Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
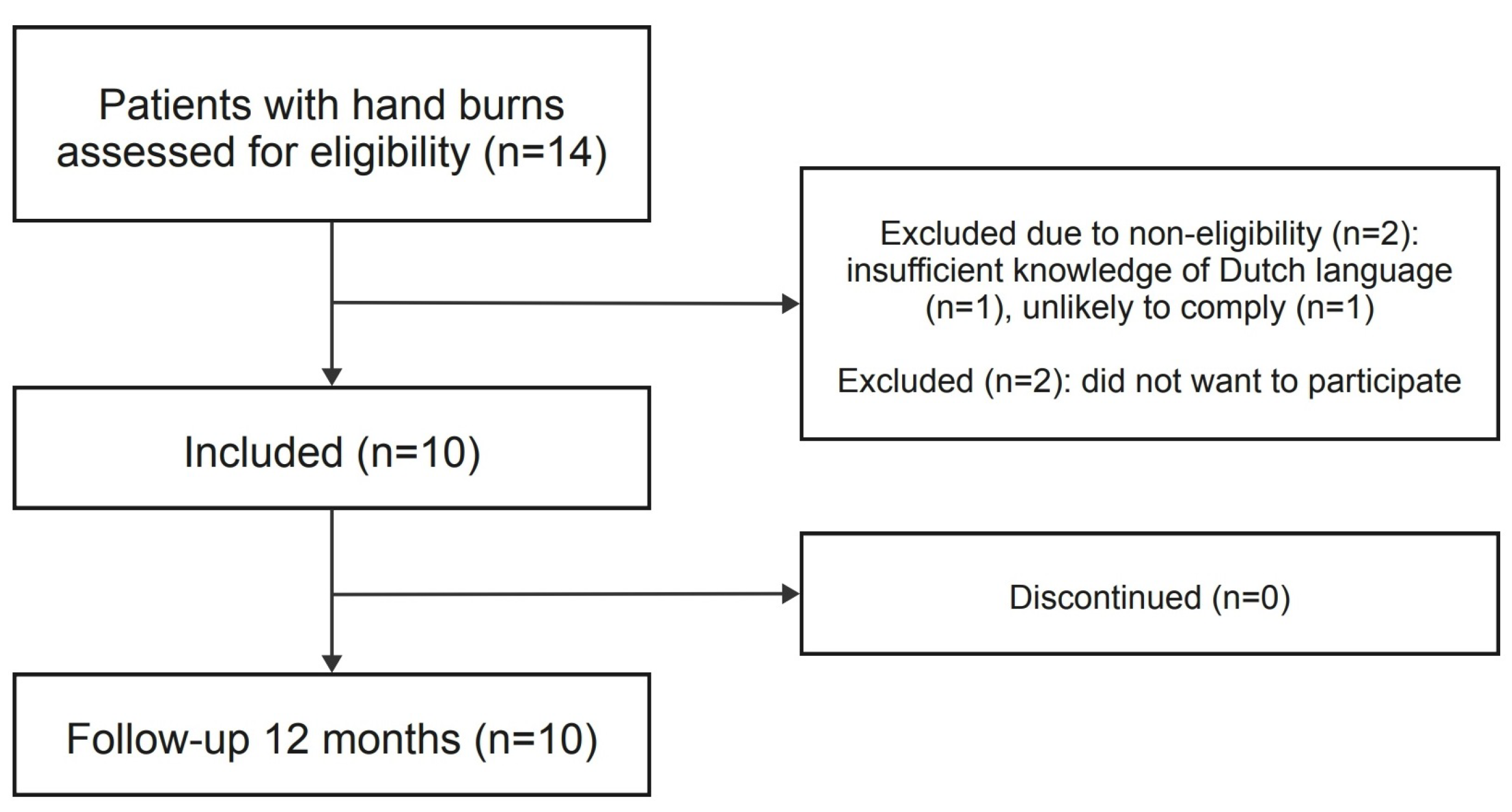
2.1. Study Design and Participants
2.2. Treatment Protocol
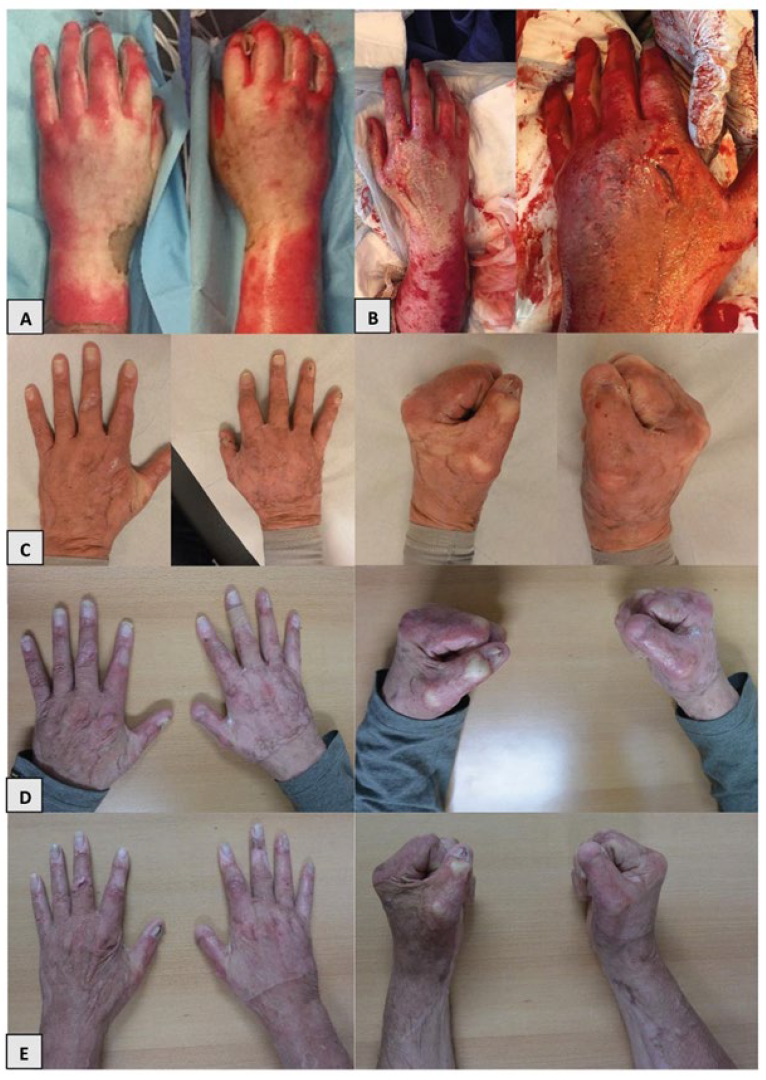
2.3. Clinical Characteristics and Outcomes
2.4. Study Outcomes
2.4.1. Hand Function
- Jebsen-Taylor Hand Function test (JTHFT): The JTHFT is a standardized and objective test with 7 items representative of various hand activities, which include (1) writing a short sentence, (2) turning over 3 × 5-inch index cards, (3) picking up small objects (paperclip, coin), (4) stacking checkers, (5) simulating eating, (6) picking up large light objects (empty cans), and (7) picking up large heavy objects (full cans). The activity is measured by the time that it takes to complete the activity and is either within a normal range (1 = yes) or not within a normal range (0 = no) [20].
- Range of Motion (ROM) (goniometry): Goniometry is used to measure the passive (PROM) and active (AROM) ROM of the wrist or finger joints. The AROM angles for each finger are described using the total active motion (TAM) score, which is the sum of the MCP, IP, PIP, and DIP joints for each digit minus the extension deficit of the measured digit [21]. A TAM of 260° was considered normal for digits 2–5. The lower arm during the measurement was placed in a neutral, flexed position of 90° and the wrist in an extended position of 20°. The AROM angles were assessed in a composite manner [22].
- Modified Kapandji Index (MKI) [23]: The MKI is a combined score from three tests: (1) a thumb opposition test, by scoring from 0 (impossible to do) to 10 (completely accomplished); (2) a finger flexion test; and (3) a flat hand/extension finger test, by scoring from 0 (impossible to do) to 5 (completely accomplished). The maximum sum score is 35 points, indicating optimal function. This assessment was only performed if the patient was fully conscious.
2.4.2. Scar Quality
2.4.3. Quality of Life
- Quick Shortened Disability Arm Shoulder Hand (Q-DASH) Questionnaire: The Q-DASH is a shortened version of the DASH, which is a patient self-rated questionnaire that is specific to the function of the upper limb extremity, and has a scale from 1 (“no difficulty”) to 5 (“impossible to carry out”); it provides a minimum total score of 0 (best) and a maximum score of 100 (worst) [26]. Patients also provided a Q-DASH questionnaire filled in as if the situation was pre-burn.
- Canadian Occupational Performance Measure (COPM): The COPM is a tool used by occupational therapists to conduct a semi-structured interview to identify issues in areas of self-care, productivity, and leisure for individual patients. Each of these problems is rated based on performance and satisfaction on a scale from 0 (worst) to 10 (best). Mean scores were calculated per patient, independently of the number of problems that they reported [27].
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Clinical Outcomes
3.3. Return to Work
3.4. Study Outcomes
3.4.1. Hand Function
- JTHFT: Cochran’s Q test determined that there was a statistically significant difference in the outcome of the test over time in picking up large light objects (n = 14, X2(2) = 7.60, p = 0.022) and picking up large heavy objects (n = 14, X2(2) = 6.50, p = 0.039). The post hoc analysis revealed no statistically significant differences between the time points. At 12 months, 11 (78.6%), 8 (57.1%), 8 (57,1%), and 7 (50%) hands achieved a normal range in items 1, 2, 6, and 7, respectively.
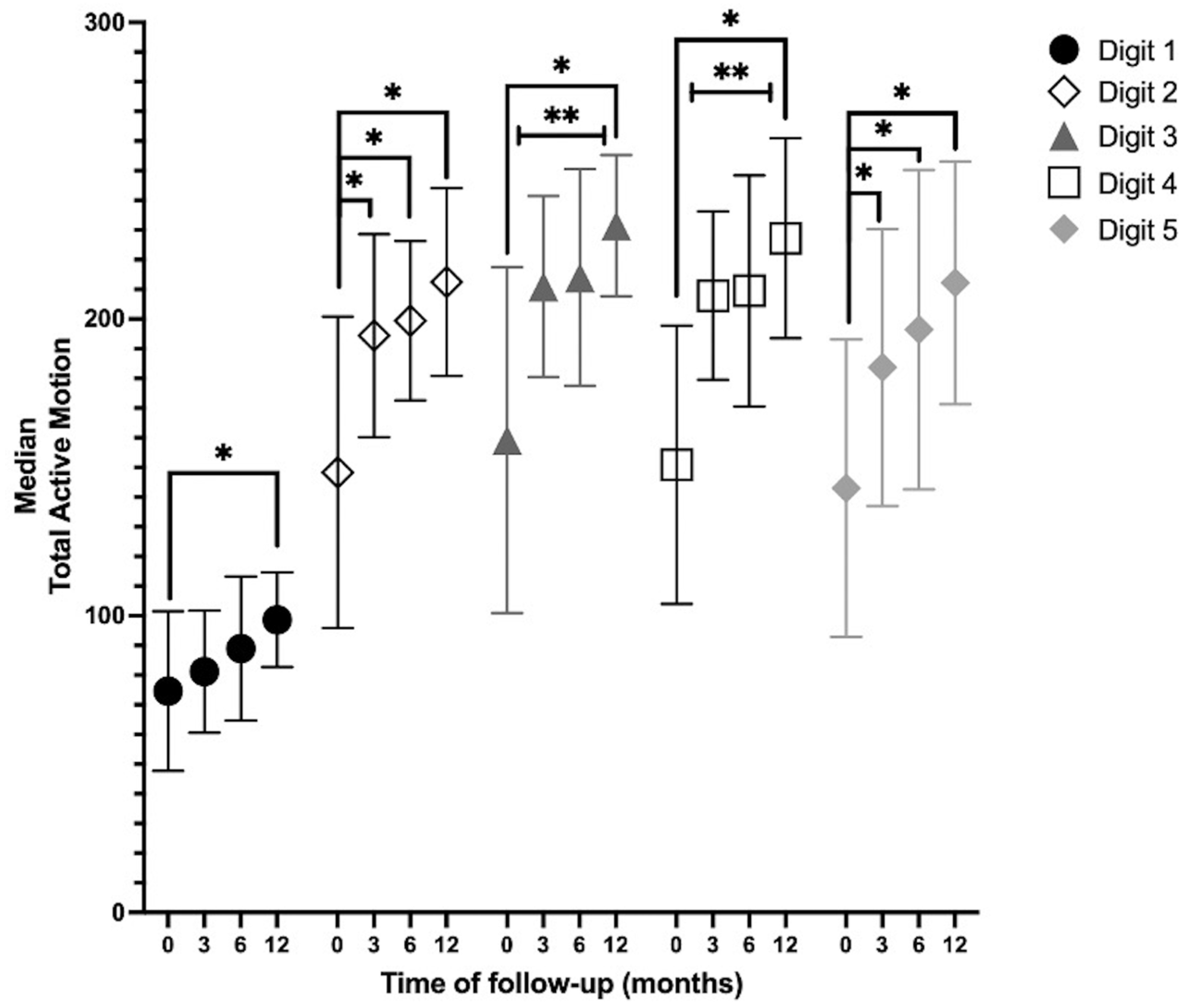
- AROM: Digits 2, 3, and 5 showed an increase in the median TAM during all measurements. Digits 1 and 4 showed an increase in the median TAM during all measurements, except for the measurements between 3 months and 6 months. In digits 3 and 4, there was a statistically significant increase between baseline and 3, 6, and 12 months; between 3 months and 12 months; and between 6 months and 12 months. In digits 2 and 5, there was a statistically significant increase in the TAM over time between baseline and 3, 6, and 12 months. At 12 months, the TAMs of digits 3 and 4 returned to near normal (260°) (Figure 3).
- MKI: There was a statistically significant increase over time between baseline and 6 months (p = 0.008) and between baseline and 12 months (p = 0.002) (Figure 4).
3.4.2. Scar Quality
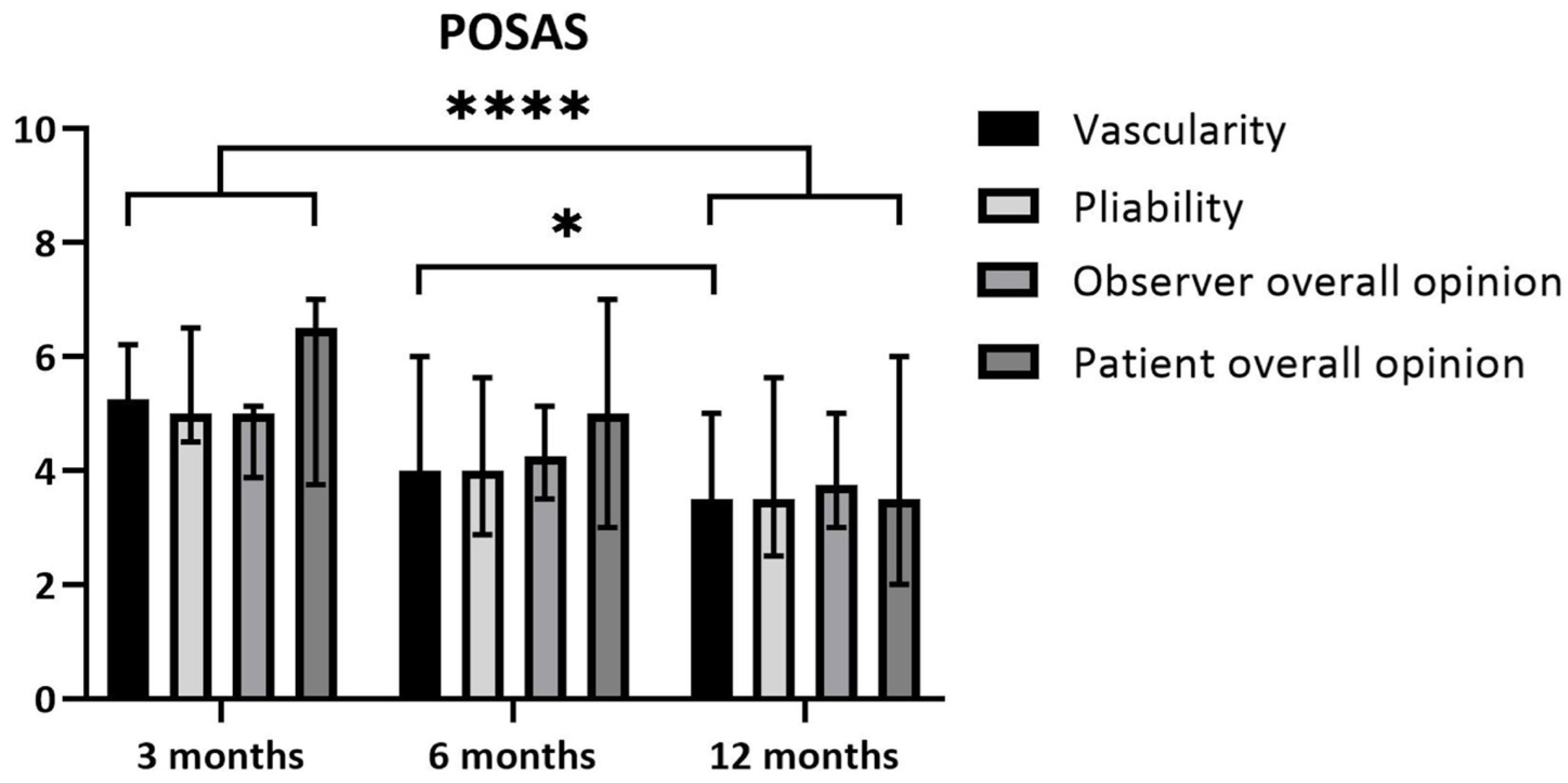
- Patient scores: The overall opinions of the patients regarding their scars 3 months after the burn yielded a median of 6.5 (IQR 3.8–7.0) and median of 3.5 (IQR 2.0–6.0) after 12 months. This improvement did not reach statistical significance based on the corrected threshold p-value (p = 0.029; threshold p < 0.017) (Figure 5).
- Observer scores: The overall opinion of the observer regarding the scars 3 months after the burn yielded a median of 5.0 (IQR 3.9–5.1) and a median of 3.8 (IQR 3.0–5.0) after 12 months. This difference over time was statistically significant, with p = 0.011. The scores for pliability improved between 3 and 12 months, with a median of 5.0 (IQR 4.5–6.5) at 3 months and a median of 3.5 (IQR 2.5–5.6) at 12 months. This difference was statistically significant (p = 0.011). The scores for vascularity showed a median of 5.3 (IQR 3.9–6.1) at 3 months and a median of 3.5 (IQR 1.9–5.0) at 12 months (p = 0.002). Vascularity also improved significantly between 6 and 12 months post-burn. The median at 6 months was 4.0 (IQR 3.8–6.0) and the median at 12 months was 3.5 (IQR 1.9–5.0) (p = 0.009) (Figure 5).
3.4.3. Quality of Life
- Q-DASH: There was a statistically significant increase (p = 0.005) between the pre-burn period (median 0.0, IQR 0.0–2.8) and 3 months (median 39.1, IQR 18.6–58.4) and a reduction between 3 months and 12 months (median 12.3, IQR 5.8–36.1) (p = 0.005) (Figure 4).
- COPM: There was a statistically significant increase (p = 0.005) in the performance scores between 3 months (median 6.1, IQR 3.8–7.4) and 12 months (median 8.8, IQR 7.9–9.8) and between 6 months (median 7.0, IQR 5.0–8.5) and 12 months (p = 0.005). There was a statistically significant increase (p = 0.011) in the satisfaction scores between 3 months (median 5.5, IQR 1.5–8.0) and 6 months (median 8.0, IQR 5.5–8.6) and between 3 and 12 months (median 8.3, IQR 7.6–9.9) (p = 0.005), but not between 6 and 12 months (p = 0.021).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| AROM | Active Range of Motion |
| COPM | Canadian Occupational Performance Measure |
| ED | Enzymatic Debridement |
| JTHFT | Jebsen-Taylor Hand Function Test |
| LOS | Length of Hospital Stay |
| MKI | Modified Kapandji Index |
| POSAS | Patient and Observer Scar Assessment Scale |
| Q-DASH | Quick Shortened Disability Arm Shoulder Hand Questionnaire |
| QOL | Quality of Life |
| ROM | Range of Motion |
| SOC | Standard of Care |
| TAM | Total Active Motion |
| TBSA | Total Body Surface Area |
References
- Bain, C.J.; Wang, T.; McArthur, G.; Williams, G.; Atkins, J.; Jones, I. Safety and efficacy of excision and direct closure in acute burns surgery: Outcome analysis in a prospective series of 100 patients and a survey of UK burns surgeons’ attitudes. Burns 2014, 40, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Deitch, E.A.; Wheelahan, T.M.; Rose, M.P.; Clothier, J.; Cotter, J. Hypertrophic burn scars: Analysis of variables. J. Trauma 1983, 23, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Gahankari, D.; Herndon, D.N. Operative Wound Management. In Total Burn Care, 3rd ed.; Greenwood, G., Ed.; Philadelphia Saunders Elsevier: Beijing, China, 2007; pp. 177–195. [Google Scholar]
- Kwa, K.A.A.; Goei, H.; Breederveld, R.S.; Middelkoop, E.; van der Vlies, C.H.; van Baar, M.E. A systematic review on surgical and nonsurgical debridement techniques of burn wounds. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1752–1762. [Google Scholar] [CrossRef]
- Agorgianitis, L.; Vasdeki, D.; Georgiou, P.; Kalofonou, M. Hand burns: Treatment, challenges and our experience in their management. Hand Surg. Rehabil. 2023, 42, 640. [Google Scholar] [CrossRef]
- van Zuijlen, P.P.; Kreis, R.W.; Vloemans, A.F.; Groenevelt, F.; Mackie, D.P. The prognostic factors regarding long-term functional outcome of full-thickness hand burns. Burns 1999, 25, 709–714. [Google Scholar] [CrossRef]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef]
- Krieger, Y.; Bogdanov-Berezovsky, A.; Gurfinkel, R.; Silberstein, E.; Sagi, A.; Rosenberg, L. Efficacy of enzymatic debridement of deeply burned hands. Burns 2012, 38, 108–112. [Google Scholar] [CrossRef]
- Cordts, T.; Horter, J.; Vogelpohl, J.; Kremer, T.; Kneser, U.; Hernekamp, J.F. Enzymatic debridement for the treatment of severely burned upper extremities—Early single center experiences. BMC Dermatol. 2016, 16, 8. [Google Scholar] [CrossRef]
- Mediwound. NexoBrid (Debrase): A debriding agent for removal of eschar in deep partial thickness and full thickness burns. In Investigator's Brochure; Mediwound: Yavne, Israel, 2017. [Google Scholar]
- Schulz, A.; Shoham, Y.; Rosenberg, L.; Rothermund, I.; Perbix, W.; Christian Fuchs, P.; Lipensky, A.; Schiefer, J.L. Enzymatic Versus Traditional Surgical Debridement of Severely Burned Hands: A Comparison of Selectivity, Efficacy, Healing Time, and Three-Month Scar Quality. J. Burn. Care Res. 2017, 38, e745–e755. [Google Scholar] [CrossRef]
- Anthonissen, M.; Daly, D.; Janssens, T.; Van den Kerckhove, E. The effects of conservative treatments on burn scars: A systematic review. Burns 2016, 42, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Edstrom, L.E.; Robson, M.C.; Macchiaverna, J.R.; Scala, A.D. Prospective randomized treatments for burned hands: Nonoperative vs. operative. Preliminary report. Scand. J. Plast. Reconstr. Surg. 1979, 13, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Malfeyt, G.A. Burns of the dorsum of the hand treated by tangential excision. Br. J. Plast. Surg. 1976, 29, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.; Lapid, O.; Bogdanov-Berezovsky, A.; Glesinger, R.; Krieger, Y.; Silberstein, E.; Sagi, A.; Judkins, K.; Singer, A.J. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: A preliminary report. Burns 2004, 30, 843–850. [Google Scholar] [CrossRef]
- Frist, W.; Ackroyd, F.; Burke, J.; Bondoc, C. Long-term functional results of selective treatment of hand burns. Am. J. Surg. 1985, 149, 516–521. [Google Scholar] [CrossRef]
- Corrales-Benítez, C.; González-Peinado, D.; González-Miranda, Á.; Martínez-Méndez, J.R. Evaluation of burned hand function after enzymatic debridement. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1048–1056. [Google Scholar] [CrossRef]
- Heitzmann, W.; Schulz, A.; Fuchs, P.C.; Schiefer, J.L. Assessing the Effect of Enzymatic Debridement on the Scar Quality in Partial-Thickness Burns to Deep Dermal Burns of the Hand: A Long-Term Evaluation. Medicina 2024, 60, 481. [Google Scholar] [CrossRef]
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar]
- Adams, L.; Greene, L.; Topoozian, E. Clinical Assessment Recommendations. American Society of Hand Therapists: Mt. Laurel, NJ, USA, 1992; pp. 56–70. [Google Scholar]
- Richard, R.; Parry, I.S.; Santos, A.; Dewey, W.S. Burn Hand or Finger Goniometric Measurements: Sum of the Isolated Parts and the Composite Whole. J. Burn. Care Res. 2017, 38, e960–e965. [Google Scholar] [CrossRef]
- Lefevre-Colau, M.M.; Poiraudeau, S.; Oberlin, C.; Demaille, S.; Fermanian, J.; Rannou, F.; Revel, M. Reliability, validity, and responsiveness of the modified Kapandji index for assessment of functional mobility of the rheumatoid hand. Arch. Phys. Med. Rehabil. 2003, 84, 1032–1038. [Google Scholar] [CrossRef]
- van de Kar, A.L.; Corion, L.U.; Smeulders, M.J.; Draaijers, L.J.; van der Horst, C.M.; van Zuijlen, P.P. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast. Reconstr. Surg. 2005, 116, 514–522. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, M.B.; Tuinebreijer, W.E.; Bloemen, M.C.; Verhaegen, P.D.; Middelkoop, E.; van Zuijlen, P.P. Rasch analysis of the Patient and Observer Scar Assessment Scale (POSAS) in burn scars. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2012, 21, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Edgar, D.W.; Wood, F.M. The QuickDASH is an appropriate tool for measuring the quality of recovery after upper limb burn injury. Burns 2007, 33, 843–849. [Google Scholar] [CrossRef]
- Carswell, A.; McColl, M.A.; Baptiste, S.; Law, M.; Polatajko, H.; Pollock, N. The Canadian Occupational Performance Measure: A research and clinical literature review. Can. J. Occup. Ther. Rev. Can. D’ergotherapie 2004, 71, 210–222. [Google Scholar] [CrossRef]
- Schulz, A.; Perbix, W.; Shoham, Y.; Daali, S.; Charalampaki, C.; Fuchs, P.C.; Schiefer, J. Our initial learning curve in the enzymatic debridement of severely burned hands-Management and pit falls of initial treatments and our development of a post debridement wound treatment algorithm. Burns 2017, 43, 326–336. [Google Scholar] [CrossRef]
- Krieger, Y.; Rubin, G.; Schulz, A.; Rosenberg, N.; Levi, A.; Singer, A.J.; Rosenberg, L.; Shoham, Y. Bromelain-based enzymatic debridement and minimal invasive modality (mim) care of deeply burned hands. Ann. Burn. Fire Disasters 2017, 30, 198–204. [Google Scholar]
- Dadras, M.; Wagner, J.M.; Wallner, C.; Sogorski, A.; Sacher, M.; Harati, K.; Lehnhardt, M.; Behr, B. Enzymatic debridement of hands with deep burns: A single center experience in the treatment of 52 hands. J. Plast. Surg. Hand Surg. 2020, 54, 220–224. [Google Scholar] [CrossRef]
- Rivas-Nicolls, D.; Aguilera-Sáez, J.; Gallardo-Calero, I.; Serracanta, J.; Gomez, P.; Palao, R.; Barret, J.P. Does Enzymatic Debridement Allow Us To Perform Conservative Treatment On Clinically Deep Hand Burns? A Retrospective Review. Ann. Burn. Fire Disasters 2020, 33, 239–244. [Google Scholar]
- Malsagova, A.T.; El-Habbassi, A.; Billner, M.; Berns, M.; Pueski, T.; Bodenschatz, K.J.; Heidekrueger, P.I.; Ehrl, D. Long-Term Functional Outcomes Following Enzymatic Debridement of Deep Hand Burns Using Nexobrid®: A Retrospective Analysis. J. Clin. Med. 2024, 13, 4729. [Google Scholar] [CrossRef]
- Ghalayini, G.; O’Brien, L.; Bourke-Taylor, H.M. Recovery in the first six months after hand and upper limb burns: A prospective cohort study. Aust. Occup. Ther. J. 2019, 66, 201–209. [Google Scholar] [CrossRef]
- Claes, K.; Hoeksema, H.; Lafaire, C.; De Cuyper, L.; De Groote, K.; Vyncke, T.; De Decker, I.; Verbelen, J.; De Coninck, P.; Depypere, B.; et al. The process to obtain reimbursement for enzymatic debridement in clinically deep burns. Acta Chir. Belg. 2023, 123, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Claes, K.E.Y.; De Decker, I.; Monstrey, S.; Shoham, Y.; Vyncke, T.; Depypere, B.; De Wolf, E.; Decuypere, F.; Lannau, B.; Hoeksema, H. Helpful hints in deciding what and when to operate after enzymatic debridement. Burns 2023, 49, 80–90. [Google Scholar] [CrossRef] [PubMed]
- De Decker, I.; De Graeve, L.; Hoeksema, H.; Monstrey, S.; Verbelen, J.; De Coninck, P.; Vanlerberghe, E.; Claes, K.E.Y. Enzymatic debridement: Past, present, and future. Acta Chir. Belg. 2022, 122, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.; Bruton, A. A study to compare the reliability of composite finger flexion with goniometry for measurement of range of motion in the hand. Clin. Rehabil. 2002, 16, 562–570. [Google Scholar] [CrossRef]
- van de Ven-Stevens, L.A.; Munneke, M.; Terwee, C.B.; Spauwen, P.H.; van der Linde, H. Clinimetric properties of instruments to assess activities in patients with hand injury: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2009, 90, 151–169. [Google Scholar] [CrossRef]
- Margarita, M.; Petros, D.; Filio, A.; Chris, A.; Sevasti, S.; Kyriaki, K. Therapeutic management of hand burns. The significant role of physiotherapy and occupational therapy. Hand Surg. Rehabil. 2023, 42, 657. [Google Scholar] [CrossRef]
 ** significant for Q-DASH; p < 0.017.
** significant for Q-DASH; p < 0.017.
 ** significant for Q-DASH; p < 0.017.
** significant for Q-DASH; p < 0.017.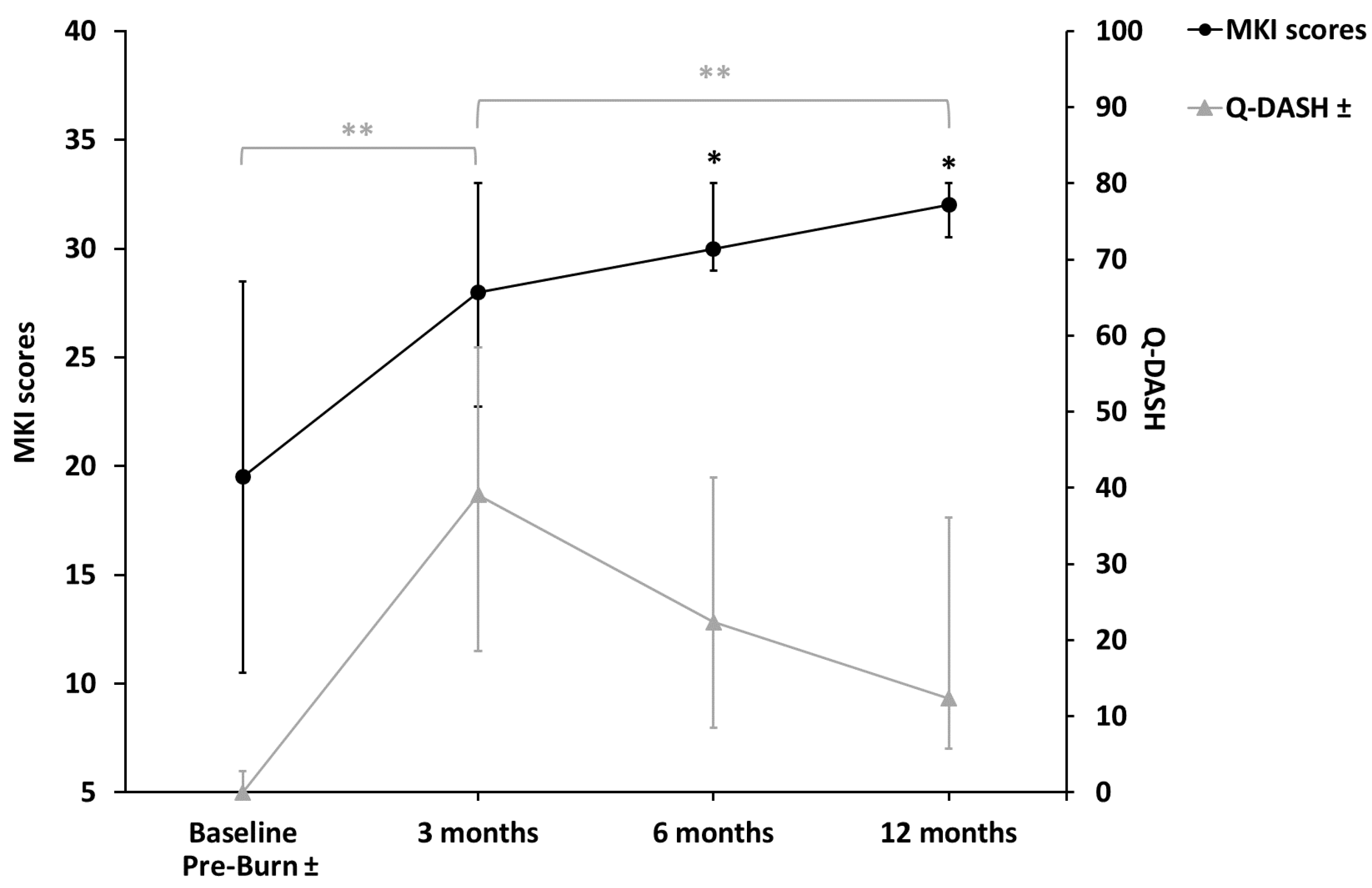
| General (n = 10, 14 Enzymatically Treated Hands) | |
| Male (n) | 9 |
| Age (years) | 56.3 ± 12.5 |
| Smoking (yes, n) | 3 |
| Comorbidities (yes, n): | |
| Diabetes mellitus | 1 |
| Cardiovascular disease | 2 |
| Cause of burn: | |
| Flame (n) | 9 |
| Scald (n) | 1 |
| Right hand dominance (n) | 9 |
| TBSA * burned total (%) | 11.0 ± 8.1 |
| Time to wound healing (days) | 35.1 ± 12.6 |
| Length of hospital stay (days) | 25.3 ± 15.6 |
| Enzymatically Treated Hands (n = 14) | |
| TBSA * burned (%) | 1.8, 1.5–2.5 |
| TBSA * burned 2nd degree (%) | 1.5, 1.0–2.5 |
| TBSA * burned 3rd degree (%) | 0, 0–1.0 |
| TBSA * excised (%) | 0.3, 0–1.3 |
| TBSA * skin grafted (%) | 1.0 ± 0.6 |
| TBSA* skin grafted in percentage of TBSA burned (%) | 57.6 ± 31.7 |
| Time to wound healing (days) | 31.0, 24.0–39.0 |
| Wound colonization pathogenic bacteria (n) | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwa, K.A.A.; Reuvers, A.C.; Borst-van Breugel, J.; Pijpe, A.; van Zuijlen, P.P.M.; Breederveld, R.S.; Meij-de Vries, A. Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study. Eur. Burn J. 2025, 6, 36. https://doi.org/10.3390/ebj6020036
Kwa KAA, Reuvers AC, Borst-van Breugel J, Pijpe A, van Zuijlen PPM, Breederveld RS, Meij-de Vries A. Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study. European Burn Journal. 2025; 6(2):36. https://doi.org/10.3390/ebj6020036
Chicago/Turabian StyleKwa, Kelly Aranka Ayli, Annika Catherina Reuvers, Jorien Borst-van Breugel, Anouk Pijpe, Paul P. M. van Zuijlen, Roelf S. Breederveld, and Annebeth Meij-de Vries. 2025. "Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study" European Burn Journal 6, no. 2: 36. https://doi.org/10.3390/ebj6020036
APA StyleKwa, K. A. A., Reuvers, A. C., Borst-van Breugel, J., Pijpe, A., van Zuijlen, P. P. M., Breederveld, R. S., & Meij-de Vries, A. (2025). Hand Function Recovers to Near Normal in Patients with Deep Dermal Hand Burns Treated with Enzymatic Debridement: A Prospective Cohort Study. European Burn Journal, 6(2), 36. https://doi.org/10.3390/ebj6020036







