Abstract
Functional Optical Balance (FOB) is a novel personalized strategy for intraocular lens (IOL) selection in cataract surgery, designed to reconcile the trade-off between optical quality and spectacle independence. FOB is a core concept aiming to maximize visual performance by treating the two eyes as a synergistic pair. One eye (often the dominant eye) is optimized for pristine optical quality (typically distance vision with a high-contrast monofocal or low-add IOL). In contrast, the fellow eye is optimized for extended depth of focus and pseudoaccommodation (using an extended depth-of-focus or multifocal/trifocal IOL) to reduce dependence on glasses. This review introduces the rationale and theoretical basis for FOB, including the interplay of depth of focus and optical aberrations, binocular summation, ocular dominance, and neuroadaptation. We discuss the clinical implementation of FOB: how the first-eye results guide the second-eye IOL choice in a tailored “mix-and-match” approach, as well as practical workflow considerations such as patient selection, ocular measurements, and decision algorithms. We also review current evidence from the literature on asymmetric IOL combinations (e.g., monofocal plus multifocal, or EDOF plus trifocal), highlighting visual outcomes, patient satisfaction, and remaining evidence gaps. Overall, FOB represents a paradigm shift toward binocular, patient-customized refractive planning. Early clinical reports suggest it can deliver a continuous range of vision without significantly compromising visual quality, though careful patient counseling and case selection are essential. Future directions include the integration of advanced diagnostics, artificial intelligence-driven IOL planning tools, and adaptive optics simulations to refine this personalized approach further. The promise of FOB is to improve cataract surgery outcomes by achieving an optimal balance: one that provides each patient with excellent visual quality and functional vision across distances, tailored to their lifestyle and expectations.
1. Introduction
Achieving both excellent optical quality and full spectacle independence remains one of the central challenges in modern cataract surgery [1,2,3]. While advanced presbyopia-correcting intraocular lenses (IOLs), including multifocal, trifocal, and extended depth-of-focus (EDOF) designs, have expanded the range of uncorrected vision, they often come at the cost of optical compromises [4]. Despite notable progress in IOL technology, there is still no perfect lens capable of delivering high-quality vision at all focal points without side effects. The trade-off between range and quality of vision remains a fundamental limitation [5].
Conventional strategies each present well-established drawbacks. Monovision, for instance, uses two monofocal IOLs with different refractive targets, typically assigning the dominant eye for distance and the non-dominant eye for near [6]. This approach consistently improves spectacle independence, with reported success rates frequently above 80% and minimal dysphotopsias [6]. However, monovision can impair binocular visual function [7]. Many patients report diminished stereopsis or describe a “waxy” visual quality due to the persistent defocus in one eye [7]. In cases with greater refractive disparity, the loss of stereoacuity and contrast sensitivity can be considerable, making monovision less suitable for patients with high demands for binocular precision [7].
Conversely, multifocal and trifocal IOLs distribute incoming light across multiple focal points to restore near and intermediate vision [8]. Bilateral trifocal implantation typically yields excellent distance, intermediate, and near acuity, often reaching 20/20 vision across all ranges [8]. However, this optical distribution reduces the contrast allocated to each focal point and frequently induces dysphotopsias such as halos and glare, particularly under mesopic conditions [9]. In bilateral multifocal implantation, neither eye receives a full-contrast image, which can potentially diminish the visual cortex’s ability to resolve detail [9]. Patients may describe this as a reduction in the perceived “quality” of vision [9]. Moreover, if the patient does not tolerate the photic disturbances of multifocality, the bilateral nature of the implantation offers no fallback, there is no “escape” eye with uncompromised clarity, which can significantly contribute to dissatisfaction [10]. Consequently, multifocal IOLs require careful patient selection [10]. Relative contraindications include irregular corneas, macular pathology, and large pupil diameters or angle kappa values, which are associated with greater risk for photic phenomena or reduced visual quality [10].
EDOF lenses have emerged as an intermediate solution [4]. These lenses generate an elongated focal zone, providing continuous vision from distance to intermediate, with only mild limitations at nearb [4]. Devices such as the Tecnis PureSee and Alcon Vivity represent leading examples, using advanced refractive optics to create a smooth gradient of focus [4]. EDOF lenses typically offer better contrast sensitivity and fewer halos than trifocal designs, although they may still fall short in terms of sharp near vision, often requiring reading glasses for fine print [4].
In essence, all current presbyopia-correcting IOLs involve trade-offs [11]. Monofocals optimize image clarity but limit range, trifocals maximize range at the expense of contrast, and EDOF designs balance the two but do not eliminate the need for near correction [11]. No available IOL design fully resolves presbyopia without some degree of compromise [11].
To address this limitation, the concept of Functional Optical Balance (FOB) has been proposed [12]. This approach customizes the IOL selection for each eye in a complementary fashion, rather than using identical implants or relying solely on conventional monovision [12]. The core idea of FOB is to assign one eye, typically the dominant, to receive a lens that prioritizes optical quality, maximizing contrast and minimizing aberrations and dysphotopsias, while the fellow eye receives an IOL that extends depth of focus to cover near and intermediate ranges [12]. This strategy, often referred to in the literature as “hybrid monovision” or “blended vision,” creates an intentional asymmetry that allows the visual system to integrate high-quality distance vision with functional range coverage [13]. This concept is grounded in a fundamental equation: the greater the pseudoaccommodation (functional ability to see at near distances post-IOL implantation, without true accommodative change, often achieved through increased depth of focus, residual myopia, or optical aberrations), the lower the optical quality; conversely, the greater the optical quality, the lower the pseudoaccommodation [14]. This inverse relationship defines the essence of FOB.
Crucially, FOB is not a fixed formula but a strategic framework. It requires thorough preoperative assessment of the patient’s visual demands and ocular characteristics, careful lens pairing, and precise refractive targeting to achieve an optimal binocular outcome. This review aims to present the theoretical and clinical foundation of FOB, highlighting the physiological underpinnings of binocular integration, the staged use of first-eye outcomes to guide second-eye planning, and the emerging clinical evidence supporting asymmetric IOL implantation strategies.
2. Methods
This review was conducted as an integrative synthesis of the current literature on FOB in cataract surgery. A comprehensive search was performed in May 2025 using the PubMed, Embase, and Cochrane Library databases. Search terms included ‘Functional Optical Balance,’ ‘blended vision,’ ‘cataract surgery,’ ‘asymmetric intraocular lens implantation,’ ‘monovision,’ ‘EDOF IOL’, ‘mix-and-match’, and ‘multifocal IOL.’ Relevant clinical trials, observational studies, and narrative reviews published in English over the past 15 years were considered. The selection prioritized studies that addressed asymmetric IOL strategies, mix-and-match outcomes, binocular visual performance, and neural adaptation in pseudophakic patients. Articles were selected based on their clinical relevance, originality, and contribution to the conceptual and practical understanding of the FOB approach.
Quality Assessment
The methodological rigor of this narrative review was evaluated using the SANRA (Scale for the Assessment of Narrative Review Articles) instrument, which consists of six criteria scored from 0 to 2, for a maximum total of 12 points. This manuscript received the maximum score of 12, reflecting high standards in justification of the topic, statement of objectives, source selection, presentation of relevant data, discussion of evidence, and use of appropriate scientific language.
3. Review
3.1. Background
3.1.1. Depth of Focus and Optical Aberrations
At the core of the FOB concept lies a fundamental optical trade-off: increasing depth of focus generally necessitates accepting a certain degree of optical aberration. In contrast, minimizing aberrations produces the sharpest possible image at a single focal distance but narrows the range of clear vision [4]. The human cornea naturally exhibits positive spherical aberration, averaging approximately 0.27 to 0.31 microns for a 6 mm pupil. Aspheric monofocal IOLs are engineered with negative spherical aberration to neutralize this corneal profile, thereby enhancing contrast and acuity at a specific distance focus [4].
While fully correcting spherical aberration produces a sharp focal point, it also limits the depth of field, analogous to a high-end camera lens with a wide aperture [15]. In contrast, introducing or tolerating a small amount of defocus or higher-order aberration can extend the range over which vision remains functionally clear [15]. This principle is intentionally applied in EDOF lens design, which uses refractive or diffractive optics to elongate the focal zone [15]. By balancing positive and negative spherical aberrations across the lens surface, these designs allow for continuous but slightly softer focus across a range of distances [15].
Kanclerz et al. summarized this balance: correcting spherical aberration provides the sharpest image at one point, whereas allowing certain aberrations can improve depth of focus at the expense of peak clarity [4]. The FOB strategy capitalizes on this optical principle by placing a high-quality, low-aberration lens in one eye and a depth-enhancing lens in the other, thus offering the patient a binocular solution that spans both ends of the visual performance spectrum.
3.1.2. IOL Optical Profiles and Visual Range
Modern IOLs can be conceptualized along a continuum between “single focus, maximum clarity” and “extended range, multifocal optics.” Each design has specific benefits and trade-offs.
Aspheric monofocal IOLs have a single focal point and are optimized for maximum clarity and contrast sensitivity at a specific distance [16]. When used in an eye with otherwise healthy optics, these lenses provide excellent image quality, particularly under mesopic and scotopic conditions, with minimal risk of halos or glare [16]. However, they do not extend the range of vision unless combined with monovision or other strategies [16].
Enhanced monofocal or “monofocal-plus” IOLs, such as Tecnis Eyhance (Johnson & Johnson) and Vivinex Impress (Hoya), offer a slight increase in depth of field by moding the central 2.0 mm of the optic, without introducing diffractive structures [17]. These lenses apply negative spherical aberration in the central zone to gently extend depth of focus while preserving contrast sensitivity similar to standard monofocals [17]. Another example is the EMV lens from Rayner, which incorporates positive spherical aberration in the central optic to enhance intermediate vision [17]. These lenses are ideal candidates for the dominant “quality” eye in a FOB strategy, as they improve intermediate function with minimal contrast loss and a low risk of photic phenomena [17].
EDOF IOLs offer continuous focus from distance to intermediate, typically at a range of 50–60 cm. Refractive EDOF models, such as Alcon Vivity and Tecnis PureSee (Johnson & Johnson), rely on wavefront-shaping or surface modulation technologies to create a non-discrete extension of the focal range [18]. These lenses preserve contrast sensitivity and reduce photic disturbances compared to trifocal designs, though they may still fall short in terms of sharp near vision, often requiring reading glasses for small print [18].
Multifocal and trifocal IOLs provide the broadest range of uncorrected vision by splitting light into two or three focal zones, usually for distance, 60 cm, and 40 cm. Although they increase spectacle independence, they inherently reduce contrast sensitivity and are associated with higher rates of photic disturbances [19]. High-add designs (+3.5 D) offer better near vision but more halos, while low-add designs (+2.5 D) strike a compromise between range and comfort. In the FOB context, these lenses are typically implanted in the non-dominant “range” eye [19].
3.1.3. Binocular Summation and Ocular Dominance
Vision in daily life is inherently binocular [20]. The brain integrates input from both eyes, a phenomenon known as binocular summation, to enhance visual acuity and contrast sensitivity beyond what either eye can achieve alone [20]. This principle is central to the FOB strategy. Studies show that binocular contrast sensitivity may exceed monocular sensitivity by as much as 40%, depending on conditions and the degree of symmetry between the inputs [20].
Ocular dominance plays a key role in this integration. The dominant eye typically provides the primary visual reference for tasks that require precision, particularly at a distance [21]. For this reason, FOB strategies prioritize optical quality in the dominant eye, assigning it a monofocal or enhanced monofocal lens to deliver a high-contrast, aberration-minimized image [21]. The non-dominant eye, referred to as the “range eye,” receives a lens with broader focal capabilities, providing support for near and intermediate distances (Figure 1) [21].
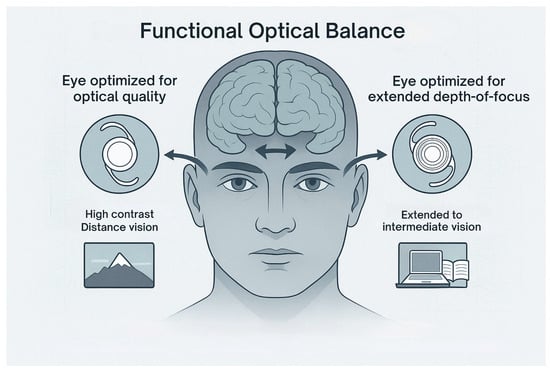
Figure 1.
Illustration of the Functional Optical Balance principle applied to IOL selection. The dominant eye is targeted for maximum contrast and optical clarity with a monofocal IOL, supporting distance vision. The non-dominant eye receives an extended depth-of-focus (EDOF) IOL to enhance intermediate and near visual performance.
This division of labor is most successful when interocular differences are intentionally designed to be fusion-compatible. Strong dominance should be carefully assessed preoperatively using standard sighting tests, as patients with unbalanced dominance may struggle with asymmetric inputs. The brain typically suppresses the less dominant input under conditions of conflict. If the dominant eye receives an image with halos or blur, the entire binocular experience may be compromised. Therefore, placing the “cleaner” optical profile in the dominant eye enhances the overall success of the FOB strategy.
3.1.4. Neural Adaptation
The human visual cortex exhibits remarkable neuroplasticity when adapting to new optical inputs, a process especially relevant following intraocular lens implantation [22]. After cataract surgery, patients typically undergo a period of neural adaptation, during which the brain learns to suppress unfocused or duplicated images and enhance attentional resources toward dominant or clearer inputs [22]. This is particularly critical in asymmetric IOL strategies like FOB, where each eye delivers distinct optical information [22].
With multifocal IOLs, for example, the retina receives simultaneous near and distance images [23]. The visual cortex must learn to disregard the defocused image depending on the visual task [23]. Many patients initially report visual disturbances such as halos or ghost images, but these often fade over time as the brain suppresses competing inputs and enhances signal interpretation [23]. This process relies on attentional modulation and potentially higher-order cortical adaptations, though the exact neural mechanisms remain incompletely understood [23].
In the context of FOB, neural adaptation must reconcile the optical asymmetry between the eyes. The ideal outcome is one of perceptual integration, in which the patient becomes unaware of the interocular difference and experiences seamless, functional binocular vision instead. This depends heavily on whether the disparity between the eyes falls within the individual’s adaptive range.
Clinical experience suggests that moderate, intentionally planned anisometropia, typically up to 1.5 to 2.0 diopters, is generally well tolerated [24]. Techniques such as mini-monovision or micro-anisometropia can enhance intermediate vision without compromising binocular fusion [24]. Several studies indicate that most parents can successfully adapt to gradual differences when those differences are introduced with purpose and purposefully [24]. However, excessive optical disparity may result in binocular inhibition, in which the visual system suppresses one eye entirely, leading to discomfort or dissatisfaction [24].
Additional considerations include image size differences (anisokonia) and distortion between the two eyes [25]. Significant discrepancies in retinal image perception can interfere with fusion and reduce adaptation success [25]. For this reason, preoperative evaluation should carefully assess the degree of expected disparity [25]. The goal of FOB is not merely to implant lenses with differing optical properties, but to ensure that the resulting binocular visual environment remains within a tolerable range for neural integration [25].
Ultimately, the neuroadaptive capacity of the individual patient is a critical determinant of FOB success. Surgeons must assess visual dominance, refractive goals, prior tolerance to asymmetry (e.g., from contact lens monovision), and overall cognitive adaptability. When executed thoughtfully, the brain can merge high-quality input from one eye with extended focus from the other into a coherent and satisfying visual experience. The theoretical underpinning of FOB thus merges optical physics with neurophysiology, relying on the brain’s ability to transform asymmetric optics into functional, binocular perception.
3.1.5. Evidence from the Literature
The concept of mixing and matching IOLs to improve visual outcomes has been explored for over a decade. While most studies do not use the term “Functional Optical Balance” explicitly, a growing body of evidence supports many of its foundational principles, particularly in the context of hybrid monovision, blended vision, and asymmetric IOL implantation strategies (Table 1).
3.1.6. Mix-and-Match vs. Bilateral Identical IOLs
Several comparative studies have evaluated whether combining different presbyopia-correcting IOLs in each eye can yield visual outcomes equal or superior to bilateral implantation of the same lens.
Nuijts et al. (2016) compared bilateral low-add multifocals (Alcon AcrySof IQ ReSTOR) to a blended approach combining a +2.5 D in one eye and a +3.0 D in the other [26]. Both groups achieved similar distance and intermediate vision, with a slight improvement in near acuity in the blended group. Importantly, patient satisfaction remained high across both cohorts, suggesting that mild asymmetry in add power does not compromise outcomes and may enhance near performance.
Gundersen and Potvin (2016) compared bilateral trifocal implantation (PhysIOL FineVision) to a blend of two apodised bifocal lenses (Alcon ReSTOR) with different add powers [5]. Their results demonstrated comparable visual acuity across distances, with the blended group reporting fewer instances of dysphotopsia. This supported the notion that tailored combinations could reduce photic phenomena while preserving range of vision.
De Medeiros et al. (2017) conducted a study comparing bilateral trifocal implantation to a hybrid approach using one EDOF lens (Johnson & Johnson Tecnis Symfony ZXR00) and one diffractive bifocal (Tecnis ZMB00) [27]. The blended group achieved visual outcomes comparable to the bilateral trifocal group in distance and intermediate ranges, with slightly reduced near vision. Notably, the hybrid group reported fewer halos and higher satisfaction with night vision, underscoring the potential benefits of asymmetric implantation.
Hayashi et al. (2019) assessed various combinations of bifocal IOLs (Alcon ReSTOR) and found that mixing different designs could enhance depth perception and patient comfort without compromising acuity, further validating the flexibility of asymmetric approaches [28].
Acar et al. (2021) published a pivotal study comparing bilateral trifocals (AT LISA) to a mixed strategy using an EDOF lens (AT LARA) in the dominant eye and a trifocal (AT LISA) in the non-dominant eye [29]. Both groups achieved excellent binocular acuity across all ranges. However, the mixed group demonstrated superior intermediate vision and significantly better contrast sensitivity, especially in mesopic conditions. These patients also reported fewer night-driving difficulties and higher overall satisfaction with nighttime vision. The authors concluded that combining EDOF and trifocal IOLs may offer the most balanced solution for functional vision with reduced visual disturbances.
Kim et al. (2022) corroborated these findings comparing bilateral trifocal implantation (FineVision Triumf and FineVision HP) to a hybrid of trifocal and EDOF (ZEISS AT LARA 829MP + AT LISA tri839MP) [30]. Their results mirrored those of Acar et al., demonstrating equivalent overall acuity but improved contrast sensitivity and fewer photic complaints in the mixed group. The defocus curve analysis showed that the hybrid strategy maintained good functional acuity across a broad range, including distance to approximately 40 cm.
3.1.7. Hybrid Monovision (Monofocal + Multifocal)
Another body of evidence supports combining a monofocal IOL in the dominant eye with a multifocal or EDOF lens in the non-dominant eye, an approach sometimes referred to as hybrid monovision.
Iida et al. (2011) implanted a monofocal lens (AQ310Ai) in the dominant eye and a diffractive multifocal (Tecnis ZM900) in the fellow eye [7]. Patients achieved greater spectacle independence than those with bilateral monofocals, yet reported fewer dysphotopsias and better contrast sensitivity than those with bilateral multifocals. The study suggested that hybrid monovision could be ideal for patients who seek functional near vision but are intolerant of bilateral multifocality.
Labiris et al. (2017) conducted a systematic review on pseudophakic monovision and multifocal outcomes [6]. They concluded that monovision strategies achieve high satisfaction with minimal visual disturbances, while multifocal lenses optimize near acuity at the expense of photic side effects. Hybrid monovision emerged as a compromise that preserves contrast in one eye while extending range in the other.
3.1.8. Blending Different Add Powers or Designs
Even within multifocal-only strategies, combining lenses with different add powers has been explored to soften side effects and optimize visual performance.
Vilar et al. (2017) compared bilateral trifocal (TNFT00) implantation to a blend (SV25T0 and SN6AD1) of two different bifocals (+3.0 D and +4.0 D) [31]. Both groups achieved similar acuity, but the blended group exhibited slightly better reading speed and subjective comfort with near tasks, supporting the idea that nuanced customization of IOLs can fine-tune patient outcomes.

Table 1.
Visual outcomes of mix-and-match presbyopia-correcting IOL strategies (asymmetric combination vs. bilateral same IOL) and visual outcomes of hybrid monovision strategies (monofocal in one eye combined with multifocal/EDOF in fellow eye).
Table 1.
Visual outcomes of mix-and-match presbyopia-correcting IOL strategies (asymmetric combination vs. bilateral same IOL) and visual outcomes of hybrid monovision strategies (monofocal in one eye combined with multifocal/EDOF in fellow eye).
| Visual Outcomes of Mix-and-Match Presbyopia-Correcting IOL Strategies (Asymmetric Combination vs. Bilateral Same IOL) | ||||||
| Study (Year) | IOL Combination Compared | Distance VA | Intermediate VA | Near VA | Patient Satisfaction | Dysphotopsias/Side Effects |
| [5] | Bilateral trifocal vs. Blended bifocals (+2.5 D & +3.0 D) | ~20/20 both eyes | ~20/20 both trifocal slightly better 2 m–67 cm range | ~20/25 both | High in both groups; no difference in NEI VFQ or overall QoV scores (trifocal group noted better vision at dashboard distance) | Low overall; trifocal group had more patients reporting visual disturbances as “bothersome” (halos) despite similar incidence. |
| [26] | Bilateral low-add (+2.5) vs. Mixed adds (+2.5/+3.0 D bifocal mix) | ~20/20 both groups | ~20/20 both groups | Slightly better in mixed-add (improved near acuity vs. bilateral low-add) | High in both groups; mild asymmetry did not compromise satisfaction | Minimal in both; no increase in halos with mixed adds (trend toward fewer complaints in mix). |
| [27] | Bilateral trifocal vs. Hybrid EDOF + bifocal (Symfony EDOF in one eye, diffractive bifocal in other) | ~20/20 | 20/20 | Trifocal ~20/20; hybrid slightly reduced (≈20/30) near acuity | High in both; hybrid group noted better night-vision confidence | Hybrid combo had fewer halos and night halos/glare complaints compared to bilateral trifocal. |
| [29] | Bilateral trifocal (AT LISA) vs. Mix & match (EDOF AT LARA in dominant eye + trifocal AT LISA in other) | ~20/20 both groups | Better in mix: e.g., 20/20 vs. 20/25 at 60 cm | ~20/20 both | Very high in both; mixed IOL group rated night driving vision significantly higher | Mixed group had fewer halos and better mesopic contrast sensitivity than bilateral trifocal. |
| [30] | Two mix-and-match trifocal + EDOF combos (FineVision Triumf + HP vs. ZEISS AT LARA + AT LISA) | ~20/20 (both combinations) | Both provided functional ~20/20 intermediate; one mix (AT LARA-LISA) slightly better −1.0 to −1.5 D (intermediate range) | Both ~20/20; the other mix (FineVision) slightly better at −3.0 to −4.0 D (near range) | High in both groups (binocular spectacle independence achieved in all cases—retrospective study) | No significant differences reported between the two mix-and-match regimens; asymmetric trifocal + EDOF provided good far/intermediate/near with minimal photic complaints. |
| [31] | Bilateral trifocal vs. Blended bifocals (+3.0 D & +4.0 D in opposite eyes) | ~20/20 both groups | ~20/20 both | ~20/25 both | High in both; blended-group patients reported slightly greater reading comfort | Similar low incidence of halos; blended adds group had marginally less near strain (subjective reports). |
| Visual Outcomes of Hybrid Monovision Strategies (Monofocal in One Eye Combined with Multifocal/EDOF in Fellow Eye) | ||||||
| Study (Year) | IOL Combination (Hybrid vs. Control) | Distance VA | Intermediate VA | Near VA | Patient Satisfaction | Dysphotopsias/Notes |
| [7] | Monofocal (dominant) + Multifocal (non-dom); vs. bilateral monofocal or multifocal (historical) | ~20/20 (dominant eye provides crisp distance) | ~20/30 (functional at intermediate distances) | ~20/30–20/40 (significantly improved near vs. bilateral mono) | High-greater spectacle independence than bilateral monofocal; patients preferred hybrid to needing reading glasses | Fewer halos and better contrast than bilateral multifocal; hybrid monovision seen as an ideal compromise for those intolerant of full multifocality. |
| [6] | Monovision vs. Multifocal IOLs (systematic review data) | ~20/20 (monovision eye targets distance) | ~20/40 (monovision eye can target intermediate with mini-monovision) | ~20/40 (non-dominant eye targeted for near in monovision) | Monovision: ~80% success/satisfaction (high spectacle independence); Multifocal IOLs: high near satisfaction but more complaints | Monovision: minimal halos/glare; reduced stereopsis at larger anisometropia. Multifocal IOLs: more halos/glare. Hybrid monovision is highlighted as balancing these effects. |
| [13] | Mix: Enhanced monofocal (Eyhance) + Diffractive EDOF (Synergy) vs. Bilateral Synergy trifocals | ~20/20 both groups | ~20/20 both (no sig. difference in defocus to −2.0 D) | Near advantage with bilateral trifocals: ~20/25 at 40–50 cm vs. ~20/32 with mix | Good in both: slightly higher “completely satisfied” rate in bilateral trifocal group (owing to sharper near vision) | Halos/glare: significantly more in bilateral trifocal group; mix combo had milder night halos at the cost of a little near clarity. |
| [12] | Extended Monovision: Monofocal (dominant eye) + Asymmetric refractive EDOF (Lentis Comfort MF15) at −1.25 D target (non-dom) | 20/22 | 20/15 | 20/20 | High: 83% overall satisfaction; ~89% reported no glasses needed for daily tasks | Low dysphotopsias: Halo intensity was mild (asymmetric refractive design); patients showed strong neuroadaptation. Study proposes this combo as a promising alternative to diffractive multifocals. |
3.1.9. Patient-Reported Outcomes and Subjective Visual Quality
Beyond objective measures of acuity and contrast, numerous studies emphasize patient-reported outcomes, including spectacle independence, night-driving confidence, and overall satisfaction. These metrics are especially relevant in evaluating asymmetric strategies like FOB, where individual perception of comfort and visual functionality often determines success.
In the Acar et al. (2021) study, both the bilateral and mixed IOL groups reported high satisfaction. However, the group with the EDOF–trifocal combination demonstrated superior ratings for night vision, likely due to the preservation of contrast and fewer halos in the dominant eye [29]. Similarly, de Medeiros et al. (2017) found that patients in the hybrid group reported fewer photic disturbances and greater confidence during nighttime activities [27].
A consistent pattern emerges when one eye delivers a clear, high-contrast image, typically the dominant eye, patients perceive their overall vision as sharper and more stable. This “safety net” effect appears to buffer against the dysphotopsias that may arise from multifocality in the fellow eye. Many patients in blended groups report that they are reassured by having at least one eye with reliable clarity, which increases tolerance for visual phenomena in the range eye.
Nevertheless, some patients do notice interocular differences and may become fixated on comparing eyes. A subset of patients in mix-and-match trials has reported asymmetry-related complaints, such as one eye being sharper or more disturbed by halos than the other, even when binocular acuity is excellent. This highlights the importance of patient counseling, expectation management, and psychological readiness.
3.1.10. Limitations and Gaps in the Current Evidence
Despite encouraging results, the current literature supporting Functional Optical Balance has notable limitations.
Terminology Inconsistencies: Most existing studies do not label their methods explicitly as FOB, instead referring to “blended vision”, “hybrid monovision”, or “mix-and-match”. This lack of standardized terminology complicates meta-analyses and hinders protocol development.
Short Follow-Up Periods: Many studies report outcomes at 3 to 6 months postoperatively. Long-term durability of satisfaction, adaptation, and visual function, particularly as posterior capsular opacification develops, or other ocular changes occur remains understudied.
Patient Selection Criteria: Few trials rigorously analyze which patient profiles are most likely to succeed with asymmetric strategies. Retrospective observations suggest that patients with visually demanding lifestyles (e.g., pilots or drivers) may prefer quality-preserving combinations, while those prioritizing near vision might accept more photic phenomena. However, these trends lack formal validation.
Objective Measures of Neuroadaptation: While subjective reports and clinical impressions support the feasibility of neural adaptation in FOB, few studies employ objective tools, such as neurofunctional imaging or detailed binocular fusion testing, to quantify the cortical processing of asymmetric optics.
Inter-Manufacturer Variability: As noted, differences in chromatic properties, edge design, or light distribution between IOL platforms may influence binocular integration. These factors are seldom accounted for in comparative studies, yet they may significantly impact visual comfort and summation.
Despite these gaps, the cumulative evidence strongly supports the efficacy, safety, and patient satisfaction associated with intentionally asymmetric IOL strategies when implemented with care. The data suggest that many patients can achieve spectacle independence across all ranges, improved night vision, and fewer dysphotopsias by leveraging the complementary strengths of different lens types in each eye. A carefully balanced mix, typically preserving clarity in the dominant eye and extending range in the non-dominant eye, appears to offer the best of both worlds.
Implementing the FOB strategy in clinical practice demands a systematic and highly individualized approach, encompassing preoperative evaluation, intraoperative decision-making, and postoperative refinement. Its success hinges on meticulous patient selection, precise biometric planning, and thoughtful lens pairing tailored to each patient’s visual goals.
3.1.11. Preoperative Evaluation and Candidate Selection
Ideal candidates for FOB are patients who desire spectacle independence across multiple distances but are also highly sensitive to visual quality. These individuals often have active lifestyles or visually demanding occupations and may have previously been dissatisfied with conventional bilateral multifocal approaches. Crucially, they must possess healthy ocular anatomy, including regular corneas, normal macular structure, and no significant neuro-ophthalmic disorders.
Psychological readiness and adaptability are equally important. Patients with realistic expectations and prior tolerance to visual asymmetry, such as those who used monovision contact lenses, often adapt more readily to asymmetric IOL implantation. Previous successful implantation in one eye can also serve as a valuable predictor of overall tolerance.
Once candidacy is established, a detailed preoperative workup is essential. Accurate axial length and keratometry measurements, using devices such as the IOLMaster 700 or Lenstar, are foundational to effective refractive targeting [32]. Even small refractive inaccuracies can destabilize the delicate binocular balance required for FOB. Corneal topography or tomography helps exclude irregular astigmatism and quantify higher-order aberrations, which can affect premium IOL performance [32].
Wavefront aberrometry provides insight into the corneal spherical aberration profile, aiding lens selection [33]. For example, a patient with high positive spherical aberration may benefit from an aspheric IOL with compensatory negative aberration. Ocular dominance must also be established, typically through simple sighting methods such as the hole-in-the-card test, and should guide lens assignment, with the dominant eye receiving the lens that prioritizes contrast and clarity [33].
Additional preoperative tests include mesopic pupillometry, which helps assess the risk of photic disturbances, and angle kappa measurements [34]. Additional preoperative tests include mesopic pupillometry and angle kappa assessment. While a large angle kappa is traditionally associated with increased risk of dysphotopsias in diffractive multifocal IOLs, due to misalignment of their concentric optical zones, refractive lenses are not exempt from similar issues [34], in eyes with a large angle kappa, careful IOL centration and design selection are critical, regardless of the optical platform used.
3.1.12. Surgical Planning and Staged Customization
The dominant eye, referred to as the “quality eye”, is generally implanted with a monofocal, enhanced monofocal, or low-halo EDOF lens, aiming for maximum contrast sensitivity and night vision performance. The non-dominant “range eye” receives a lens that extends focal depth, such as an EDOF or trifocal, depending on the patient’s near-vision needs and tolerance for dysphotopsias.
Refractive targeting is a critical component of the strategy. While both eyes are often targeted for emmetropia, it may be advantageous to induce mild myopia (e.g., −0.25 to −0.50 diopters) in the range eye to enhance near performance. This creates a layered approach, combining optical lens design with subtle anisometropia, to broaden the patient’s functional vision. Concepts like “mini-monovision” and “nanovision” (anisometropia below 1.0 D) aim to stretch depth of focus while preserving fusion and stereopsis.
The procedure itself must be precise. In the first eye, particular care should be taken with centration, especially if implanting a multifocal or EDOF lens. Even minor decentration can degrade performance. Astigmatism correction, whether via toric IOLs or limbal relaxing incisions, should be performed with high accuracy. Intraoperative aberrometry, when available, can refine IOL power selection and axis alignment, enhancing refractive precision.
Importantly, FOB favors staged surgery, using the first-eye outcome to inform the second-eye strategy. After initial implantation, the surgeon should assess both objective results (visual acuity and contrast sensitivity) and subjective feedback (patient satisfaction and visual disturbances). If the first eye provides excellent distance vision but inadequate near function, the second eye can be adjusted accordingly, either by choosing a lens with stronger near focus or by modifying the refractive target. Conversely, if halos or contrast loss are problematic, the second eye may receive a simpler optic.
3.1.13. Postoperative Assessment and Refinement
Following bilateral implantation, the binocular visual outcome must be thoroughly evaluated. This includes measuring uncorrected visual acuity at distance (6 m), intermediate (approximately 66–80 cm), and near (40 cm), as well as assessing contrast sensitivity. Real-world functional testing, such as reading smartphone text, navigating street signs, or evaluating night-driving ability, can offer more meaningful insights than Snellen charts alone.
Patients may initially experience mild visual phenomena or interocular imbalance. These often diminish with time due to neural adaptation, particularly if the asymmetry was carefully planned. Nonetheless, if residual refractive error or disturbing symptoms persist, enhancements such as LASIK or PRK may be warranted. Even small adjustments, like fine-tuning the spherical equivalent of the quality eye or inducing slight myopia in the range eye, can significantly improve overall satisfaction.
Technological tools are increasingly supporting FOB planning and execution. Software platforms such as Panfocal Vision Analyzer or IOL-Match integrate lifestyle data and IOL defocus profiles to simulate visual outcomes and recommend optimal lens combinations. Intraoperative aberrometry systems like ORA can confirm alignment and power in real-time, while digital guidance platforms assist with toric axis placement. These technologies help minimize variability and personalize outcomes.
Patient education remains fundamental. Visual simulators, trial lens demonstrations, and defocus glasses can help set expectations preoperatively, especially when describing how each eye will function postoperatively [35]. Understanding that the goal is not identical vision in both eyes, but a complementary binocular outcome, is key to acceptance and satisfaction.
When carefully executed, FOB offers a structured yet flexible approach to achieving high-quality vision across all distances. It minimizes the compromises typically associated with bilateral multifocal implantation and aligns with the broader trend toward individualized, patient-centered refractive cataract surgery.
4. Discussion
The FOB strategy represents a paradigm shift in cataract surgery by embracing intentional asymmetry between the eyes. Rather than relying on symmetric IOL implantation for optical consistency, FOB seeks to exploit the visual system’s innate capacity for integration and adaptation, assigning distinct but complementary roles to each eye. When appropriately implemented, this asymmetry can enhance overall visual performance, offering a compelling alternative for patients who prioritize both clarity and spectacle independence.
Traditional approaches to presbyopia correction often force a binary trade-off between range and quality. Bilateral trifocal implantation maximizes visual range but frequently reduces contrast and induces photic phenomena [36]. Monovision enhances independence but sacrifices stereopsis and binocular summation [36]. EDOF lenses preserve image clarity but leave gaps in near vision [36]. FOB challenges this dichotomy by leveraging neural plasticity and binocular fusion to combine the strengths of multiple lens profiles, minimizing their individual weaknesses [36].
One of the central mechanisms behind FOB’s success is binocular summation. The brain does not perceive vision as a simple average of the two eyes, but rather as a unified percept that often exceeds the performance of either eye individually [37]. By assigning the dominant eye the role of high-contrast “quality eye” and the non-dominant eye the role of “range eye,” the visual system is provided with a reliable distance anchor while gaining access to extended focus through the fellow eye (Figure 2) [37].
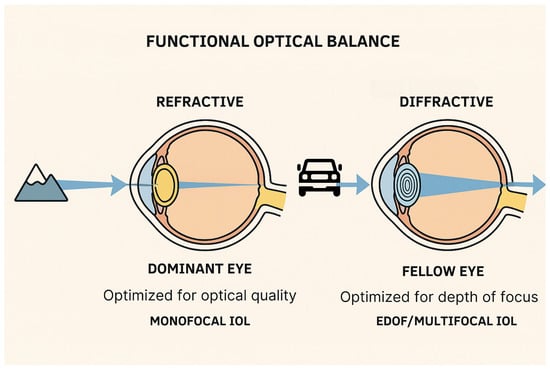
Figure 2.
Schematic representation of the Functional Optical Balance (FOB) strategy in cataract surgery. One eye is optimized for high optical quality and distance vision using a monofocal or enhanced monofocal intraocular lens (IOL), while the fellow eye is implanted with an IOL that extends the depth of focus (EDOF or multifocal) to improve near and intermediate visual function.
Clinical and physiological data support the premise that this combination results in improved overall function and higher patient satisfaction, especially when the asymmetry is subtle, deliberate, and designed to stay within the fusion threshold [37]. None of the studies reviewed explicitly evaluated FOB in the context of refractive lens exchange in eyes without significant cataract. However, in current practice, some surgeons offer presbyopia-correcting IOL combinations to patients with clear lenses typically middle-aged hyperopes seeking spectacle independence. In these cases, where surgery is performed electively on eyes with good baseline acuity, patient expectations are especially high, and tolerance for suboptimal outcomes (e.g., halos or residual refractive error) is often lower.
Neural adaptation further reinforces the viability of this strategy. Although the asymmetry between eyes introduces optical disparity, most patients can neurologically adapt to these differences [38]. Over time, the brain learns to suppress visual input from the eye that is less suited for a particular task while emphasizing the dominant, clearer signal [38]. This dynamic allocation of visual attention enables patients to perform daily activities with little to no awareness of which eye is contributing more at any given moment [38].
Still, the success of FOB is contingent on careful planning and patient-specific tailoring. Excessive interocular difference can disrupt fusion, resulting in rivalry, discomfort, or binocular inhibition. Strong ocular dominance may limit adaptability if the dominant eye receives a degraded image, emphasizing the need for precise dominance testing and appropriate lens selection. Furthermore, patients with limited neuroplasticity, such as those of advanced age or with neurologic comorbidities, may struggle to adapt and should be counseled accordingly.
Another critical advantage of FOB lies in its staged, feedback-informed nature. By evaluating the outcome of first-eye surgery, both subjectively and objectively, surgeons can refine their approach for the second eye [39]. This dynamic model supports real-time customization based on patient response, enhancing the likelihood of achieving a successful binocular result [39]. It reflects a broader trend in refractive cataract surgery: shifting from fixed, symmetric algorithms toward individualized, responsive care.
Moreover, FOB enables flexibility in lens selection based on ocular anatomy and lifestyle demands [40]. For instance, patients with large pupils or higher angle kappa may benefit from avoiding bilateral diffractive multifocals but can still achieve spectacle independence using an EDOF in the range eye and an enhanced monofocal in the quality eye [40]. Similarly, patients who prioritize night driving or image sharpness may receive a monofocal in the dominant eye and a low-add multifocal or EDOF in the fellow eye, balancing contrast with functional range [40].
Technological advances are poised to optimize the execution of FOB further. Visual simulation tools, biometric planning software, intraoperative aberrometry, and AI-assisted lens selection platforms are making it increasingly feasible to predict and customize visual outcomes with precision [41]. Emerging lens technologies, such as adjustable optics and future accommodating IOLs, may expand the toolbox available for constructing asymmetric visual systems with even finer control [41].
Nonetheless, the challenges and limitations of FOB must be acknowledged. The increased complexity of surgical planning and postoperative assessment demands greater expertise and time investment from the surgical team. Health systems with rigid reimbursement models may not support mixing IOL types from different manufacturers, limiting surgeon flexibility. Moreover, long-term data on neural adaptation, optical stability, and satisfaction beyond six months remain limited, underscoring the need for ongoing prospective studies and real-world outcome reporting. Thus, while FOB represents a promising strategy to enhance visual outcomes by leveraging asymmetric IOL implantation, it is not a universal solution. Its success depends heavily on individualized planning, precise dominance testing, and careful patient selection. Not all patients are suitable candidates, particularly those with limited neuroadaptive capacity, high sensitivity to interocular differences, or unrealistic expectations. Moreover, the outcome of the first-eye surgery plays a pivotal role in determining the success of the overall strategy. If the dominant eye, typically optimized for clarity, results in residual refractive error or poor contrast, the entire binocular balance may be compromised. Such a mismatch can undermine the intended synergy between eyes, limiting the functional benefit of FOB and potentially leading to patient dissatisfaction. For example, an unintended myopic shift in the dominant eye can blur distance vision, negating the foundational “quality eye” concept and disrupting the planned binocular synergy. In such cases, the second eye may be unable to compensate adequately, and the overall visual outcome may fall short of expectations. Therefore, staged surgery with thorough evaluation after the first procedure is essential to guide second-eye planning and preserve the integrity of the strategy.
Despite these barriers, the core concept of FOB, customizing each eye’s optics to complement the other, aligns seamlessly with the evolution of cataract surgery toward personalization and functional integration. It offers a sophisticated yet practical response to the limitations of current IOL technologies and recognizes the brain’s powerful role in shaping visual experience. When executed with precision and guided by both anatomical data and patient preferences, FOB can deliver a visual outcome that is not only functionally robust but also subjectively gratifying, enabling patients to enjoy the full spectrum of vision with minimized compromise.
5. Conclusions
FOB represents a refined evolution in cataract surgery, leveraging binocular integration, neural adaptation, and personalized IOL selection to achieve both clarity and range. By assigning distinct roles to each eye, typically clarity in the dominant eye and extended focus in the fellow, FOB minimizes the compromises of traditional symmetric strategies. When implemented adequately through careful preoperative assessment, staged decision-making, and individualized refractive targeting, FOB can reduce photic disturbances, preserve contrast sensitivity, and deliver functional vision across all distances. Technological advances such as aberrometry, visual simulators, and adaptive optics further enhance its precision and adaptability. Although further research is needed to define optimal pairings, validate long-term outcomes, and standardize terminology, FOB provides a compelling framework for delivering high-quality, personalized binocular vision that aligns with the functional demands and neuroadaptive capacity of each patient.
Author Contributions
Conceptualization, D.C.A. and A.G.S.; methodology, D.C.A. and P.L.M.M.; validation, D.C.A., P.L.M.M., A.G.S. and R.N.L.; formal analysis, D.C.A. and A.G.S.; investigation, D.C.A., F.M.T.d.M. and M.R.A.; resources, D.C.A., F.M.T.d.M. and M.R.A.; data curation, D.C.A., A.G.S., F.M.T.d.M. and M.R.A.; writing—original draft preparation, D.C.A., A.G.S. and P.L.M.M.; writing—review and editing, P.L.M.M., A.G.S., A.B.d.C.N., J.G., F.M.T.d.M., M.R.A. and R.N.L.; visualization, P.L.M.M.; supervision, R.N.L., J.G.; project administration, D.C.A., A.G.S., F.M.T.d.M. and M.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All relevant data supporting the findings of this study are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| D | Diopter |
| EDOF | Extended Depth of Focus |
| EMV | Enhanced Monovision Lens (Rayner) |
| FOB | Functional Optical Balance |
| IOL | Intraocular Lens |
| IOLMaster | Intraocular Lens Biometry Device (Carl Zeiss) |
| LASIK | Laser-Assisted In Situ Keratomileusis |
| Symfony | Extended Depth Focus Intraocular Lens (Johnson & Johnson) |
| Tecnis | Brand Family of Intraocular Lenses (Johnson & Johnson) |
| Vivity | Extended Depth of Focus Intraocular Lens (Alcon) |
| Eyhance | Enhanced Monofocal Intraocular Lens (Johnson & Johnson) |
| AT LISA | Trifocal Intraocular Lens (Carl Zeiss) |
| AT LARA | Extended Depth-of-Focus Intraocular Lens (Carl Zeiss) |
References
- Amaral, D.C.; Cheidde, L.; Ferreira, B.F.A.; Júnior, P.P.L.; Menezes, I.; Gomes, V.; Esporcatte, B.L.B.; Alves, M.R.; Monteiro, M.L.R.; Yamamoto, J.H.; et al. Cataract in HIV Patients: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e72370. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.M.; Alves, R.V.E.; Cunha, A.D.; Goldfarb, C.L.; do Rêgo Castro, C.E.; Jaime, G.; Noguera, L.R.; Carricondo, P.C. The use of cold saline solutions in cataract surgery by phacoemulsification: A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Ophthalmol. 2025, 20, 133–140. [Google Scholar] [CrossRef]
- Guedes, J.; Pereira, S.F.; Amaral, D.C.; Hespanhol, L.C.; Faneli, A.C.; Oliveira, R.D.C.; Mora-Paez, D.J.; Fontes, B.M. Phaco-Chop versus Divide-and-Conquer in Patients Who Underwent Cataract Surgery: A Systematic Review and Meta-Analysis. Clin. Ophthalmol. 2024, 18, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Toto, F.; Grzybowski, A.; Alio, J.L. Extended Depth-of-Field Intraocular Lenses: An Update. Asia. Pac. J. Ophthalmol. 2020, 9, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K.G.; Potvin, R. Comparison of visual outcomes and subjective visual quality after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of apodized diffractive bifocal intraocular lenses. Clin. Ophthalmol. 2016, 10, 805–811. [Google Scholar] [CrossRef]
- Labiris, G.; Toli, A.; Perente, A.; Ntonti, P.; Kozobolis, V.P. A systematic review of pseudophakic monovision for presbyopia correction. Int. J. Ophthalmol. 2017, 10, 992–1000. [Google Scholar] [CrossRef]
- Iida, Y.; Shimizu, K.; Ito, M. Pseudophakic monovision using monofocal and multifocal intraocular lenses: Hybrid monovision. J. Cataract. Refract. Surg. 2011, 37, 2001–2005. [Google Scholar] [CrossRef]
- Zhu, M.; Fan, W.; Zhang, G. Visual outcomes and subjective experience with three intraocular lenses based presbyopia correcting strategies in cataract patients. Sci. Rep. 2022, 12, 19625. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Wang, Y.; Chen, W.; Xiao, W.; Xiang, Y.; Zhu, Y.; Chen, C.; Dong, X.; Liu, Y.; et al. Comparison of Visual Neuroadaptations After Multifocal and Monofocal Intraocular Lens Implantation. Front. Neurosci. 2021, 15, 648863. [Google Scholar] [CrossRef]
- Woodward, M.A.; Randleman, J.B.; Stulting, R.D. Dissatisfaction after multifocal intraocular lens implantation. J. Cataract. Refract. Surg. 2009, 35, 992–997. [Google Scholar] [CrossRef]
- Fernández, J.; Ribeiro, F.J.; Rodríguez-Vallejo, M.; Dupps, W.J.; Werner, L.; Srinivasan, S.; Kohnen, T. Standard for collecting and reporting outcomes of IOL-based refractive surgery: Update for enhanced monofocal, EDOF, and multifocal IOLs. J. Cataract. Refract. Surg. 2022, 48, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Nagyova, D.; Tappeiner, C.; Blaha, A.; Goldblum, D.; Kyroudis, D. Outcome of a Mix-and-Match Approach with a Monofocal Aspherical and a Bifocal Extended Depth-of-Focus Intraocular Lens to Achieve Extended Monovision in Cataract Patients. Klin. Monbl. Augenheilkd. 2025, 242, 372–378. [Google Scholar] [CrossRef]
- Hida, W.T.; Moscovici, B.K.; Cortez, C.M.; Colombo-Barboza, G.N.; Tzelikis, P.F.M.; Motta, A.F.P.; De Medeiros, A.L.; Nose, W.; Carricondo, P.C. Comparison of visual outcomes of bilateral dual-technology diffractive intraocular lens vs blended enhanced monofocal with dual-technology intraocular lens. J. Cataract. Refract. Surg. 2024, 50, 401–406. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Mukhija, R.; Ashena, Z.; Bunce, C.; Spalton, D.J. Incidence and factors for pseudoaccommodation after monofocal lens implantation: The Monofocal Extended Range of Vision study. J. Cataract. Refract. Surg. 2023, 49, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Camps, V.J.; García, C.; Caballero, M.T.; Gonzalez-Leal, J.M. Analysis and comparison of monofocal, extended depth of focus and trifocal intraocular lens profiles. Sci. Rep. 2022, 12, 8654. [Google Scholar] [CrossRef]
- McCabe, C.M.; Peterson, R.; Hull, J.; Bala, C. Impact of Aspheric Monofocal Intraocular Lens Implantation on Uncorrected Intermediate Visual Acuity: A Combined Analysis. Clin. Ophthalmol. 2024, 18, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Morelli, A.; Cennamo, M.; Roszkowska, A.M.; Favuzza, E. Enhanced Monofocal Intraocular Lenses: A Retrospective, Comparative Study between Three Different Models. J. Clin. Med. 2023, 12, 3588. [Google Scholar] [CrossRef]
- Kohnen, T.; Petermann, K.; Böhm, M.; Hemkeppler, E.; Ahmad, W.; Hinzelmann, L.; Pawlowicz, K.; Jandewerth, T.; Lwowski, C. Nondiffractive wavefront-shaping extended depth-of-focus intraocular lens: Visual performance and patient-reported outcomes. J. Cataract. Refract. Surg. 2022, 48, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Friedman, D.S.; Jin, S.; Yusufu, M.; Zhang, J.; Wang, J.; Hou, S.; Zhu, G.; Wang, B.; Xiong, Y.; et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: A system review and meta-analysis based on randomized controlled trials. Surv. Ophthalmol. 2019, 64, 647–658. [Google Scholar] [CrossRef]
- Sverdlichenko, I.; Mandelcorn, M.S.; Issashar Leibovitzh, G.; Mandelcorn, E.D.; Markowitz, S.N.; Tarita-Nistor, L. Binocular visual function and fixational control in patients with macular disease: A review. Ophthalmic Physiol. Opt. 2022, 42, 258–271. [Google Scholar] [CrossRef]
- Song, T.; Duan, X. Ocular dominance in cataract surgery: Research status and progress. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 33–41. [Google Scholar] [CrossRef]
- Martins Rosa, A.; Silva, M.F.; Ferreira, S.; Murta, J.; Castelo-Branco, M. Plasticity in the human visual cortex: An ophthalmology-based perspective. Biomed. Res. Int. 2013, 2013, 568354. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K.G.; Potvin, R. Comparative visual performance with monofocal and multifocal intraocular lenses. Clin. Ophthalmol. 2013, 7, 1979–1985. [Google Scholar] [CrossRef][Green Version]
- Hayashi, K.; Yoshida, M.; Manabe, S.; Hayashi, H. Optimal amount of anisometropia for pseudophakic monovision. J. Refract. Surg. 2011, 27, 332–338. [Google Scholar] [CrossRef]
- Stokkermans, T.J.; Day, S.H. Aniseikonia. [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK585108/ (accessed on 1 May 2025).
- Nuijts, R.M.; Jonker, S.M.; Kaufer, R.A.; Lapid-Gortzak, R.; Mendicute, J.; Martinez, C.P.; Schmickler, S.; Kohnen, T. Bilateral implantation of +2.5 D multifocal intraocular lens and contralateral implantation of +2.5 D and +3.0 D multifocal intraocular lenses: Clinical outcomes. J. Cataract. Refract. Surg. 2016, 42, 194–202. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, A.L.; de Araújo Rolim, A.G.; Motta, A.F.P.; Ventura, B.V.; Vilar, C.; Chaves, M.A.P.D.; Carricondo, P.C.; Hida, W.T. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin. Ophthalmol. 2017, 11, 1911–1916. [Google Scholar] [CrossRef]
- Hayashi, K.; Sato, T.; Igarashi, C.; Yoshida, M. Comparison of visual outcomes between bilateral trifocal intraocular lenses and combined bifocal intraocular lenses with different near addition. Jpn. J. Ophthalmol. 2019, 63, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Acar, B.; Nurozler Tabakci, B. Clinical outcome comparison: Bilateral trifocal vs. mix-match extended depth of focus and trifocal intraocular lenses. Int. Ophthalmol. 2021, 41, 3675–3686. [Google Scholar] [CrossRef]
- Kim, J.W.; Eom, Y.; Park, W.; Song, J.S.; Jeong, J.W.; Park, S.K.; Kim, H.M. Comparison of visual outcomes after two types of mix-and-match implanted trifocal extended-depth-of-focus and trifocal intraocular lenses. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3275–3283. [Google Scholar] [CrossRef]
- Vilar, C.; Hida, W.T.; de Medeiros, A.L.; Magalhães, K.R.P.; de Moraes Tzelikis, P.F.; Chaves, M.A.P.D.; Motta, A.F.P.; Carricondo, P.C.; Alves, M.R.; Nosé, W. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin. Ophthalmol. 2017, 11, 1393–1397. [Google Scholar] [CrossRef]
- Passi, S.F.; Thompson, A.C.; Gupta, P.K. Comparison of agreement and efficiency of a swept source-optical coherence tomography device and an optical low-coherence reflectometry device for biometry measurements during cataract evaluation. Clin. Ophthalmol. 2018, 12, 2245–2251. [Google Scholar] [CrossRef]
- Schuster, A.K.; Tesarz, J.; Vossmerbaeumer, U. Ocular wavefront analysis of aspheric compared with spherical monofocal intraocular lenses in cataract surgery: Systematic review with metaanalysis. J. Cataract. Refract. Surg. 2015, 41, 1088–1097. [Google Scholar] [CrossRef]
- Qi, Y.; Lin, J.; Leng, L.; Zhao, G.; Wang, Q.; Li, C.; Hu, L. Role of angle κ in visual quality in patients with a trifocal diffractive intraocular lens. J. Cataract. Refract. Surg. 2018, 44, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Louzada, R.N.; Alves, M.R. Sobre a necessidade de políticas nacionais de educação em saúde ocular no Brasil. eOftalmo 2024, 10, 1–4. [Google Scholar] [CrossRef]
- Martínez Palmer, A.; Gómez Faiña, P.; España Albelda, A.; Comas Serrano, M.; Nahra Saad, D.; Castilla Céspedes, M. Visual function with bilateral implantation of monofocal and multifocal intraocular lenses: A prospective, randomized, controlled clinical trial. J. Refract. Surg. 2008, 24, 257–264. [Google Scholar] [CrossRef]
- Azen, S.P.; Varma, R.; Preston-Martin, S.; Ying-Lai, M.; Globe, D.; Hahn, S. Binocular visual acuity summation and inhibition in an ocular epidemiological study: The Los Angeles Latino Eye Study. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1742–1748. [Google Scholar]
- Barbot, A.; Pirog, J.T.; Ng, C.J.; Yoon, G. Neural adaptation to the eye’s optics through phase compensation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Frampton, G.; Harris, P.; Cooper, K.; Lotery, A.; Shepherd, J. The clinical effectiveness and cost-effectiveness of second-eye cataract surgery: A systematic review and economic evaluation. Health Technol. Assess. 2014, 18, 1–206. [Google Scholar] [CrossRef] [PubMed]
- Tarib, I.; Kasier, I.; Herbers, C.; Hagen, P.; Breyer, D.; Kaymak, H.; Klabe, K.; Lucchesi, R.; Teisch, S.; Diakonis, V.F.; et al. Comparison of Visual Outcomes and Patient Satisfaction After Bilateral Implantation of an EDOF IOL and a Mix-and-Match Approach. J. Refract. Surg. 2019, 35, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.R.; Pineda, R. Intraoperative aberrometry: An update on applications and outcomes. Curr. Opin. Ophthalmol. 2023, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).