Abstract
Substantial research interest has been shown over the past ten years in the management of burn injuries. This bibliometric analysis aims to identify and evaluate the most cited articles that have significantly advanced the field of burn injury management. The 100 most cited articles published from January 2014 to September 2024 were collated using the Web of Science database. The full text of each article was meticulously analyzed for descriptive parameters including subject matter, journal of publication, authorship, institutional affiliation, country of origin, and year of publication. The 100 most cited articles had an average of 203 citations, with the most cited article reaching 754 citations and the least cited article cited 105 times. The subjects ranged from enhancing wound care outcomes to metabolic support, fluid management, and infection prevention and management. These articles were distributed across 59 source journals, with 44% of articles having been published in just ten prominent journals. While bibliometric analyses do not accurately gauge scientific merit, this study illuminates the significant contributions to burn management over the past decade and provides valuable insights into research trends in the field.
1. Introduction
Burns can be associated with extensive soft tissue injuries and contribute to significant morbidity and mortality. Research on the management of burns has garnered significant interest over the last decade, as demonstrated in Figure 1. However, identifying the articles that have significantly impacted and advanced the field remains challenging. While the aim of academic research is the generation of robust literature with a high level of evidence, only a minority of publications substantially contribute to the existing body of scientific knowledge.

Figure 1.
Substantial increase in the number of publications in the field of burn management over the past decade.
The importance of articles published in a particular domain is echoed in the quantity of citations received from peers. Citations serve as an acknowledgement by the authors to their colleagues who previously published endeavors in that academic domain. The more times an article is cited, the greater the presumed significance of the article in that particular academic domain. In this context, a bibliometric analysis can be a valuable tool for identifying impactful studies. Therefore, this study focuses on the 100 most cited articles published in the field of burn management from the past decade, aiming to provide insight into significant advancements and key contributions.
2. Materials and Methods
The Web of Science database (Clarivate, Philadelphia, PA, USA) was utilized to perform a comprehensive search and collate articles in the field of burn management published between January 2014 and September 2024 with the highest number of citations. Three authors independently conducted the search using the keyword “burn” to identify all articles in the English language. Articles unrelated to the field of burn management were excluded after a thorough review of the full text of each article. A preliminary list of articles was compiled by combining the results from all three authors. Discrepancies were resolved through a consensus conference of all authors, culminating in the final list of 100 articles relevant to the field of burn management with the highest number of citations. The methodology was based on the approach previously described by Joyce et al. [1]. Each article was systematically analyzed to extract descriptive data, including the topic, the journal and publication year, and the authors, their institutional affiliations, and their country of origin. This process is demonstrated as a PRISMA flow diagram in Figure 2. This study was conducted in accordance with the World Medical Association Declaration of Helsinki and the Good Clinical Practice guidelines.
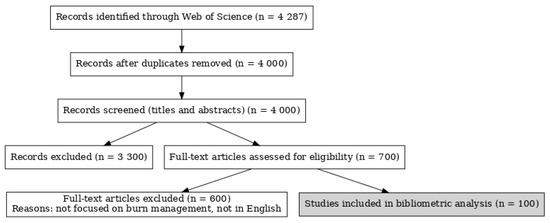
Figure 2.
PRISMA flow diagram.
3. Results
In the past decade, the 100 most cited papers in the field of burn management, as demonstrated in Table 1, received an average of 203 citations with a standard deviation of 117 citations. The article with the most citations garnered 754 citations, while the article with the fewest citations had 107 citations.

Table 1.
The top 100 articles on burn management with the most citations from 2014 to 2024.
The top 100 articles on burn management with the most citations in the past ten years were distributed across 59 source journals. Table 2 demonstrates the most prominent ten journals, with impact factors ranging from 2.0 to 98.4, which contributed to 44% of these articles. Further analysis of this list demonstrates that 60% of these articles constituted “review articles” and 66% of these articles were available “open access”. Elsevier and Springer Nature published 53% of these articles. Funding was a significant feature, with 20% of the articles receiving financial support from the National Institutes of Health (NIH), USA, and a further 20% of the articles receiving financial support from the United States Department of Health and Human Services.

Table 2.
Source journals contributing most frequently to the top 100 articles on burn management with the most citations from 2014 to 2024.
Authorship analysis revealed that seven authors contributed to 29% of the 100 most cited articles on burns management from 2014 to 2024, as demonstrated in Table 3. A gender predilection was noted, with only 23% of the contributions from female authors. Geographically, 42% of the articles originated from European authors, followed by 28% from North American authors, and 23% from Asian authors, as demonstrated in Figure 3. Contributions came from a variety of disciplines, with plastic and reconstructive surgeons and general surgeons making equal contributions to the literature on burn management. Additional contributions were notes from trauma surgeons, pain and rehabilitation physicians, and academics or researchers in basic science and pharmacotherapy.

Table 3.
Authors contributing most frequently to the top 100 articles on burn management with the most citations from 2014 to 2024.
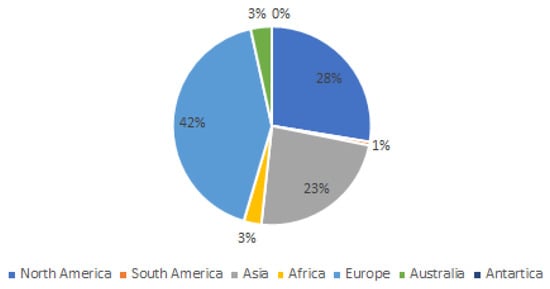
Figure 3.
Geographic distribution of authorship (based on continent) that contributed to the list of top 100 articles on burn management with the most citations from 2014 to 2024.
4. Discussion
This bibliometric analysis of burn management identified the 100 most cited articles published over the past decade in English-language literature. Our findings offer valuable insights into significant advancements in this field and highlight key contributions and trends. This list includes major randomized control trials, systematic reviews, and clinical consensus guidelines that contribute an integral knowledge base for the burn surgeon.
Wound care and dressing innovations were a prominent theme that was noted in 17 of the top 100 most cited articles, particularly focussed on rapid healing and prevention of infections [43,76,98]. The most cited article, with 754 citations, is a review of natural and synthetic polymers for wounds and burns dressing published in the International Journal of Pharmaceutics [2]. Polysaccharides (alginates, chitin, chitosan, chondroitin, and heparin), proteoglycans and proteins (collagen, eggshell membrane, fibrin, gelatin, keratin, and silk fibroin) are natural polymers used in wounds and burns management because of their biocompatibility, biodegradability and similarity to macromolecules recognized by the body. Synthetic polymers, such as tissue-engineered skin, have been utilized in regenerative medicine for the treatment of severe skin defects or partial-thickness burn injuries.
Critical care management of burns was another prominent theme. The article with the most citations (404) in this domain explored intensive care unit-acquired weakness (ICUAW), a de novo form of muscle weakness, in a substantial number of patients admitted to the ICU with severe burns and other trauma [7]. Other articles in this theme examined the fluid resuscitation of critically ill burns patients, nutrition and metabolism, and prevention and management ICU and hospital acquired concurrent infections [5,62,71]. Another major area of research was the use of virtual reality for pain management. The article with the most citations (200) in this domain was a comprehensive literature review that explored the use of virtual reality as a distraction tool to alleviate pain and distress during medical procedures such as burns debridement and dressing change [36]. Other publications on this theme explored the use of virtual reality for pain and anxiety management in the pediatric burn population [69,70].
This study sheds light on advances since the initial work by Joyce et al. that explored the 100 most influential articles in the field burn management from 1945 to 2013 [1]. Historically, the prominent publications were limited to 27 source journals, including Annals of Surgery, Journal of Trauma, Injury, Infection and Critical Care, Lancet, Burns, and New England Journal of Medicine [1]. In the past decade, impactful work has been distributed across a broader range of journals, reflecting the rise in open-access publishing and the increased digitization of research. Prominent publications spanned 56 source journals, including Critical Care, Burns & Trauma, and Burns, with ongoing contributions from Lancet and Annals of Surgery to the most prominent burns literature.
The 100 most influential articles on burns from 1945 to 2013 ranged from 104 citations (least cited) to 746 citations (most cited) [1]. This is similar to the 100 most cited articles from 2014 to 2024, which ranged from 105 (least cited) to 754 (most cited). However, this is not similar to bibliometric analyses in other fields, such as hand surgery, during the same period, where citation numbers ranged from 47 (least cited) to 179 (most cited) citations [102].
While journal impact factor is often used as a measure of quality, this does not necessarily determine the journal’s contribution to the list of top 100 articles with the most citations. The impact factor is a measure of the quality of academic journals within an academic domain and is derived by dividing the number of citations from a journal by the number of articles published in that journal over two years [103]. Most of these metrics have significant limitations in that they attribute greater significance to work in academic domains with a larger audience. For example, Lancet (impact factor 98.4) appeals to the wider medical audience, while journals such as Annals of Surgery (impact factor 10.1) and Burns & Trauma (impact factor 6.3) enjoy a smaller, subspecialized readership, yet both have contributed substantially to the literature on burns.
This study has numerous limitations that are inherent to all bibliometric analyses. Citations were utilized as a substitute to comprehend the impact and scientific merit of published articles. Citations may reflect an author’s recognition of the study’s relevance to their research. However, it should be noted that publications from 2023 to 2024 were not included in this list due to insufficient time for the accumulation of citations. Citation bias and self-citation can exaggerate bibliometric results, and non-English articles can have limited visibility. Despite these limitations, this bibliometric analysis offers a snapshot of the impactful research in burn management. It highlights key advancements and provides researchers, surgeons, and allied health professionals with a curated reference of influential studies to inform practice and future investigations [104].
5. Conclusions
This bibliometric analysis provides an insightful overview of the 100 most cited articles in burn management from 2014 to 2024. These articles reflect significant advancements and key contributions across diverse areas, including wound care, critical care management, and pain relief innovations. By highlighting the most impactful research, this study serves as a valuable resource for surgeons, researchers, and allied health professionals, offering insights into the foundational work shaping modern burn care. While bibliometric analysis has inherent limitations, it remains a valuable tool for comprehending the evolution of scientific progress in this field.
Author Contributions
Conceptualization, A.J.N.; methodology, A.J.N. and J.A.; software, J.A.; formal analysis, A.J.N. and J.A.; investigation, A.J.N. and J.A.; resources, A.J.N.; data curation, A.J.N. and J.A.; writing—original draft preparation, A.J.N.; writing—review and editing, R.W.S., P.A.B. and L.D.-T.; visualization, J.A.; supervision, R.W.S., P.A.B., and L.D.-T.; project administration, A.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was undertaken in accordance with the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. Ethical review and approval were not applicable owing to the lack of involvement of human or animal subjects.
Data Availability Statement
The authors declare that data will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NIH | National Institutes of Health |
| ICUAW | Intensive Care Unit-Acquired Weakness |
References
- Joyce, C.W.; Kelly, J.C.; Sugrue, C. A bibliometric analysis of the 100 most influential papers in burns. Burns 2014, 40, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet 2016, 388, 1427–1436. [Google Scholar] [CrossRef]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived from Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. eBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, Y.J. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64 Pt 5, 471–497. [Google Scholar] [CrossRef]
- Preiser, J.C.; van Zanten, A.R.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and nutritional support of critically ill patients: Consensus and controversies. Crit. Care 2015, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn. Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Teot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in Burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress During Medical Procedures: A Comprehensive Literature Review. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn. Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Luginbuhl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra14. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Bassetti, M.; De Waele, J.J.; Eggimann, P.; Garnacho-Montero, J.; Kahlmeter, G.; Menichetti, F.; Nicolau, D.P.; Paiva, J.A.; Tumbarello, M.; Welte, T.; et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med. 2015, 41, 776–795. [Google Scholar] [CrossRef]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Ahuja, R.B. ISBI Practice Guidelines For Burn Care: Editorial. Burns 2016, 42, 951–952. [Google Scholar] [CrossRef]
- Rose, T.; Verbeken, G.; Vos, D.D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burn. Trauma 2014, 4, 66–73. [Google Scholar]
- Gentile, P.; De Angelis, B.; Pasin, M.; Cervelli, G.; Curcio, C.B.; Floris, M.; Di Pasquali, C.; Bocchini, I.; Balzani, A.; Nicoli, F.; et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical evaluation for cell-based therapies in patients with scars on the face. J. Craniofac. Surg. 2014, 25, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Makitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control Release 2016, 244 Pt B, 292–301. [Google Scholar] [CrossRef]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 5. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair. Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]
- Walker, P.F.; Buehner, M.F.; Wood, L.A.; Boyer, N.L.; Driscoll, I.R.; Lundy, J.B.; Cancio, L.C.; Chung, K.K. Diagnosis and management of inhalation injury: An updated review. Crit. Care 2015, 19, 351. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Lewis, S.R.; Pritchard, M.W.; Evans, D.J.; Butler, A.R.; Alderson, P.; Smith, A.F.; Roberts, I. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst. Rev. 2018, 8, CD000567. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107 Pt B, 2008–2019. [Google Scholar] [CrossRef]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef]
- Bittner, E.A.; Shank, E.; Woodson, L.; Martyn, J.A. Acute and perioperative care of the burn-injured patient. Anesthesiology 2015, 122, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatolog. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Shan, Y.H.; Peng, L.H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef]
- Arane, K.; Behboudi, A.; Goldman, R.D. Virtual reality for pain and anxiety management in children. Can. Fam. Physician 2017, 63, 932–934. [Google Scholar]
- Jeffs, D.; Dorman, D.; Brown, S.; Files, A.; Graves, T.; Kirk, E.; Meredith-Neve, S.; Sanders, J.; White, B.; Swearingen, C.J. Effect of virtual reality on adolescent pain during burn wound care. J. Burn. Care Res. 2014, 35, 395–408. [Google Scholar] [CrossRef]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and metabolism in burn patients. Burn. Trauma 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Pinto, R.; Kraft, R.; Nathens, A.B.; Finnerty, C.C.; Gamelli, R.L.; Gibran, N.S.; Klein, M.B.; Arnoldo, B.D.; Tompkins, R.G.; et al. Morbidity and survival probability in burn patients in modern burn care. Crit. Care Med. 2015, 43, 808–815. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Hampson, P.; Dinsdale, R.J.; Wearn, C.M.; Bamford, A.L.; Bishop, J.R.B.; Hazeldine, J.; Moiemen, N.S.; Harrison, P.; Lord, J.M. Neutrophil Dysfunction, Immature Granulocytes, and Cell-free DNA are Early Biomarkers of Sepsis in Burn-injured Patients: A Prospective Observational Cohort Study. Ann. Surg. 2017, 265, 1241–1249. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Hultman, C.S.; Friedstat, J.S.; Edkins, R.E.; Cairns, B.A.; Meyer, A.A. Laser resurfacing and remodeling of hypertrophic burn scars: The results of a large, prospective, before-after cohort study, with long-term follow-up. Ann. Surg. 2014, 260, 519–529; discussion 529–532. [Google Scholar]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Haq, Z.; Shirzadeh, E.; Huvard, M.J.; Djalilian, A.R. Current and Upcoming Therapies for Ocular Surface Chemical Injuries. Ocul. Surf. 2017, 15, 48–64. [Google Scholar] [CrossRef]
- Hop, M.J.; Polinder, S.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Costs of burn care: A systematic review. Wound Repair. Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, M.; Agarwal, T.; Sangwan, V.S.; Vajpayee, R.B. Treatment of acute ocular chemical burns. Surv. Ophthalmol. 2018, 63, 214–235. [Google Scholar] [CrossRef]
- Gold, M.H.; Berman, B.; Clementoni, M.T.; Gauglitz, G.G.; Nahai, F.; Murcia, C. Updated international clinical recommendations on scar management: Part 1—Evaluating the evidence. Dermatol. Surg. 2014, 40, 817–824. [Google Scholar]
- Rousseau, A.F.; Prescott, H.C.; Brett, S.J.; Weiss, B.; Azoulay, E.; Creteur, J.; Latronico, N.; Hough, C.L.; Weber-Carstens, S.; Vincent, J.L.; et al. Long-term outcomes after critical illness: Recent insights. Crit. Care 2021, 25, 108. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Lee, J.M.; Moon, B.M.; Park, H.J.; Lee, O.J.; Kim, J.H.; Kim, D.K.; Park, C.H. 3D electrospun silk fibroin nanofibers for fabrication of artificial skin. Nanomedicine 2015, 11, 681–691. [Google Scholar] [CrossRef]
- Fairbairn, N.G.; Randolph, M.A.; Redmond, R.W. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 662–675. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burn. Trauma 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Lantieri, L.; Grimbert, P.; Ortonne, N.; Suberbielle, C.; Bories, D.; Gil-Vernet, S.; Lemogne, C.; Bellivier, F.; Lefaucheur, J.P.; Schaffer, N.; et al. Face transplant: Long-term follow-up and results of a prospective open study. Lancet 2016, 388, 1398–1407. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Bonomo, R.A.; Tolmasky, M.E. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules 2020, 10, 720. [Google Scholar] [CrossRef]
- Lambden, S.; Creagh-Brown, B.C.; Hunt, J.; Summers, C.; Forni, L.G. Definitions and pathophysiology of vasoplegic shock. Crit. Care 2018, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Han, Y.D.; Yan, X.L.; Ren, J.; Zeng, Q.; Li, X.D.; Pei, X.T.; Han, Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef]
- Mehta, Y.; Gupta, A.; Todi, S.; Myatra, S.; Samaddar, D.P.; Patil, V.; Bhattacharya, P.K.; Ramasubban, S. Guidelines for prevention of hospital acquired infections. Indian. J. Crit. Care Med. 2014, 18, 149–163. [Google Scholar] [PubMed]
- Malbrain, M.; Langer, T.; Annane, D.; Gattinoni, L.; Elbers, P.; Hahn, R.G.; De Laet, I.; Minini, A.; Wong, A.; Ince, C.; et al. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann. Intensive Care 2020, 10, 64. [Google Scholar] [CrossRef]
- Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF Family: From Drug Development to Clinical Application. Int. J. Mol. Sci. 2018, 19, 1875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef]
- Ali, S.S.; Morsy, R.; El-Zawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): A novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017, 12, 6059–6073. [Google Scholar] [CrossRef]
- Lee, K.C.; Dretzke, J.; Grover, L.; Logan, A.; Moiemen, N. A systematic review of objective burn scar measurements. Burn. Trauma 2016, 4, 14. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Stanojcic, M.; Abdullahi, A.; Rehou, S.; Parousis, A.; Jeschke, M.G. Pathophysiological Response to Burn Injury in Adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Neriamparambil, A.J.; Antony, J.; Wijenayake, L.J. The 100 most cited articles in hand surgery from 2012–2022: A bibliometric analysis. Australas. J. Plast. Surg. 2024, 7, 2. Available online: https://ajops.com/article/92973-the-100-most-cited-articles-in-hand-surgery-from-2012-2022-a-bibliometric-analysis (accessed on 10 April 2025). [CrossRef]
- Roldan-Valadez, E.; Salazar-Ruiz, S.Y.; Ibarra-Contreras, R.; Rios, C. Current concepts on bibliometrics: A brief review about impact factor, Eigenfactor score, CiteScore, SCImago Journal Rank, Source-Normalised Impact per Paper, H-index, and alternative metrics. Ir. J. Med. Sci. 2019, 188, 939–951. [Google Scholar] [CrossRef]
- Hassan, W.; Duarte, A.E. Bibliometric analysis: A few suggestions. Curr. Probl. Cardiol. 2024, 49, 102640. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
