An Integrated Approach for Designing and Analyzing Lumbar Vertebral Biomodels with Artificial Disc Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Model Generation
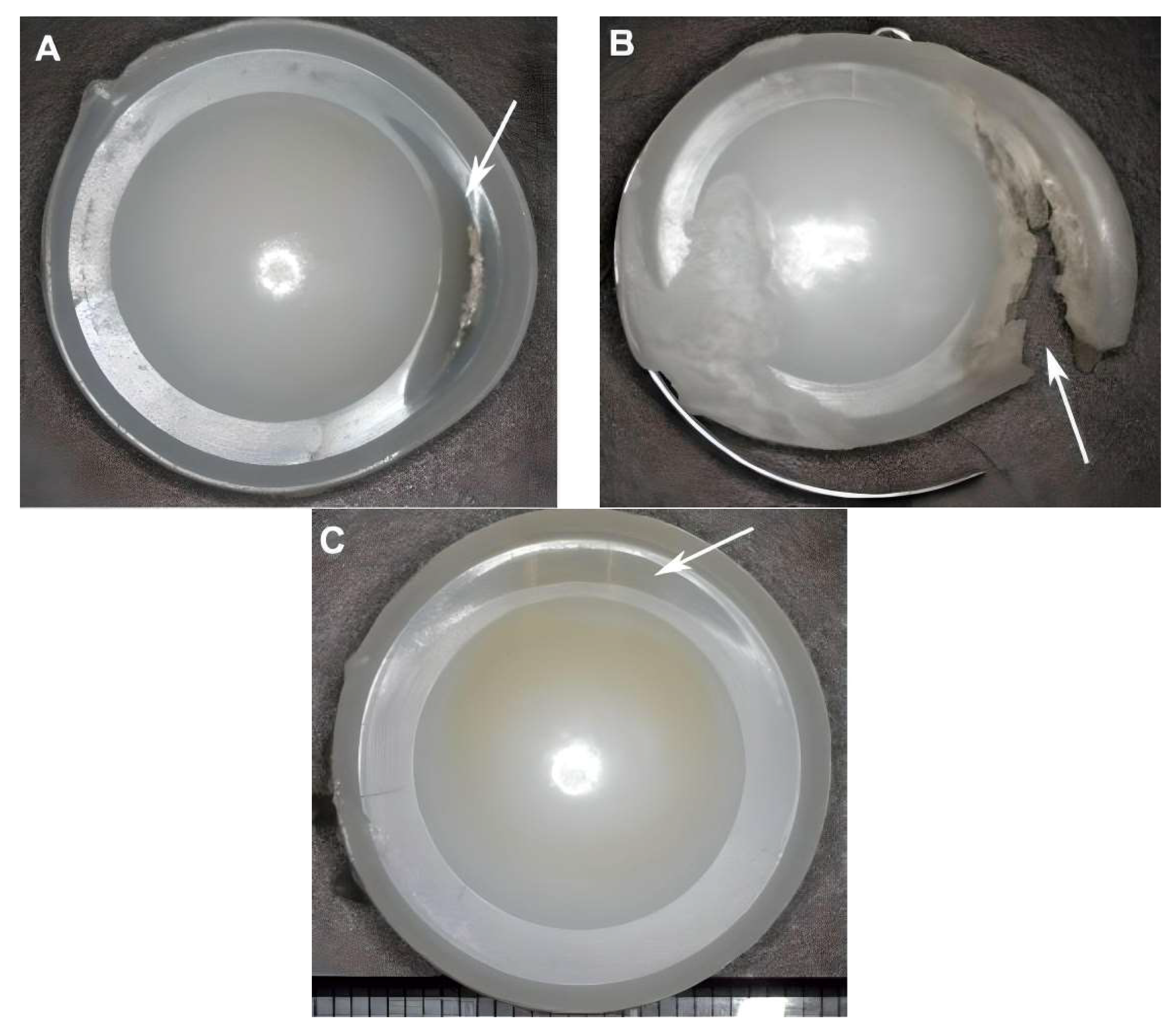
2.3. Validation Study
2.4. Boundary Conditions
2.5. Materials Properties
3. Results
3.1. Range of Motion
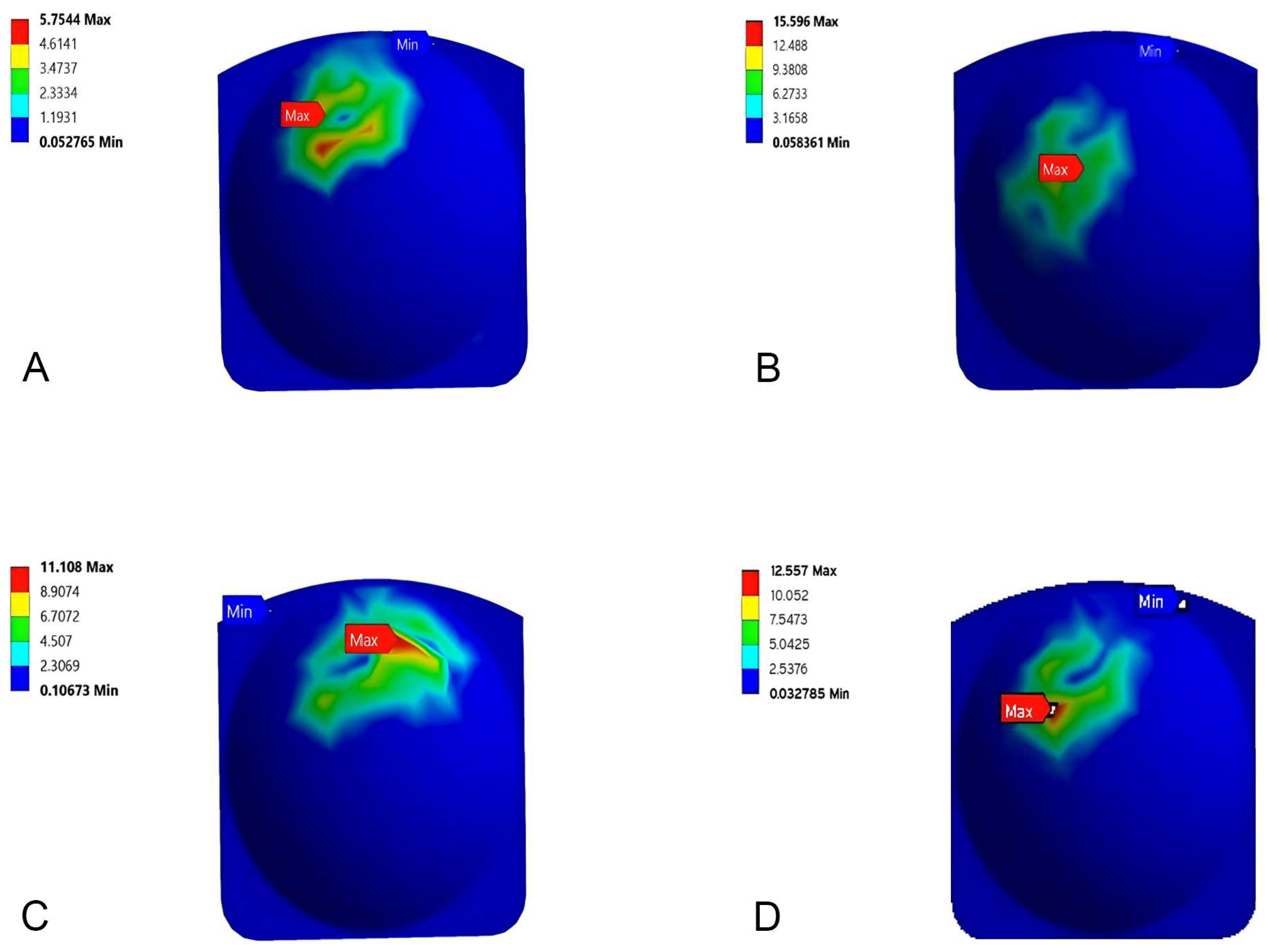
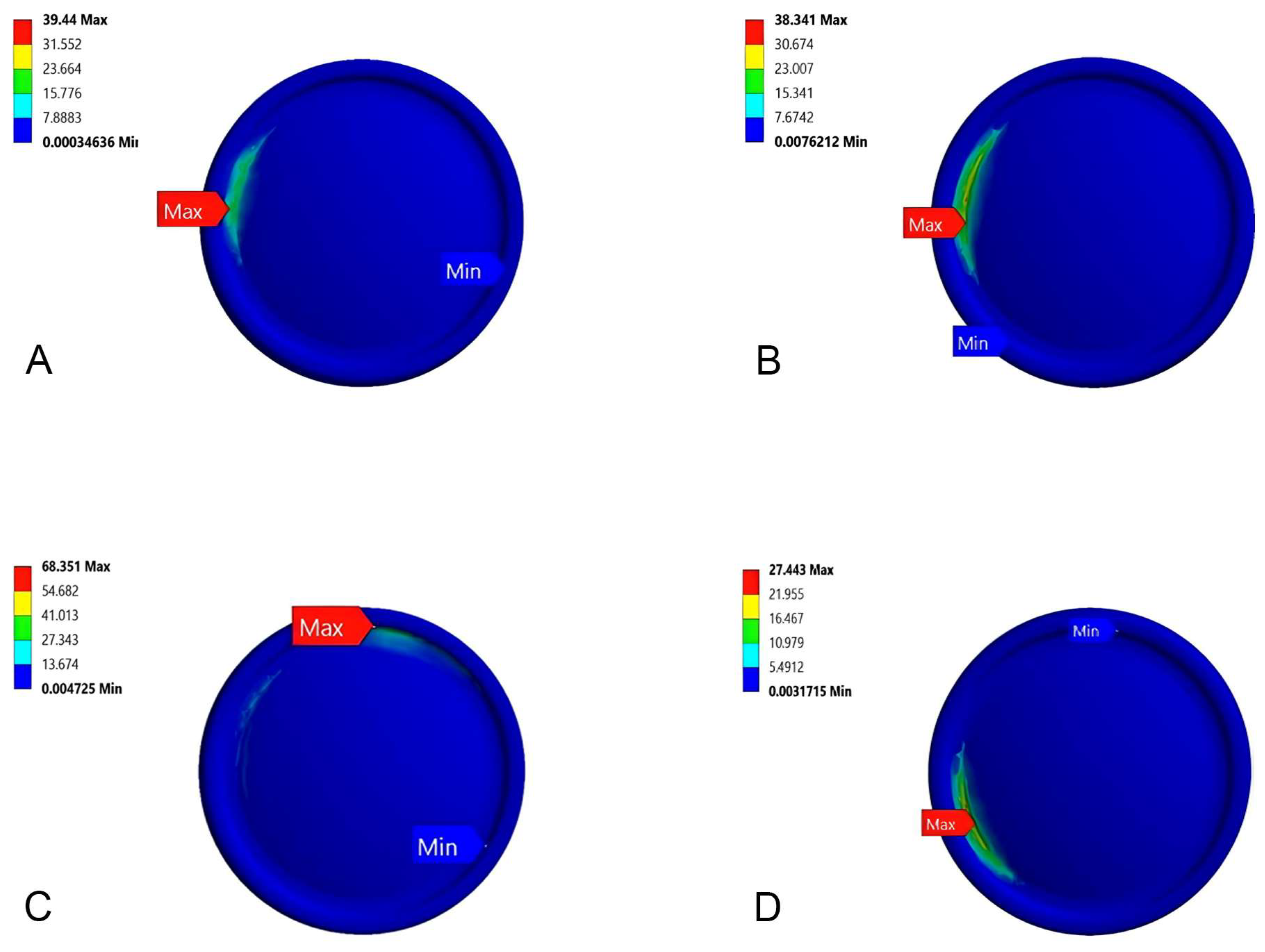
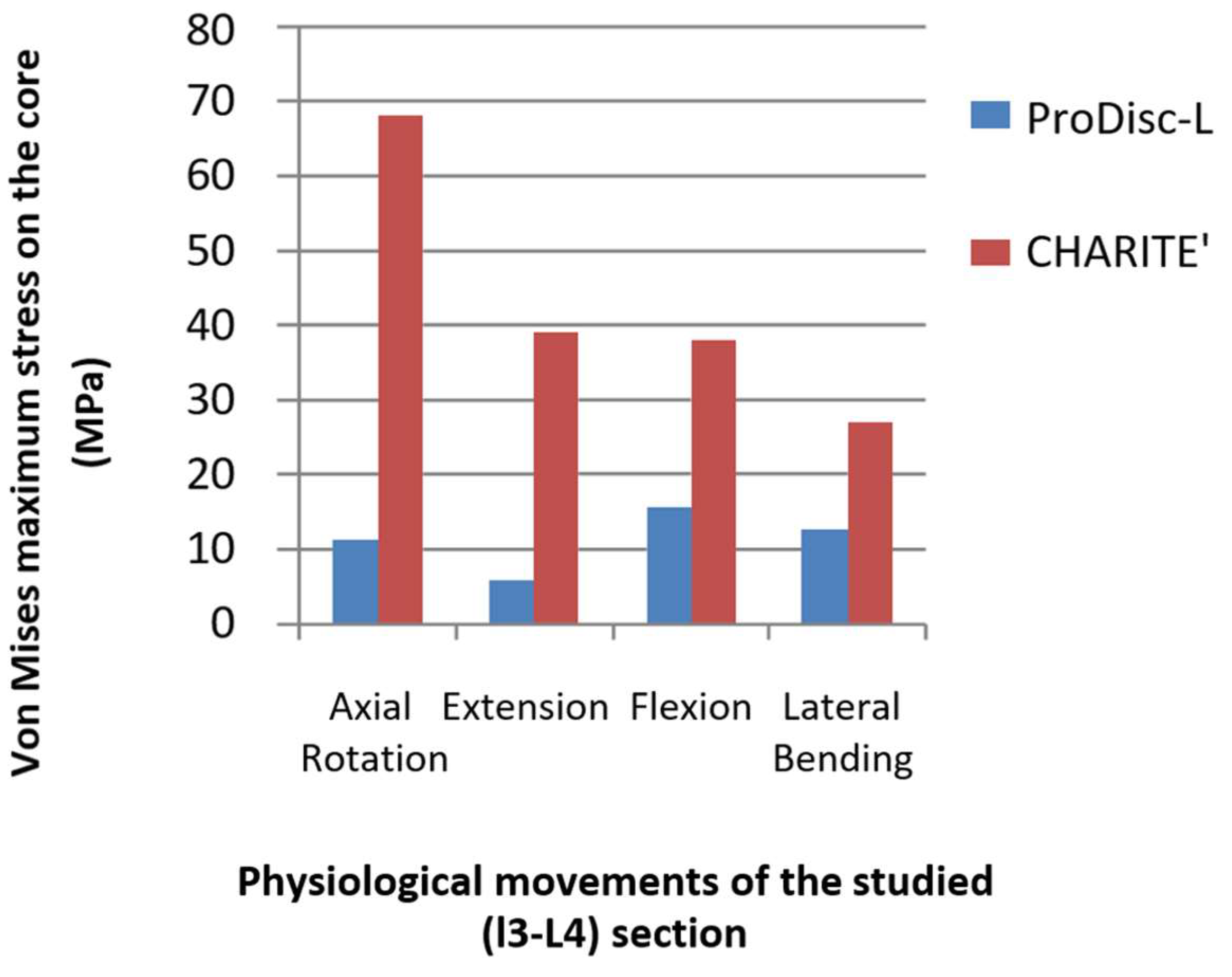
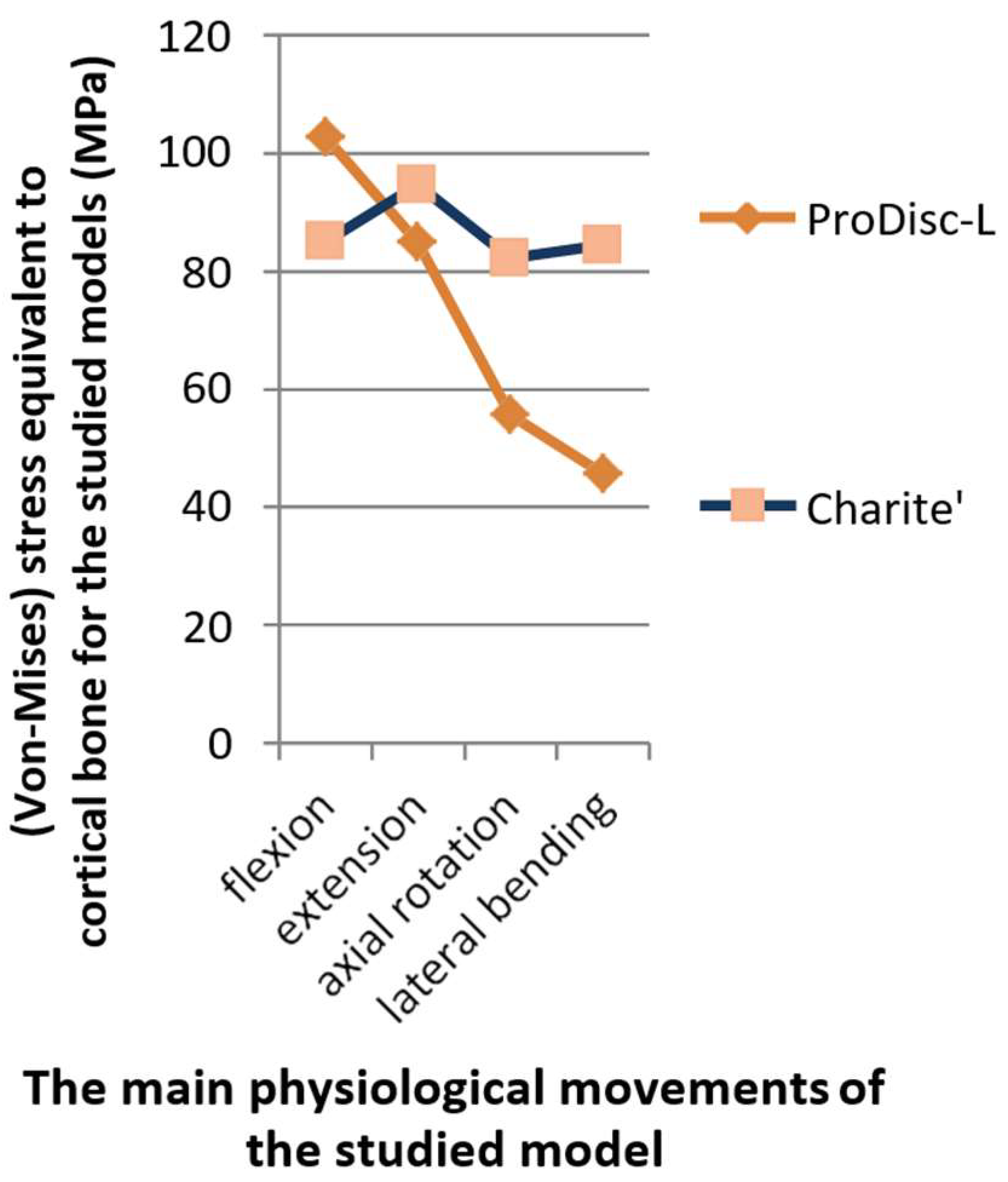
3.2. Stress Distribution
3.3. Bone Fracture Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ament, J.D.; Yang, Z.; Nunley, P.; Stone, M.B.; Kim, K.D. Cost-Effectiveness of Cervical Total Disc Replacement vs. Fusion for the Treatment of 2-Level Symptomatic Degenerative Disc Disease. JAMA Surg. 2014, 149, 1231–1239. [Google Scholar] [CrossRef]
- Patel, V.V.; Wuthrich, Z.R.; McGilvray, K.C.; Lafleur, M.C.; Lindley, E.M.; Sun, D.; Puttlitz, C.M. Cervical Facet Force Analysis after Disc Replacement versus Fusion. Clin. Biomech. 2017, 44, 52–58. [Google Scholar] [CrossRef] [PubMed]
- DiAngelo, D.J.; Chung, C.; Hoyer, D.; Carson, T.; Foley, K.T. 164. Biomechanical Analysis of the Endplate Fixation Methods of Cervical Total Disc Replacement (TDR) Prostheses to Shear Force Expulsion. Spine J. 2021, 21, S82–S83. [Google Scholar] [CrossRef]
- Kim, D.-W.; Lee, K.-Y.; Jun, Y.; Lee, S.J.; Park, C.K. Friction and Wear Characteristics of UHMWPE against Co-Cr Alloy under the Wide Range of Contact Pressures in Lumbar Total Disc Replacement. Int. J. Precis. Eng. Manuf. 2011, 12, 1111–1118. [Google Scholar] [CrossRef]
- Lazennec, J.Y. Lumbar and Cervical Viscoelastic Disc Replacement: Concepts and Current Experience. World J. Orthop. 2020, 11, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Reeks, J.; Liang, H. Materials and Their Failure Mechanisms in Total Disc Replacement. Lubricants 2015, 3, 346–364. [Google Scholar] [CrossRef]
- Kölle, L.; Ignasiak, D.; Ferguson, S.J.; Helgason, B. Ceramics in Total Disc Replacements: A Scoping Review. Clin. Biomech. 2022, 100, 105796. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Camarero-Espinosa, S.; Foster, E. Materials for the Spine: Anatomy, Problems, and Solutions. Materials 2019, 12, 253. [Google Scholar] [CrossRef]
- Pham, M.H.; Mehta, V.A.; Tuchman, A.; Hsieh, P.C. Material Science in Cervical Total Disc Replacement. Biomed Res. Int. 2015, 2015, 719123. [Google Scholar] [CrossRef]
- Biswas, J.K.; Malas, A.; Majumdar, S.; Rana, M. A Comparative Finite Element Analysis of Artificial Intervertebral Disc Replacement and Pedicle Screw Fixation of the Lumbar Spine. Comput. Methods Biomech. Biomed. Engin. 2022, 25, 1812–1820. [Google Scholar] [CrossRef]
- Coric, D.; Parish, J.; Boltes, M.O. M6-C Artificial Disc Placement. Neurosurg. Focus 2017, 42, V6. [Google Scholar] [CrossRef]
- Stephen, D.G.; Prakash; Das, N.K.; Shukla, S. Wear Performance of UHMWPE and PCU Artificial Disc Materials. J. Inst. Eng. (India) Ser. D 2022, 103, 383–394. [Google Scholar] [CrossRef]
- Beatty, S. We Need to Talk about Lumbar Total Disc Replacement. Int. J. Spine Surg. 2018, 12, 201–240. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, K.; Allain, J.; Longis, P.-M.; Steib, J.-P.; Beaurain, J.; Delécrin, J. Comparison between Total Disc Replacement and Hybrid Construct at Two Lumbar Levels with Minimum Follow-up of Two Years. Orthop. Traumatol. Surg. Res. 2017, 103, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Plais, N.; Thevenot, X.; Cogniet, A.; Rigal, J.; Le Huec, J.C. Maverick Total Disc Arthroplasty Performs Well at 10 Years Follow-up: A Prospective Study with HRQL and Balance Analysis. Eur. Spine J. 2018, 27, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Kiyani, S.; Taheri-Behrooz, F.; Asadi, A. Analytical and Finite Element Analysis of Shape Memory Polymer for Use in Lumbar Total Disc Replacement. J. Mech. Behav. Biomed. Mater. 2021, 122, 104689. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Treon, K.; Craig, N.J.A. Low Back Pain: Current Surgical Approaches. Asian Spine J. 2015, 9, 645–657. [Google Scholar] [CrossRef]
- Hollenbeck, J.F.M.; Fattor, J.A.; Patel, V.; Burger, E.; Rullkoetter, P.J.; Cain, C.M.J. Validation of Pre-Operative Templating for Total Disc Replacement Surgery. Int. J. Spine Surg. 2019, 13, 84–91. [Google Scholar] [CrossRef]
- Prokopovich, P.; Perni, S.; Fisher, J.; Hall, R.M. Spatial Variation of Wear on Charité Lumbar Discs. Acta Biomater. 2011, 7, 3914–3926. [Google Scholar] [CrossRef]
- Siskey, R.; Peck, J.; Mehta, H.; Kosydar, A.; Kurtz, S.; Hill, G. Development of a Clinically Relevant Impingement Test Method for a Mobile Bearing Lumbar Total Disc Replacement. Spine J. 2016, 16, 1133–1142. [Google Scholar] [CrossRef]
- Alexander, T.; Antonis, L.; Savvas, S.; Nikolaos, M. Nonintrusive 3D Reconstruction of Human Bone Models to Simulate Their Bio-Mechanical Response. 3D Res. 2012, 3, 5. [Google Scholar] [CrossRef]
- Rundell, S.A.; Auerbach, J.D.; Balderston, R.A.; Kurtz, S.M. Total Disc Replacement Positioning Affects Facet Contact Forces and Vertebral Body Strains. Spine 2008, 33, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.Z.; Goel, V.K. Ability of the Finite Element Models to Predict Response of the Human Spine to Sinusoidal Vertical Vibration. Spine 2003, 28, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, D.-A.; Kim, S. Biomechanical Effects of the Geometry of Ball-and-Socket Artificial Disc on Lumbar Spine: A Finite Element Study: A Finite Element Study. Spine 2017, 42, E332–E339. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, W.; Wang, Z.-H.; Wei, H.-W. Clinical Study of a New Transpedicular Nonfusion Posterior Dynamic Stabilization System for Treating Herniated Lumbar Intervertebral Disks. Neurosurg. Q. 2015, 25, 427–431. [Google Scholar] [CrossRef]
- Auerbach, J.D.; Ballester, C.M.; Hammond, F.; Carine, E.T.; Balderston, R.A.; Elliott, D.M. The Effect of Implant Size and Device Keel on Vertebral Compression Properties in Lumbar Total Disc Replacement. Spine J. 2010, 10, 333–340. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Kuo, Y.-J.; Chen, C.-H.; Wu, L.-C.; Chiang, C.-J.; Lin, C.-L. Biomechanical Assessment of Vertebroplasty Combined with Cement-Augmented Screw Fixation for Lumbar Burst Fractures: A Finite Element Analysis. Appl. Sci. 2020, 10, 2133. [Google Scholar] [CrossRef]
- Goreham-Voss, C.M.; Hyde, P.J.; Hall, R.M.; Fisher, J.; Brown, T.D. Cross-Shear Implementation in Sliding-Distance-Coupled Finite Element Analysis of Wear in Metal-on-Polyethylene Total Joint Arthroplasty: Intervertebral Total Disc Replacement as an Illustrative Application. J. Biomech. 2010, 43, 1674–1681. [Google Scholar] [CrossRef]
- Niosi, C.A.; Zhu, Q.A.; Wilson, D.C.; Keynan, O.; Wilson, D.R.; Oxland, T.R. Biomechanical Characterization of the Three-Dimensional Kinematic Behaviour of the Dynesys Dynamic Stabilization System: An In Vitro Study. Eur. Spine J. 2006, 15, 913–922. [Google Scholar] [CrossRef]
- Noailly, J.; Planell, J.A.; Lacroix, D. On the Collagen Criss-Cross Angles in the Annuli Fibrosi of Lumbar Spine Finite Element Models. Biomech. Model. Mechanobiol. 2011, 10, 203–219. [Google Scholar] [CrossRef]
- Kurtz, S.M.; van Ooij, A.; Ross, R.; de Waal Malefijt, J.; Peloza, J.; Ciccarelli, L.; Villarraga, M.L. Polyethylene Wear and Rim Fracture in Total Disc Arthroplasty. Spine J. 2007, 7, 12–21. [Google Scholar] [CrossRef]
- Schuermans, V.N.E.; Smeets, A.Y.J.M.; van de Kar, L.G.C.; Hermans, S.M.M.; Curfs, I.; Boselie, T.F.M.; van Santbrink, H. A Systematic Review on Neurological Outcomes for Cervical Degenerative Myelopathy after Anterior Decompression Surgery: Motion Preservation vs. Fusion. Int. J. Spine Surg. 2022, 16, 969–976. [Google Scholar] [CrossRef]
- Kurtz, S.M.; MacDonald, D.; Ianuzzi, A.; van Ooij, A.; Isaza, J.; Ross, E.R.; Regan, J. The Natural History of Polyethylene Oxidation in Total Disc Replacement. Spine 2009, 34, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Highly Cross-Linked Ultrahigh Molecular Weight Polyethylene with Improved Fatigue Resistance for Total Joint Arthroplasty: Recipient of the 2006 Hap Paul Award. J. Arthroplasty 2008, 23, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical Basis of Bone Strength: Influence of Bone Material, Bone Structure and Muscle Action. J. Musculoskelet. Neuron. Interact. 2017, 17, 114–139. [Google Scholar]
- Karim, L.; Vashishth, D. Role of Trabecular Microarchitecture in the Formation, Accumulation, and Morphology of Microdamage in Human Cancellous Bone: Trabecular Microarchitecture Affects Microdamage. J. Orthop. Res. 2011, 29, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Maknickas, A.; Alekna, V.; Ardatov, O.; Chabarova, O.; Zabulionis, D.; Tamulaitienė, M.; Kačianauskas, R. FEM-Based Compression Fracture Risk Assessment in Osteoporotic Lumbar Vertebra L1. Appl. Sci. 2019, 9, 3013. [Google Scholar] [CrossRef]
- Kundu, J.; Pati, F.; Shim, J.-H.; Cho, D.-W. Rapid Prototyping Technology for Bone Regeneration. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 254–284. ISBN 9780857095992. [Google Scholar]
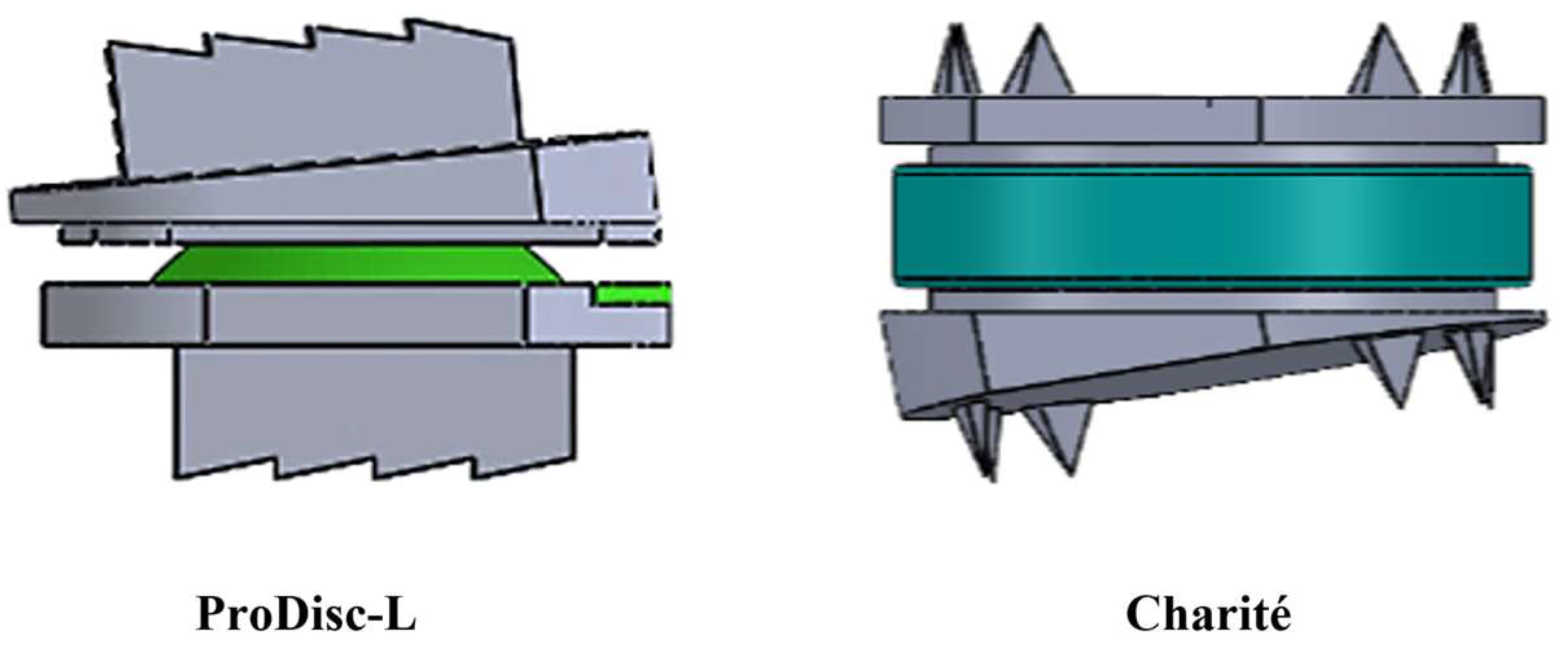
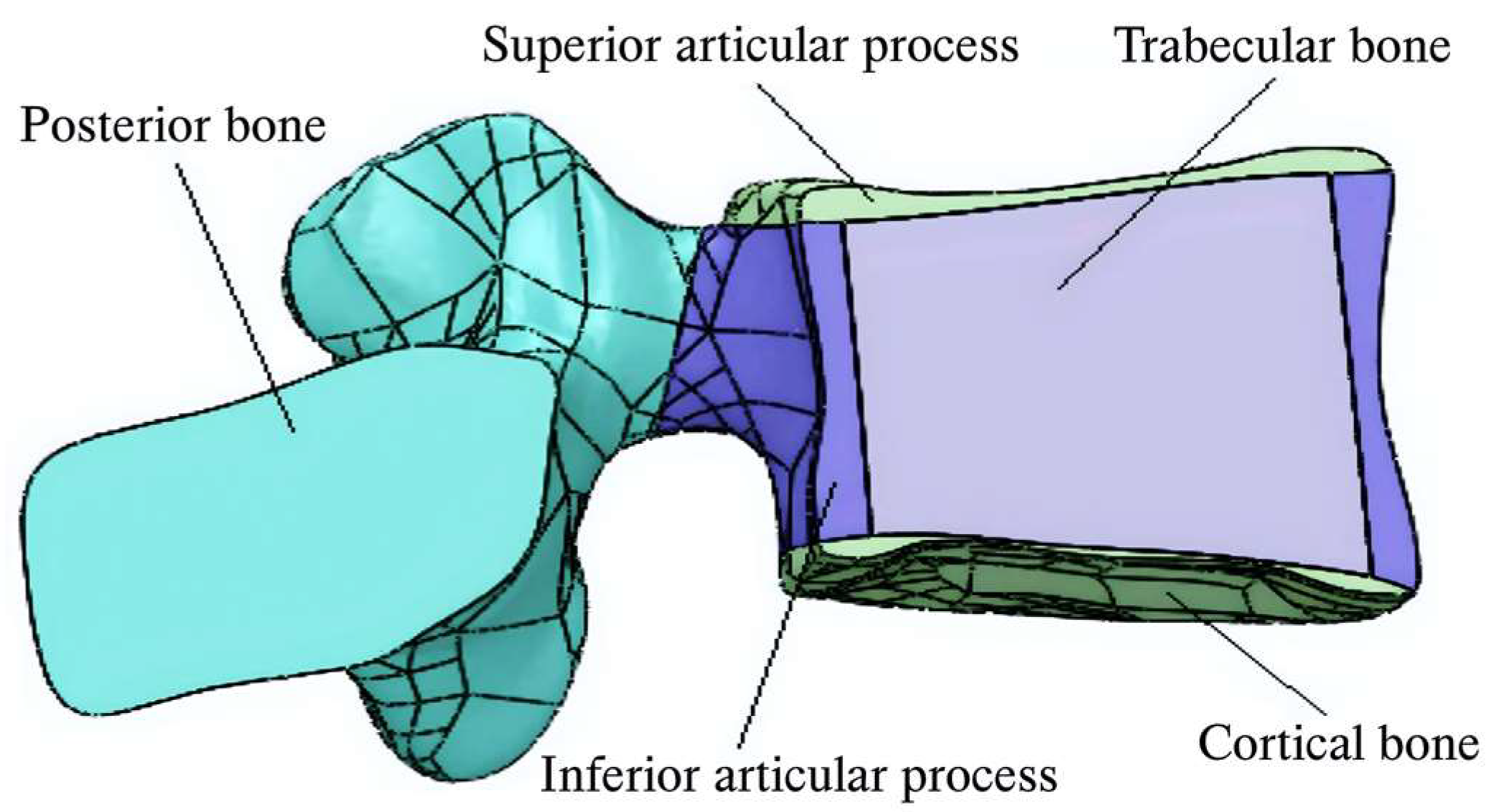
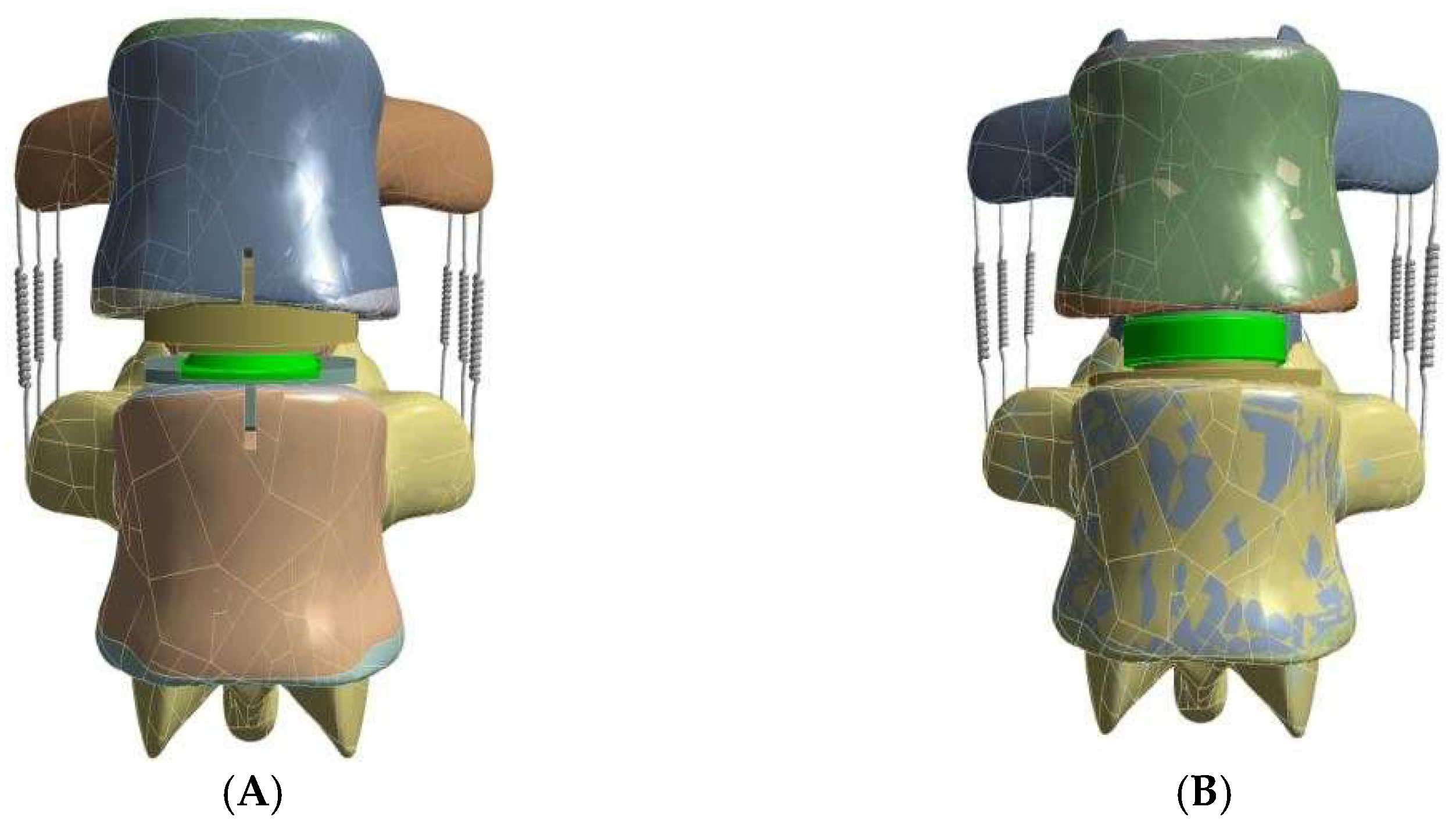
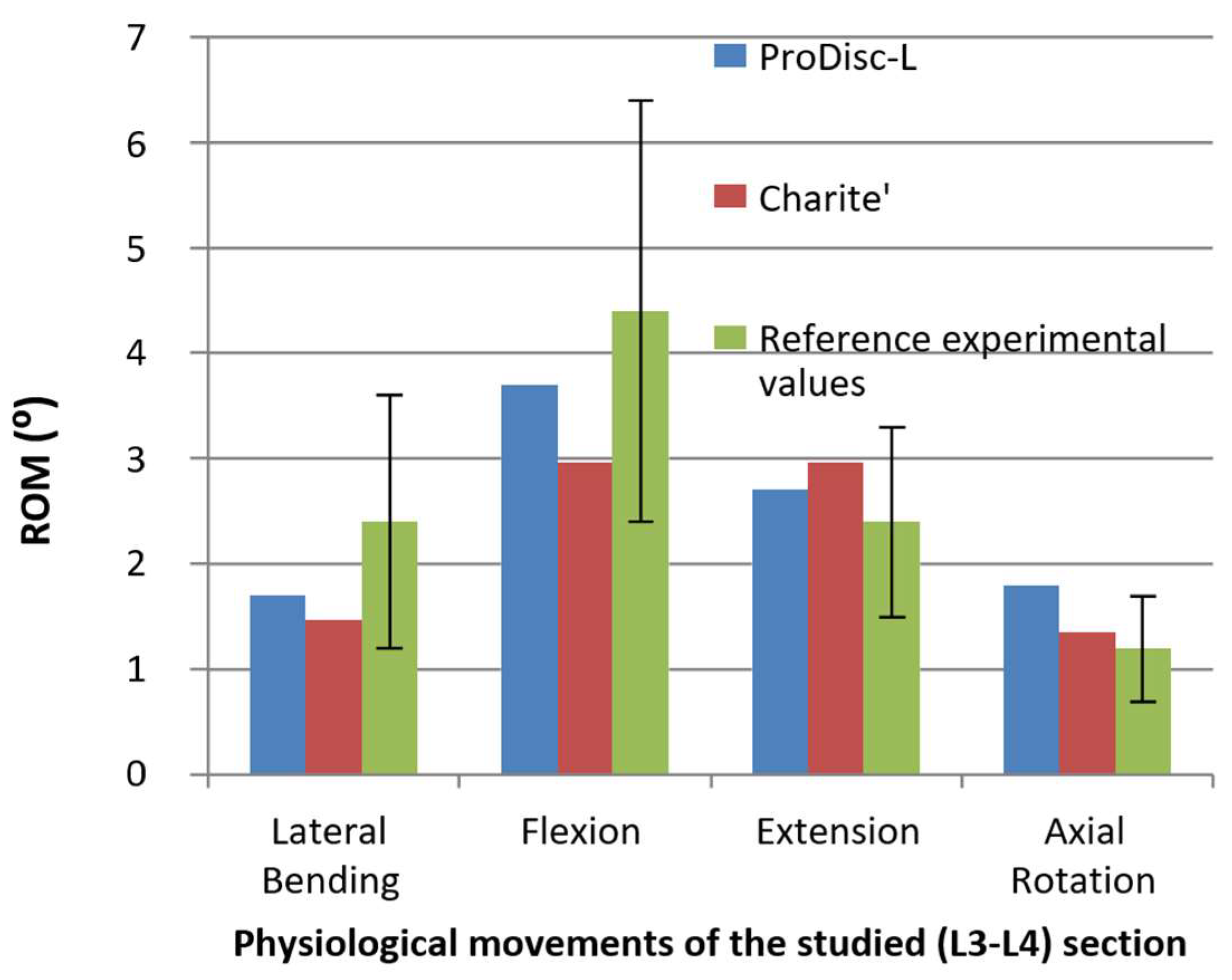
| Component | Elements | Nodes | Component | Elements | Nodes | ||
|---|---|---|---|---|---|---|---|
| L3 | Cortical bone | 3135 | 6148 | L3 | Cortical bone | 3299 | 6465 |
| Cancellous bone | 528 | 1062 | Cancellous bone | 372 | 787 | ||
| Lower endplate1 | 991 | 2100 | Lower endplate | 2287 | 4582 | ||
| Lower endplate2 | 1218 | 2469 | Upper endplate | 1215 | 2563 | ||
| Upper endplate | 1139 | 2391 | Posterior bone | 6643 | 12,227 | ||
| Posterior bone | 4885 | 9059 | Cortical bone | 2259 | 4560 | ||
| L4 | Cortical bone | 2502 | 4947 | L4 | Cancellous bone | 503 | 1022 |
| Cancellous bone | 556 | 1103 | Lower endplate | 1321 | 2801 | ||
| Lower endplate | 915 | 1966 | Upper endplate | 635 | 1438 | ||
| Upper endplate1 | 459 | 1054 | Posterior bone | 5720 | 10,467 | ||
| Upper endplate2 | 412 | 968 | Core | 29,517 | 48,609 | ||
| Posterior bone | 3584 | 6662 | Lower endplate | 373 | 1163 | ||
| ProDisc-L | Core | 2555 | 1521 | CHARITÉ | Upper endplate | 378 | 1196 |
| Lower endplate | 148 | 369 | Cortical bone | 3299 | 6465 | ||
| Upper endplate | 307 | 734 | Cancellous bone | 372 | 787 | ||
| Material | Density (g/cm3) | Young Modulus (MPa) | Poisson Ratio |
|---|---|---|---|
| Cortical bone | 1.7 | 12,000 | 0.3 |
| Cancellous bone | 1.1 | 100 | 0.2 |
| Posterior bone | 1.4 | 3500 | 0.25 |
| Upper and lower endplate | 1.7 | 12,000 | 0.3 |
| CoCrMo alloy | 8.9 | 210,000 | 0.3 |
| UHMWPE | 0.94 | 1200 | 0.46 |
| Material | Density (g/cm3) | Young Modulus (MPa) | Poisson Ratio | Cross-Section Area (mm2) |
|---|---|---|---|---|
| LF | 1 | 50 | 0.3 | 60 |
| TL | 1 | 50 | 0.3 | 10 |
| IS | 1 | 28 | 0.3 | 35.5 |
| SS | 1 | 28 | 0.3 | 35.5 |
| CL | 1 | 20 | 0.3 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwich, M.A.; Ebrahem, K.; Shash, M.; Nazha, H.M.; Szávai, S.; Zhang, Y.; Juhre, D. An Integrated Approach for Designing and Analyzing Lumbar Vertebral Biomodels with Artificial Disc Replacement. Appl. Mech. 2023, 4, 1227-1239. https://doi.org/10.3390/applmech4040063
Darwich MA, Ebrahem K, Shash M, Nazha HM, Szávai S, Zhang Y, Juhre D. An Integrated Approach for Designing and Analyzing Lumbar Vertebral Biomodels with Artificial Disc Replacement. Applied Mechanics. 2023; 4(4):1227-1239. https://doi.org/10.3390/applmech4040063
Chicago/Turabian StyleDarwich, Mhd Ayham, Katreen Ebrahem, Maysaa Shash, Hasan Mhd Nazha, Szabolcs Szávai, Yicha Zhang, and Daniel Juhre. 2023. "An Integrated Approach for Designing and Analyzing Lumbar Vertebral Biomodels with Artificial Disc Replacement" Applied Mechanics 4, no. 4: 1227-1239. https://doi.org/10.3390/applmech4040063
APA StyleDarwich, M. A., Ebrahem, K., Shash, M., Nazha, H. M., Szávai, S., Zhang, Y., & Juhre, D. (2023). An Integrated Approach for Designing and Analyzing Lumbar Vertebral Biomodels with Artificial Disc Replacement. Applied Mechanics, 4(4), 1227-1239. https://doi.org/10.3390/applmech4040063





