Predicting Clinical Outcomes and Symptom Relief in Uterine Fibroid Embolization Using Machine Learning on MRI Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Curation
2.2. Machine Learning Model Pipeline
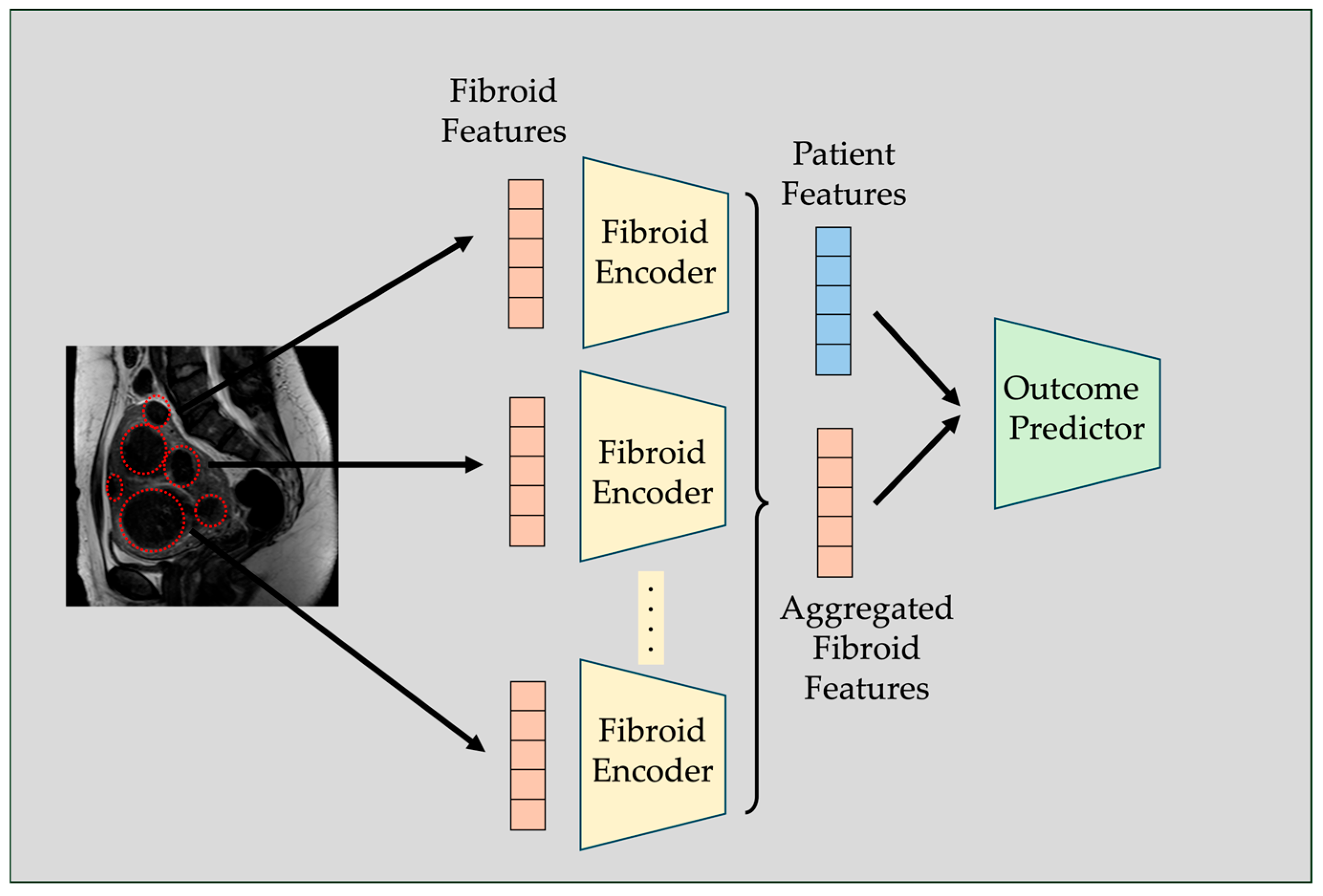
2.3. Fibroid-Level Prediction
3. Results
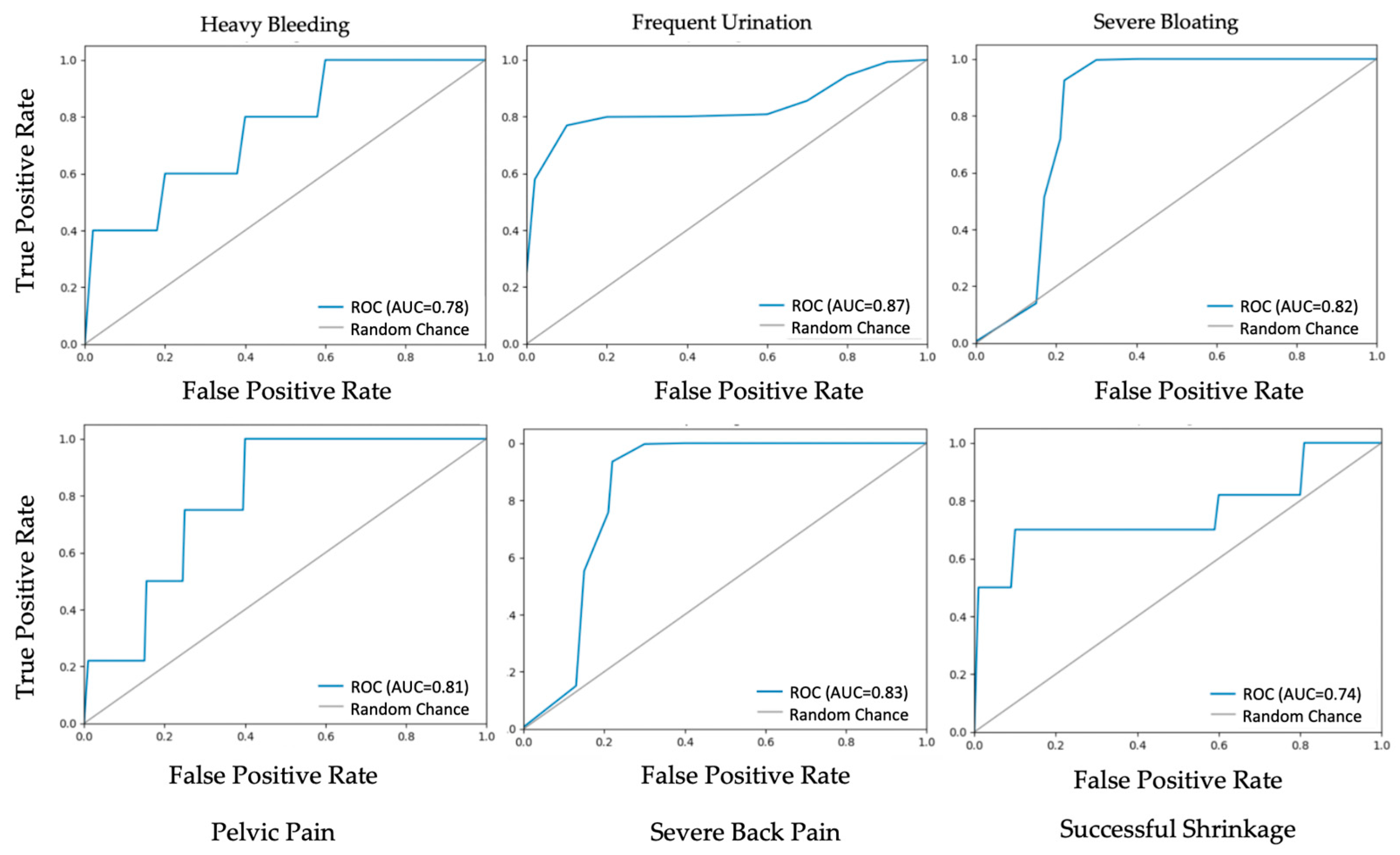
3.1. Procedure Outcome and Post-Op Symptom Prediction
3.2. Ablation Study
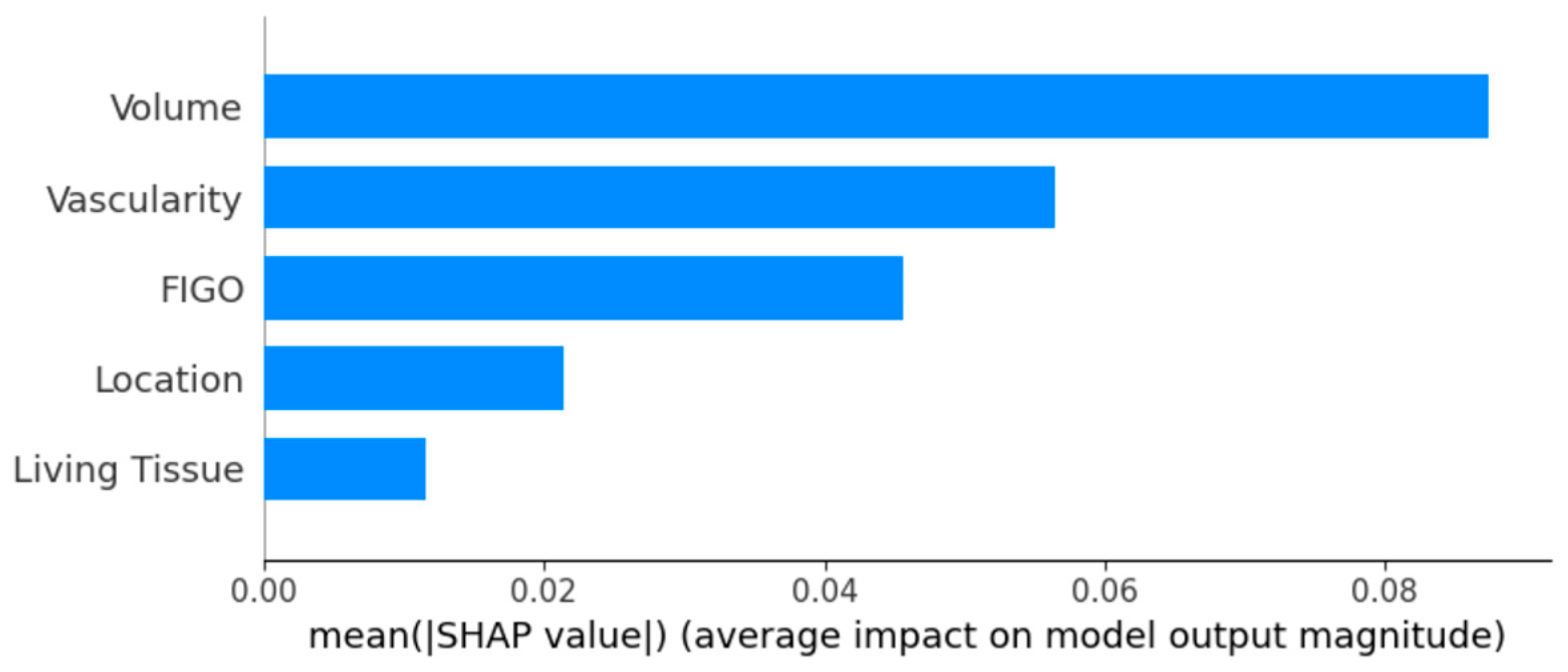
3.3. Individual Fibroid Outcome Prediction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar]
- Stewart, E.A.; Nicholson, W.K.; Bradley, L.; Borah, B.J. The burden of uterine fibroids for African-American women: Results of a national survey. J. Women’s Health 2013, 22, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Hazimeh, D.; Coco, A.; Casubhoy, I.; Segars, J.; Singh, B. The Annual Economic Burden of Uterine Fibroids in the United States (2010 Versus 2022): A Comparative Cost-Analysis. Reprod. Sci. 2024, 31, 3743–3756. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.; Scott-Barrett, S. Radiological appearances of uterine fibroids. Indian J. Radiol. Imaging 2009, 19, 222–231. [Google Scholar] [CrossRef]
- El Sabeh, M.; Borahay, M.A. The Future of Uterine Fibroid Management: A More Preventive and Personalized Paradigm. Reprod. Sci. 2021, 28, 3285–3288. [Google Scholar] [CrossRef]
- Mension, E.; Calaf, J.; Chapron, C.; Dolmans, M.M.; Donnez, J.; Marcellin, L.; Petraglia, F.; Vannuccini, S.; Carmona, F. An update on the management of uterine fibroids: Personalized medicine or guidelines? J. Endometr. Uterine Disord. 2024, 7, 100080. [Google Scholar] [CrossRef]
- Mettler, L.; Schollmeyer, T.; Tinelli, A.; Malvasi, A.; Alkatout, I. Complications of Uterine Fibroids and Their Management, Surgical Management of Fibroids, Laparoscopy and Hysteroscopy versus Hysterectomy, Haemorrhage, Adhesions, and Complications. Obstet. Gynecol. Int. 2012, 2012, 791248. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- Young, M.; Mikes, B.A. Uterine Fibroid Embolization. In StatPearls; StatPearls Publishing LLC: Petersburg, FL, USA, 2025. [Google Scholar]
- Mas, A.; Tarazona, M.; Dasí Carrasco, J.; Estaca, G.; Cristóbal, I.; Monleón, J. Updated approaches for management of uterine fibroids. Int. J. Womens Health 2017, 9, 607–617. [Google Scholar] [CrossRef]
- Balamurugan, S.; Shah, R.; Panganiban, K.; Lehrack, M.; Agrawal, D.K. Uterine Artery Embolization: A Growing Pillar of Gynecological Intervention. J. Radiol. Clin. Imaging 2025, 8, 1–17. [Google Scholar] [CrossRef]
- Raikhlin, A.; Baerlocher, M.O.; Asch, M.R. Uterine fibroid embolization: CME update for family physicians. Can. Fam. Physician 2007, 53, 250–256. [Google Scholar]
- Kubiszewski, K.; Maag, B.; Hunsaker, P.; Iwai, T.; Lim, J.; Duffey, E.; Varlamov, A.; Yoo, R. Investigating the Underutilization of Uterine Fibroid Embolization by Surveying Practice Preferences of Obstetricians/Gynecologists. J. Vasc. Interv. Radiol. 2023, 34, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.K.; Hwang, G.; Jahed, A.; Modanlou, S.; Chen, B. Abdominal myomectomy versus uterine fibroid embolization in the treatment of symptomatic uterine leiomyomas. Am. J. Roentgenol. 2003, 180, 1571–1575. [Google Scholar] [CrossRef]
- Lukies, M.; Clements, W. Current Strategies for Prevention of Infection After Uterine Artery Embolisation. Cardiovasc. Interv. Radiol. 2022, 45, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Dumousset, E.; Chabrot, P.; Rabischong, B.; Mazet, N.; Nasser, S.; Darcha, C.; Garcier, J.M.; Mage, G.; Boyer, L. Preoperative uterine artery embolization (PUAE) before uterine fibroid myomectomy. Cardiovasc. Interv. Radiol. 2008, 31, 514–520. [Google Scholar] [CrossRef]
- LaMorte, A.I.; Lalwani, S.; Diamond, M.P. Morbidity associated with abdominal myomectomy. Obstet. Gynecol. 1993, 82, 897–900. [Google Scholar] [PubMed]
- Mailli, L.; Patel, S.; Das, R.; Chun, J.Y.; Renani, S.; Das, S.; Ratnam, L. Uterine artery embolisation: Fertility, adenomyosis and size—What is the evidence? CVIR Endovasc. 2023, 6, 8. [Google Scholar] [CrossRef]
- Kaufman, C.S. Improving Access to UFE: What Are the Barriers? Endovasc. Today 2022, 21, 74–76. [Google Scholar]
- Stiglic, G.; Kocbek, P.; Fijacko, N.; Zitnik, M.; Verbert, K.; Cilar, L. Interpretability of machine learning-based prediction models in healthcare. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2020, 10, e1379. [Google Scholar] [CrossRef]
- Tonekaboni, S.; Joshi, S.; McCradden, M.D.; Goldenberg, A. What clinicians want: Contextualizing explainable machine learning for clinical end use. In Proceedings of the Machine Learning for Healthcare Conference, Ann Arbor, MI, USA, 8–10 August 2019; pp. 359–380. [Google Scholar]
- Raji, H.; Tayyab, M.; Sui, J.; Mahmoodi, S.R.; Javanmard, M. Biosensors and machine learning for enhanced detection, stratification, and classification of cells: A review. Biomed. Microdevices 2022, 24, 26. [Google Scholar] [CrossRef]
- Raji, H.; Xie, P.; Mahmoodi, S.R.; Javanmard, M. Wireless power-up and readout from a label-free biosensor. Biomedical Microdevices. 2025, 27, 2. [Google Scholar] [CrossRef]
- Tinelli, A.; Morciano, A.; Sparic, R.; Hatirnaz, S.; Malgieri, L.E.; Malvasi, A.; D’Amato, A.; Baldini, G.M.; Pecorella, G. Artificial Intelligence and Uterine Fibroids: A Useful Combination for Diagnosis and Treatment. J. Clin. Med. 2025, 14, 3454. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, H.; Yang, G.; Li, F.; Wen, Y.; Zhang, Q.; Dillenseger, J.-L.; Coatrieux, J.-L. HIFUNet: Multi-class segmentation of uterine regions from MR images using global convolutional networks for HIFU surgery planning. IEEE Trans. Med. Imaging 2020, 39, 3309–3320. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, L.; Ma, S.; Zhong, Y.; He, Z.; Li, C.; Xiao, Z.; Zheng, Y.; Lv, F. DARU-Net: A dual attention residual U-Net for uterine fibroids segmentation on MRI. J. Appl. Clin. Med. Phys. 2023, 24, e13937. [Google Scholar] [CrossRef]
- Drukker, K.; Medved, M.; Harmath, C.B.; Giger, M.L.; Madueke-Laveaux, O.S. Radiomics and quantitative multi-parametric MRI for predicting uterine fibroid growth. J. Med. Imaging 2024, 11, 054501. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Zhou, X.; Yu, R.; Huang, N.; Chen, J. Machine learning models for prediction of NPVR ≥ 80% with HIFU ablation for uterine fibroids. Int. J. Hyperth. 2025, 42, 2473754. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, E.; Bayrak, O.-C.; Nadarajan, C.; Müslümanoğlu, M.-H.; Nguyen, M.-D.; Keserci, B. Role of machine learning algorithms in predicting the treatment outcome of uterine fibroids using high-intensity focused ultrasound ablation with an immediate nonperfused volume ratio of at least 90%. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8376–8394. [Google Scholar] [PubMed]
- Li, C.; He, Z.; Lv, F.; Liu, Y.; Hu, Y.; Zhang, J.; Liu, H.; Ma, S.; Xiao, Z. An interpretable MRI-based radiomics model predicting the prognosis of high-intensity focused ultrasound ablation of uterine fibroids. Insights Into Imaging 2023, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Z.; Lv, F.; Liao, H.; Xiao, Z. Predicting the Prognosis of HIFU Ablation of Uterine Fibroids Using a Deep Learning-Based 3D Super-Resolution DWI Radiomics Model: A Multicenter Study. Acad. Radiol. 2024, 31, 4996–5007. [Google Scholar] [CrossRef]
- Shen, L.; Huang, X.; Liu, Y.; Bai, S.; Wang, F.; Yang, Q. Multi-sequence magnetic resonance imaging radiomics combined with imaging features predicts the difficulty of HIFU treatment of uterine fibroids. Sci. Rep. 2025, 15, 3259. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Lei, B. Uterine artery embolization compared with high-intensity focused ultrasound ablation for the treatment of symptomatic uterine myomas: A systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 218–227. [Google Scholar] [CrossRef]
- Luo, Y.H.; Xi, I.L.; Wang, R.; Abdallah, H.O.; Wu, J.; Vance, A.Z.; Chang, K.; Kohi, M.; Jones, L.; Reddy, S.; et al. Deep Learning Based on MR Imaging for Predicting Outcome of Uterine Fibroid Embolization. J. Vasc. Interv. Radiol. 2020, 31, 1010–1017 e1013. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef]
- Duc, N.M.; Keserci, B. Review of influential clinical factors in reducing the risk of unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids. Diagn. Interv. Radiol. 2018, 24, 283–291. [Google Scholar] [CrossRef]
- Faye, N.; Pellerin, O.; Thiam, R.; Chammings, F.; Brisa, M.; Marques, E.; Cuénod, C.A.; Sapoval, M.; Fournier, L.S. Diffusion-weighted imaging for evaluation of uterine arterial embolization of fibroids. Magn. Reson. Med. 2013, 70, 1739–1747. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, M.D.; Jung, D.C.; Lee, M.; Won, J.Y.; Park, S.I.; Lee, D.Y.; Lee, K.H. Apparent diffusion coefficient of uterine leiomyoma as a predictor of the potential response to uterine artery embolization. J. Vasc. Interv. Radiol. 2013, 24, 1361–1365. [Google Scholar] [CrossRef]
- Zhang, Y.; Hare, J.; Prugel-Bennett, A. Deep Set Prediction Networks. Adv. Neural Inf. Process. Syst. 2019, 32, 3207–3217. [Google Scholar]
- Zaheer, M.; Kottur, S.; Ravanbakhsh, S.; Poczos, B.; Salakhutdinov, R.R.; Smola, A.J. Deep Sets. Adv. Neural Inf. Process. Syst. 2017, 30, 3391–3401. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4768–4777. [Google Scholar]
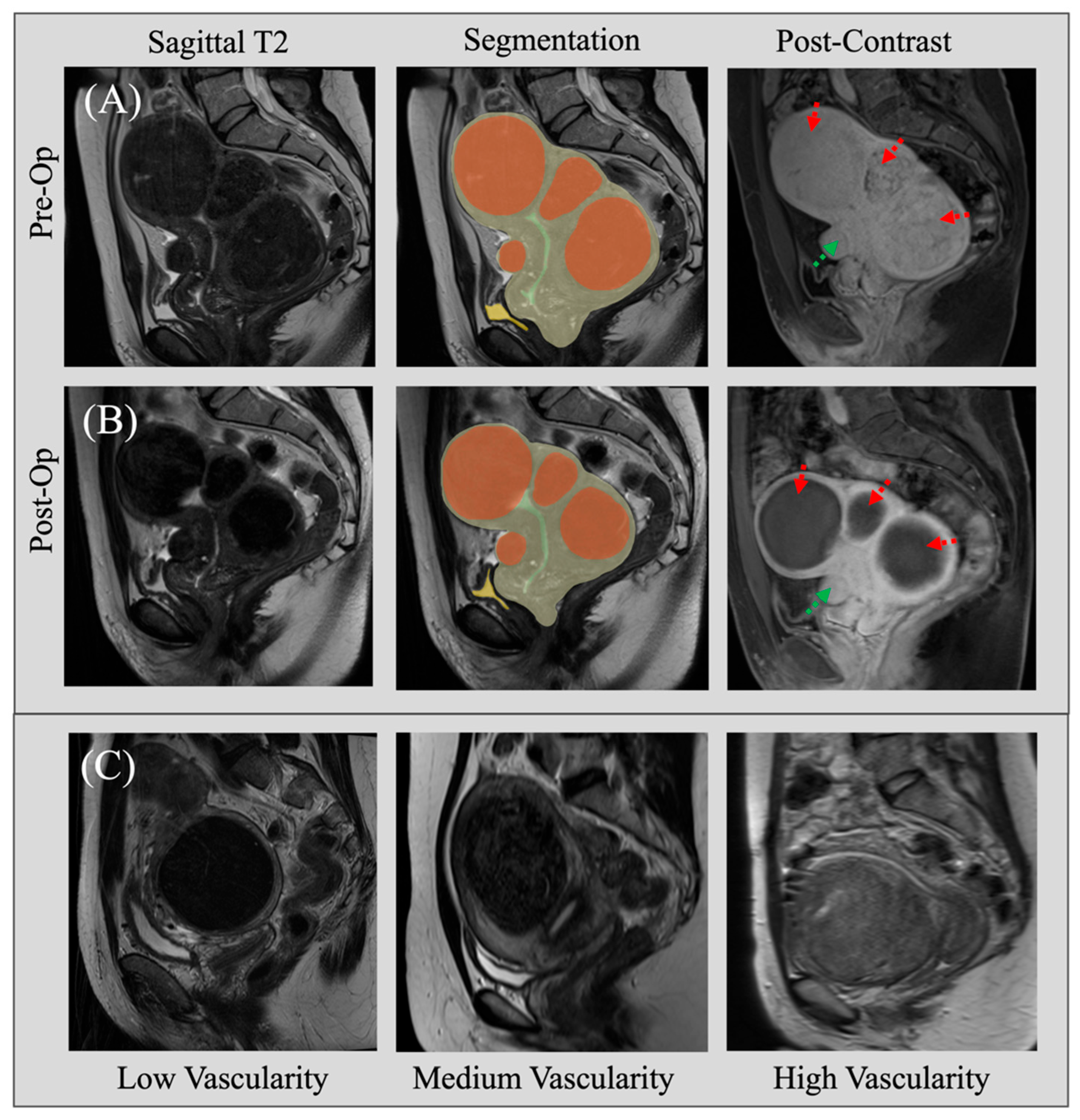
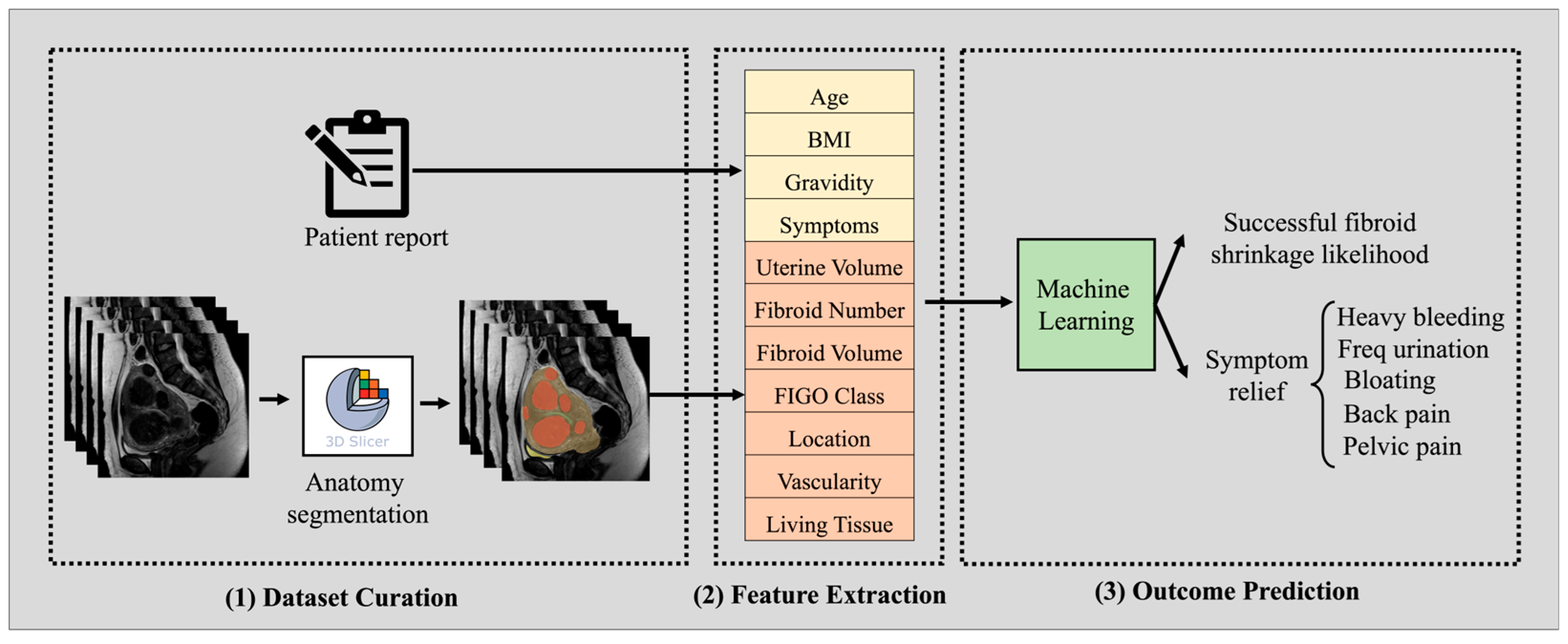
| Patient Cohort (n = 74) | Pre-Op | Post-Op |
|---|---|---|
| Uterus volume (cm3) | 629 ± 374 | 407 ± 237 |
| Fibroid number | 4.2 ± 3.4 | 3.8 ± 3.1 |
| Fibroid total volume (cm3) | 286 ± 243 | 160 ± 158 |
| Symptom Prevalence | ||
| 83.8% | 31.7% |
| 54.0% | 31.1% |
| 58.1% | 24.3% |
| 91.8% | 58.1% |
| 78.4% | 48.6% |
| Age (year) | 49.5 ± 7.0 | -- |
| Weight (kg) | 70.4 ± 15.9 | -- |
| BMI (kg/m2) | 25.9 ± 6.0 | -- |
| Gravidity | 1.7 ± 2.0 | -- |
| Racial Demographics | -- | |
| 28% | -- |
| 16% | -- |
| 13% | -- |
| 24% | -- |
| Fibroid Characteristics (n = 311) | Frequency |
|---|---|
| FIGO Classification | |
| 7% |
| 24% |
| 23% |
| 2% |
| 44% |
| Vascularity | |
| 12% |
| 32% |
| 56% |
| Location | |
| 30% |
| 27% |
| 24% |
| 13% |
| 6% |
| Living Tissue | |
| 9% |
| 30% |
| 61% |
| Model | Accuracy | Precision | Recall | F-1 Score |
|---|---|---|---|---|
| Heavy Bleeding | 81% | 85% | 81% | 78% |
| Frequent Urination | 88% | 90% | 88% | 88% |
| Severe Bloating | 81% | 91% | 81% | 83% |
| Pelvic Pain | 82% | 81% | 82% | 81% |
| Severe Back Pain | 82% | 80% | 81% | 81% |
| Successful Shrinkage | 75% | 75% | 75% | 75% |
| Model | Heavy Bleeding | Freq Urination | Bloating | Pelvic Pain | Back Pain | Successful Shrinkage |
|---|---|---|---|---|---|---|
| Deep Set Networks | 81% | 88% | 81% | 82% | 82% | 75% |
| Traditional Neural Net | 75% | 75% | 75% | 75% | 75% | 69% |
| Light GBM | 56% | 75% | 69% | 75% | 62% | 50% |
| SVM | 62% | 87% | 75% | 69% | 75% | 56% |
| Model | Heavy Bleeding | Freq Urination | Bloating | Pelvic Pain | Back Pain | Successful Shrinkage |
|---|---|---|---|---|---|---|
| Deep Set Networks | 78% | 88% | 83% | 81% | 81% | 75% |
| Traditional Neural Net | 73% | 71% | 77% | 75% | 75% | 68% |
| Light GBM | 57% | 74% | 70% | 73% | 59% | 50% |
| SVM | 53% | 85% | 77% | 65% | 75% | 55% |
| Model | Accuracy | Precision | Recall | F1 Score |
|---|---|---|---|---|
| Logistic Regression | 57% | 61% | 57% | 58% |
| Support Vector Machine | 61% | 64% | 61% | 62% |
| Random Forests | 76% | 75% | 76% | 75% |
| XGBoost | 71% | 69% | 71% | 67% |
| K-Nearest Neighbors | 63% | 65% | 63% | 64% |
| Light GBM | 66% | 67% | 65% | 66% |
| Neural Nets | 70% | 75% | 70% | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janghorbani, S.; Caprio, A.; Sam, L.; Lee, B.C.; Sabuncu, M.R.; Lamparello, N.A.; Schiffman, M.; Mosadegh, B. Predicting Clinical Outcomes and Symptom Relief in Uterine Fibroid Embolization Using Machine Learning on MRI Features. AI 2025, 6, 200. https://doi.org/10.3390/ai6090200
Janghorbani S, Caprio A, Sam L, Lee BC, Sabuncu MR, Lamparello NA, Schiffman M, Mosadegh B. Predicting Clinical Outcomes and Symptom Relief in Uterine Fibroid Embolization Using Machine Learning on MRI Features. AI. 2025; 6(9):200. https://doi.org/10.3390/ai6090200
Chicago/Turabian StyleJanghorbani, Sepehr, Alexandre Caprio, Laya Sam, Benjamin C. Lee, Mert R. Sabuncu, Nicole A. Lamparello, Marc Schiffman, and Bobak Mosadegh. 2025. "Predicting Clinical Outcomes and Symptom Relief in Uterine Fibroid Embolization Using Machine Learning on MRI Features" AI 6, no. 9: 200. https://doi.org/10.3390/ai6090200
APA StyleJanghorbani, S., Caprio, A., Sam, L., Lee, B. C., Sabuncu, M. R., Lamparello, N. A., Schiffman, M., & Mosadegh, B. (2025). Predicting Clinical Outcomes and Symptom Relief in Uterine Fibroid Embolization Using Machine Learning on MRI Features. AI, 6(9), 200. https://doi.org/10.3390/ai6090200







