [Google Scholar] [CrossRef]
- Carcia, P.F.; McCarron, E.M. Synthesis and properties of thin films polymorphs of molybdenum trioxide. Thin Solid Films 1987, 155, 53–63. [Google Scholar] [CrossRef]
- Altman, E.I.; Droubay, T.; Chambers, S.A. Growth of MoO3 films by oxygen plasma assisted molecular beam epitaxy. Thin Solid Films 2002, 414, 205–215. [Google Scholar] [CrossRef]
- Siokou, A.; Leftheriotis, G.; Papaefthimiou, S.; Yianoulis, P. Effect of the tungsten and molybdenum oxidation states on the thermal coloration of amorphous WO3 and MoO3 films. Surf. Sci. 2001, 482–485, 294–299. [Google Scholar] [CrossRef]
- Ressler, T.; Jentoft, R.E.; Wienold, J.; Günter, M.M.; Timpe, O. In situ XAS and XRD studies on the formation of Mo suboxides during reduction of MoO3. J. Phys. Chem. B 2000, 104, 6360–6370. [Google Scholar] [CrossRef]
- Anbananthan, N. Studies on oxygen deficient molybdenum oxide electrodes. Bull. Electrochem. 2003, 19, 79–84. [Google Scholar]
- Hashem, A.M.A.; Abbas, S.; Abdel-Ghany, A.; Julien, C.M. Blend formed by oxygen deficient MoO3−δ oxides as lithium-insertion compounds. J. Alloys Compd. 2016, 686, 744–752. [Google Scholar] [CrossRef]
- Alfonso, J.E.; Moreno, L.C. Preparation and chemical characterization of neodymium-doped molybdenum oxide films grown using spray pyrolysis. Rev. Mex. Fis. 2014, 60, 114–118. [Google Scholar]
- Yeh, H.L.; Tai, S.H.; Hsieh, C.M.; Chang, B.K. First-principles study of lithium intercalation and diffusion in oxygen-defective titanium dioxide. J. Phys. Chem. C 2018, 122, 19447–19454. [Google Scholar] [CrossRef]
- Luo, H.C.; Martin, M. Investigation of the phase diagram and the defect structure of nonstoichiometric Li1+xMn2-xO4-δ (0 ≤ x ≤ 0.33) spinel. Electrochem. Soc. Proc. 2003, 2003, 281–288. [Google Scholar]
- Dong, W.; Xu, J.; Wang, C.; Lu, Y.; Liu, X.; Wang, X.; Yuan, X.; Wang, Z.; Lin, T.; Sui, M.; et al. A Robust and conductive black tin oxide nanostructure makes efficient lithium-ion batteries possible. Adv. Mater. 2017, 29, 1700136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, T.; Zhao, X.; Pang, W.K.; Gao, H.; Li, S.; Zhou, Z.; Liu, H.; Guo, Z. Atomic interface engineering and electric-field effect in ultrathin Bi2MoO6 nanosheets for superior lithium ion storage. Adv. Mater. 2017, 29, 1700396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, J.; Xu, H.; Sun, S.; Xu, Y.; Zhou, M.; Gao, X.; Sun, Z. Plasma-introduced oxygen defects confined in Li4Ti5O12 nanosheets for boosting lithium-ion diffusion. ACS Appl. Mater. Interfaces 2019, 11, 17384–17392. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.-W.; Pennycook, S.J.; Lee, W.S.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Qiao, X.; Chen, J.; Li, X.; Dongge, M. Observation of hole hopping via dopant in MoOx-doped organic semiconductors: Mechanism analysis and application for high performance organic light-emitting devices. J. Appl. Phys. 2010, 107, 104505. [Google Scholar] [CrossRef]
- Guo, Y.; Robertson, J. Origin of the high work function and high conductivity of MoO3. Appl. Phys. Lett. 2014, 105, 222110. [Google Scholar] [CrossRef]
- Meduri, P.; Clark, E.; Kim, J.H.; Dayalan, E.; Sumanasekera, G.U.; Sunkara, M.K. MoO3−x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett. 2012, 12, 1784–1788. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, N.; Sun, K. Electrochemical preparation of porous MoO3 film with a high rate performance as anode for lithium ion batteries. J. Mater. Chem. A 2013, 1, 221–224. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Wang, Y.; Ma, Z.; Li, Z. In-situ microstructural investigations by electron-beam irradiation induced crystallization of amorphous MoOx thin films with high performance for Li-ion storage. Electrochim. Acta 2014, 144, 369–375. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Xing, Z.; Qian, Y. Hydrothermal synthesis of α-MoO3 and the influence of later heat treatment on its electrochemical properties. Int. J. Electrochem. Sci. 2013, 8, 9851–9857. [Google Scholar]
- Ahmed, B.; Shahid, M.; Nagaraju, D.H.; Anjum, D.H.; Hedhili, M.N.; Alshareef, H.N. Surface passivation of MoO3 nanorods by atomic layer deposition towards high rate durable Li ion battery anodes. ACS Appl. Mater. Interfaces 2015, 7, 13154–13163. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, R.; Yang, R.; Ji, W.; Jiang, M.; Ding, W.; Peng, L. Mixed molybdenum oxides with superior performances as an advanced anode material for lithium-ion batteries. Sci. Rep. 2017, 7, 44697. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Benhammou, D.; Parilla, P.A.; Mahan, A.H.; Dillon, A.C. Metal oxide nanoparticles for advanced energy applications. Thin Solid Films 2009, 517, 3591–3595. [Google Scholar] [CrossRef]
- Xue, X.-Y.; Chen, Z.-H.; Xing, L.-L.; Yuan, S.; Chen, Y.-J. SnO2/α-MoO3 core-shell nanobelts and their extraordinarily high reversible capacity as lithium-ion battery anodes. Chem. Commun. 2011, 47, 5205–5207. [Google Scholar] [CrossRef]
- Riley, L.A.; Lee, S.H.; Gedvilias, L.; Dillon, A.C. Optimization of MoO3 nanoparticles as negative-electrode material in high-energy lithium ion batteries. J. Power Sources 2010, 195, 588–592. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.H.; Deshpande, R.; Parilla, P.A.; Whitney, E.; Gillaspie, D.T.; Jones, K.M.; Mahan, A.H.; Zhang, S.B.; Dillon, A.C. Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv. Mater. 2008, 20, 3627–3632. [Google Scholar] [CrossRef]
- Ette, P.M.; Ramesha, P.G.K. Self-assembled lamellar alpha-molybdenum trioxide as high performing anode material for lithium-ion batteries. J. Power Sources 2015, 278, 630–638. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wang, X.; Wang, C.; Liang, L.; Grote, F.; Wu, M.; Mi, Y.; Lei, Y. Enhancement of sodium ion battery performance enabled by oxygen vacancies. Angew. Chem. Int. Ed. 2015, 54, 8768–8771. [Google Scholar] [CrossRef]
- Icovi, M.; Panero, S.; D’Agate, A.; Pistoia, G.; Temperoni, C. Non-Stoichiometric molybdenum oxides as cathodes for lithium cells. Part II. Secondary batteries. J. Electroanal. Chem. 1979, 102, 343–349. [Google Scholar] [CrossRef]
- Feng, C.; Gao, H.; Zhang, C.; Guo, Z.; Liu, H. Synthesis and electrochemical properties of MoO3/C nanocomposite. Electrochim. Acta 2013, 93, 101–106. [Google Scholar] [CrossRef]
- Hassan, M.F.; Guo, Z.P.; Chen, Z.; Liu, H.K. Carbon-coated MoO3 nanobelts as anode materials for lithium-ion batteries. J. Power Sources 2010, 195, 2372–2376. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wang, Y.; Zhang, C.; Li, X.-S.; Dong, W.-S. In situ synthesis of a-MoO3/graphene composites as anode materials for lithium ion battery. Mater. Chem. Phys. 2014, 143, 1111–1118. [Google Scholar] [CrossRef]
- Ma, F.; Yuan, A.; Xu, J.; Hu, P. Porous α-MoO3/MWCNT nanocomposite synthesized via a surfactant-assisted solvothermal route as a lithium-ion-battery high-capacity anode material with excellent rate capability and cyclability. ACS Appl. Mater. Interfaces 2015, 7, 15531–15541. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Khelfa, A.; Guesdon, J.P.; Gorenstein, A. Lithium intercalation in MoO3: A comparison between crystalline and disordered phases. Appl. Phys. A 1994, 59, 173–178. [Google Scholar] [CrossRef]
- Julien, C.; Nazri, G.A.; Guesdon, J.P.; Gorenstein, A.; Khelfa, A.; Hussain, O.M. Influence of the growth conditions on electrochemical features of MoO3 film-cathodes in lithium microbatteries. Solid State Ionics 1994, 73, 319–326. [Google Scholar] [CrossRef]
- Halalay, I.C.; Nazri, G.-A.; Cheng, Y.-T.; Eesley, G.L.; Meyer, M.S. Optical measurement of lithium diffusivity in cathode materials: Amorphous MoO3 films. J. Power Sources 1995, 54, 218–220. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, W.; Wu, X.; Lv, Y.; Qiu, J.; Guo, J.; Wang, X.; Jia, D.; Yan, J.; Wu, D. Ultrafine MoO3 anchored in coal-based carbon nanofibers as anode for advanced lithium-ion batteries. Carbon 2020, 156, 445–452. [Google Scholar] [CrossRef]
- Ding, J.; Abbas, S.A.; Hanmandlu, C.; Lin, L.; Lai, C.S.; Wang, P.C.; Li, L.J.; Chu, C.W.; Chang, C.C. Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J. Power Sources 2017, 348, 270–280. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, H.; Du, Z.; Zeng, Z.; Gao, C.; Zhang, Z.; Du, X.; Kulka, A.; Swierczek, K. Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 180, 947–956. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, H.; Gu, D.; Li, J.; Wang, L.; Shen, L. A new way to prepare MoO3/C as anode of lithium ion battery for enhancing the electrochemical performance at room temperature. J. Electrochem. Sci. Technol. 2016, 7, 170–178. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, C.; Liu, G.; Lu, H.; Zhang, R.; Wang, L.; Wang, H. Largely enhanced electrochemical performance in MoO3−x nanobelts formed by a “sauna reaction”: Importance of oxygen vacancies. Electrochim. Acta 2017, 239, 16–24. [Google Scholar] [CrossRef]
- Tysyachny, V.P.; Shembel, E.M.; Apostolova, R.D.; Nagirny, V.M.; Kylyvnyk, K.E.; Eskova, N.I. Studies of the lithium ion transport properties in electrolytic molybdenum oxides. Solid State Ionics 2004, 169, 135–137. [Google Scholar] [CrossRef]
- Xie, J.; Kohno, K.; Matsumura, T.; Imanishi, N.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion diffusion kinetics in LiMn2O4 thin films prepared by pulsed laser deposition. Electrochim. Acta 2008, 54, 376–381. [Google Scholar] [CrossRef]
- Julien, C.; Haro-Poniatowski, E.; Camacho-Lopez, M.A.; Escobar-Alarcon, L.; Jimenez-Jarquin, J. Growth of LiMn2O4 thin films by pulsed-laser deposition and their electrochemical properties in lithium microbatteries. Mater. Sci. Eng. B 2000, 72, 36–46. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion diffusion kinetics in LiFePO4 thin films prepared by radio frequency magnetron sputtering. Electrochim. Acta 2009, 54, 4631–4637. [Google Scholar] [CrossRef]
- Ho, C.; Raistrick, I.D.; Huggins, R.A. Application of A-C techniques to the study of lithium diffusion in tungsten trioxide thin films. J. Electrochem. Soc. 1980, 127, 343–350. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.X.; Wegner, G.; Fang, X.; Chen, C.H.; Lieberwirth, I. Determination of the diffusion coefficient of lithium ions in nano-Si. Solid State Ionics 2009, 180, 222–225. [Google Scholar] [CrossRef]
- Maier, J. Diffusion in materials with ionic and electronic disorder. Mater. Res. Soc. Symp. Proc. 1991, 210, 499. [Google Scholar] [CrossRef]
- Wu, C.; Xie, H.; Li, D.; Liu, D.; Ding, S.; Tao, S.; Chen, H.; Liu, Q.; Chen, S.; Chu, W.; et al. Atomically intercalating tin ions into the interlayer of molybdenum oxide nanobelt toward long-cycling lithium battery. J. Phys. Chem. Lett. 2018, 9, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Yebka, B.; Ziolkiewicz, S.; Doi, A. Lithium insertion in molybdenum and vanadium oxide films. Electrochem. Soc. Proc. 1997, 97-24, 862–873. [Google Scholar]
- Lee, Y.-S.; Ruy, K.-S. Study of the lithium diffusion properties and high rate performance of TiNb6O17 as an anode in lithium secondary battery. Sci. Rep. 2017, 7, 16617. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L.; Skundin, A.M.; Pleskov, Y.V.; Terukov, E.I.; Kon’kov, O.I. Lithium intercalation in thin amorphous-silicon films. Russian J. Electrochem. 2006, 42, 363–369. [Google Scholar] [CrossRef]
- Sivonxay, E.; Aykol, M.; Persson, K.A. The lithiation process and Li diffusion in amorphous SiO2 and Si from first-principles. Electrochim. Acta 2020, 331, 135344. [Google Scholar] [CrossRef]
- Ostadhossein, A.; Kim, S.-Y.; Cubuk, E.D.; Qi, Y.; van Duin, A.C.T. Atomic insight into the lithium storage and diffusion mechanism of SiO2/Al2O3 electrodes of lithium ion batteries: ReaxFF reactive force field modeling. J. Phys. Chem. A 2016, 120, 2114–2127. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M.; Nizhnikovskii, E.A.; Fesenko, A.V. Temperature effect on the lithium diffusion rate in graphite. Russ. J. Electrochem. 2006, 42, 259–262. [Google Scholar] [CrossRef]
- Gong, J.; Wu, H. Electrochemical intercalation of lithium species into disordered carbon prepared by the heat-treatment of poly (p-phenylene) at 650 °C for anode in lithium-ion battery. Electrochim. Acta 2000, 45, 1753–1762. [Google Scholar] [CrossRef]
- Yang, H.; Bang, H.J.; Prakash, J. Evaluation of electrochemical interface area and lithium diffusion coefficient for a composite graphite anode. J. Electrochem. Soc. 2004, 151, A1247–A1250. [Google Scholar] [CrossRef]
- Panchmatia, P.M.; Armstrong, A.R.; Bruce, P.G.; Islam, M.S. Lithium-ion diffusion mechanisms in the battery anode material Li1+xV1−xO2. Phys. Chem. Chem. Phys. 2014, 16, 21114–21118. [Google Scholar] [CrossRef]
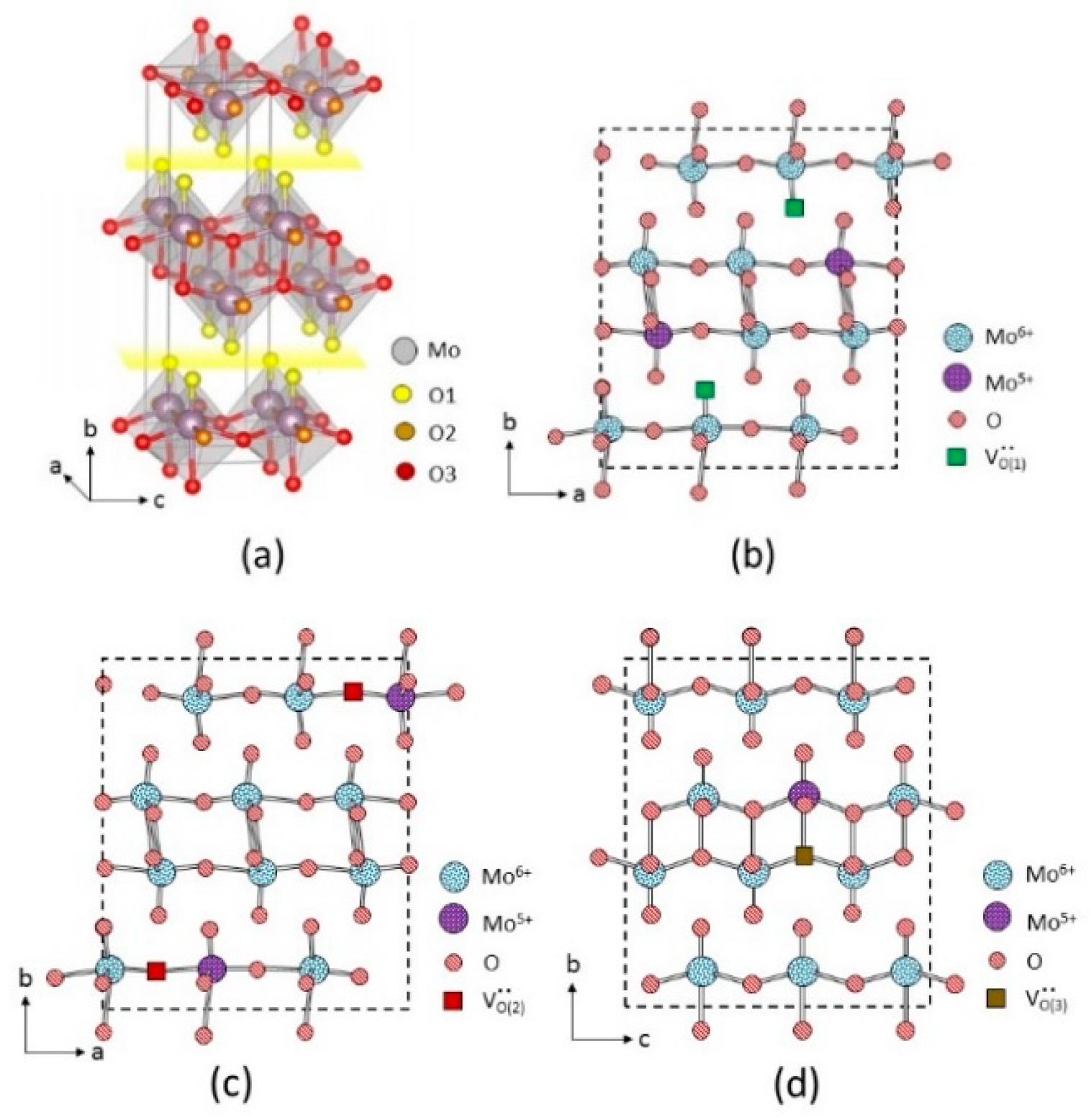

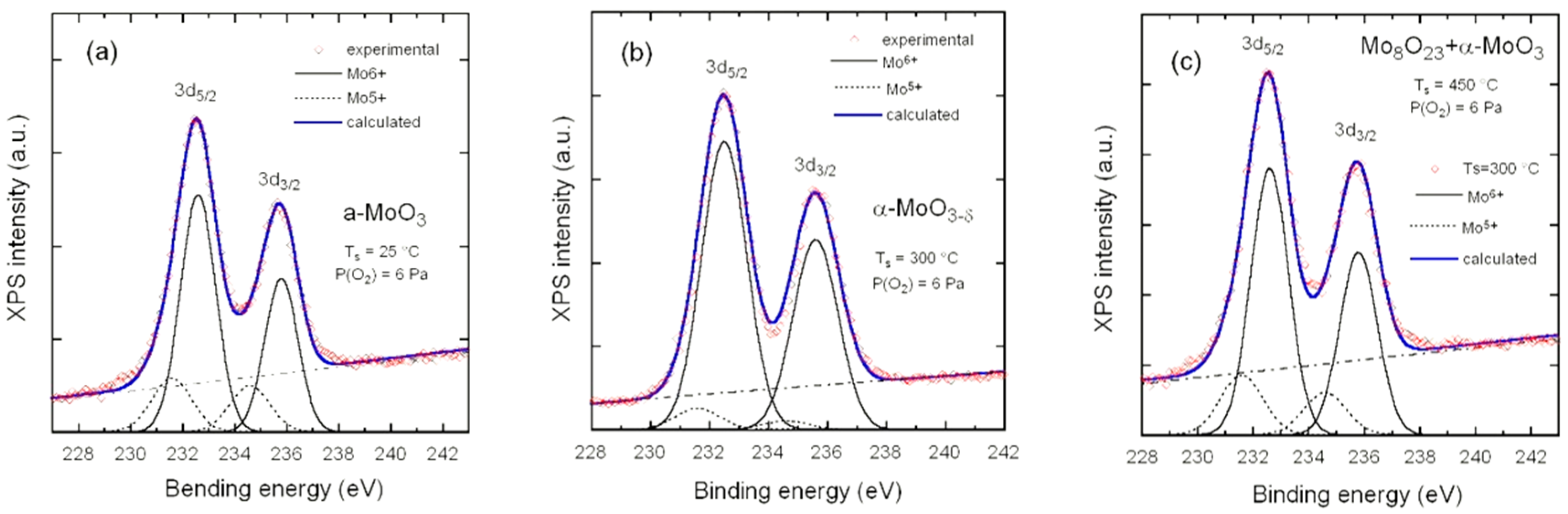


| Ts (°C) | k | δ | Composition |
|---|---|---|---|
| 25 | 0.55 | 0.178 | a-MoO2.822 |
| 300 | 0.04 | 0.018 | α-MoO2.982 |
| 450 | 0.27 | 0.106 | 0.85Mo8O23-0.15MoO3 |
| Electrode | Specific Discharge Capacity(µAh cm−2 µm−1) | CE (%) | Specific Capacity after 100 Cycles(µAh cm−2 µm−1) | |
|---|---|---|---|---|
| 1st | 2nd | |||
| MoO2.822 | 468 | 362 | 0.77 | 205 |
| MoO2.982 | 390 | 295 | 0.74 | 209 |
| MoO2.884 | 484 | 372 | 0.77 | 300 |
| Thin Film Anode | DLi+ (cm2 s−1) | W @ 0.5 V | |
|---|---|---|---|
| min | max | ||
| a-MoO3-δ | 2×10−15 | 7×10−14 | 34.5 |
| α-MoO3-δ | 4×10−14 | 6×10−12 | 21.8 |
| 0.85Mo8O23-0.15MoO3 | 7×10−15 | 1.1×10−12 | 25.2 |
| Material a | Particle Size (nm) | Electrochemical Performance | Ref. |
|---|---|---|---|
| Mo17O47 NWs | ~90 | 630 mAhg−1 @ 50 mAg−1 for 20 cycles | [123] |
| MoO3 NRs | 100–250 | 460 mAhg−1 @ 1.5 Ag−1 for 50 cycles | [127] |
| MoO2.895 | ~1000 | 600 mAhg−1 @ 0.03C for 35 cycles | [94] |
| MoO3 NSs | 5–20 | 630 mAhg−1 @ C/2 for 150 cycles | [132] |
| MoO3 NRs | 400 | 450 mAh g−1 @ 25 mA g−1 for 90 cycles | [126] |
| MoO3 NSs b | 40 | 800 mAh g−1 @ C/10 for 40 cycles | [131] |
| MoO3 NBs | 150 | 300 mAh g−1 @ C/10 for 50 cycles | [137] |
| MoO3 NFs | 20–30 | 550 mAh g−1 @ 2C for 75 cycles | [123] |
| MoO3 NBs | 150 | 300 mAh g−1 @ 50 mA g−1 for 10 cycles | [138] |
| MoO3 NSs | ~3–50 | 620 mAhg−1 @ C/2 for 150 cycles | [129] |
| α-MoO3 NBs | ~220 | 1067 mAhg−1 @ C/2 for 50 cycles | [130] |
| MoO3 | 100–500 | 450 mAh g−1 @ 200 mA g−1 for 200 cycles | [128] |
| MoOx | 100–500 | 900 mAh g−1 @ 200 mA g−1 for 200 cycles | [128] |
| MoO3 TF | 100 | 650 mAh g−1 @ 3 A g−1 for 50 cycles | [124] |
| MoOx TF | 1.1 µm thick | 347 µAh cm−2 @ 90 µA cm−2 for 100 cycles | [125] |
| a-MoO2.822 TF | amorphous | 205 µAh cm−2 µm−1 @ 1 Ag−1 for 100 cycles | this work |
| α-MoO2.982 TF | 69 | 209 µAh cm−2 µm−1 @ 1 Ag−1 for 100 cycles | this work |
| MoO3 + Mo8O23 TF | 72 | 300 µAh cm−2 µm−1 @ 1 Ag−1 for 100 cycles | this work |
| Material | Sampling | DLi (cm2 s−1) | Ref. |
|---|---|---|---|
| LixMoO3 | thin film | 10−12–10−11 | [141] |
| MoO3/a-C | nanocomposite | 3.4 × 10−14 | [149] |
| α-MoO3 | nanobelts | 3.3 × 10−14 | [155] |
| MoO3:Sn | doped nanobelts | 7.3 × 10−13 | [155] |
| porous MoO3 | electrodeposited | 7.1 × 10−11 | [124] |
| a-MoO3-δ | sputtered thin film | 3.0 × 10−14 | [156] |
| Li2.1Si | nanopowder 50 nm | 4.0 × 10−13 | [153] |
| Li2.7Si | nanopowder 50 nm | 2.0 × 10−12 | [153] |
| TiNb2O7 | ball-milled 1–5 µm | 4.6 × 10−14 | [157] |
| TiNb6O17 | ball-milled 1–5 µm | 1.3 × 10−13 | [157] |
| a-Si:H | thin film | 4.0 × 10−13 | [158] |
| LiSiO2 | DFT calculation | 2.6 × 10−11 | [159] |
| a-Li2.5SiO2 | ReaxFF calculation | 1.0 × 10−13 | [160] |
| LixC6 | graphite FG-A | 1.2 × 10−10 | [161] |
| Li1(H0.224C)6 | disordered carbon | 6.0 × 10−15 | [162] |
| LiC6 (50–250 mV) | artificial graphite | 10−10–10−12 | [163] |
| Li1.16V0.93O2 | atomistic modelling | 7.5 × 10−10 | [164] |
| a-MoO2.822 | PLD thin film | 2.0 × 10−15–7.0 × 10−14 | this work |
| α-MoO2.982 | PLD thin film | 6.0 × 10−14–6.0 × 10−12 | this work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmi-Narayana, A.; Hussain, O.M.; Ramana, C.V.; Camacho-Lopez, M.; Abdel-Ghany, A.; Hashem, A.; Mauger, A.; Julien, C.M. Molybdenum-Suboxide Thin Films as Anode Layers in Planar Lithium Microbatteries. Electrochem 2020, 1, 160-187. https://doi.org/10.3390/electrochem1020012
Lakshmi-Narayana A, Hussain OM, Ramana CV, Camacho-Lopez M, Abdel-Ghany A, Hashem A, Mauger A, Julien CM. Molybdenum-Suboxide Thin Films as Anode Layers in Planar Lithium Microbatteries. Electrochem. 2020; 1(2):160-187. https://doi.org/10.3390/electrochem1020012
Chicago/Turabian StyleLakshmi-Narayana, Ambadi, Obili M. Hussain, Chintalapalle V. Ramana, Marco Camacho-Lopez, Ashraf Abdel-Ghany, Ahmed Hashem, Alain Mauger, and Christian M. Julien. 2020. "Molybdenum-Suboxide Thin Films as Anode Layers in Planar Lithium Microbatteries" Electrochem 1, no. 2: 160-187. https://doi.org/10.3390/electrochem1020012
APA StyleLakshmi-Narayana, A., Hussain, O. M., Ramana, C. V., Camacho-Lopez, M., Abdel-Ghany, A., Hashem, A., Mauger, A., & Julien, C. M. (2020). Molybdenum-Suboxide Thin Films as Anode Layers in Planar Lithium Microbatteries. Electrochem, 1(2), 160-187. https://doi.org/10.3390/electrochem1020012







