The Reconstruction of Various Complex Full-Thickness Skin Defects with a Biodegradable Temporising Matrix: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients (Ethics Committee)
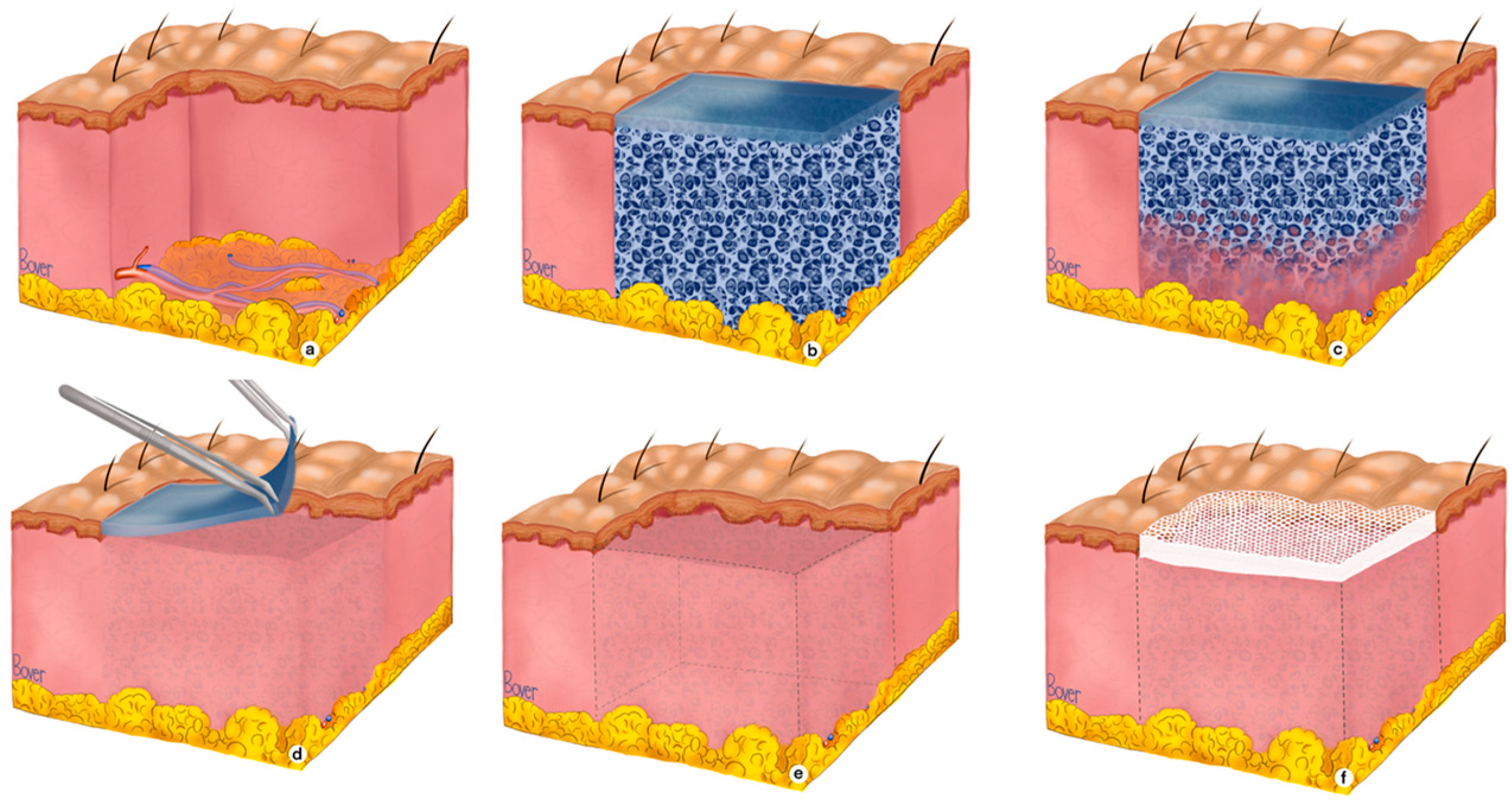
2.2. BTM Application Protocol for Complex Full-Thickness Wounds
3. Results
3.1. Case Descriptions
3.1.1. Patient 1

3.1.2. Patient 2

3.1.3. Patient 3
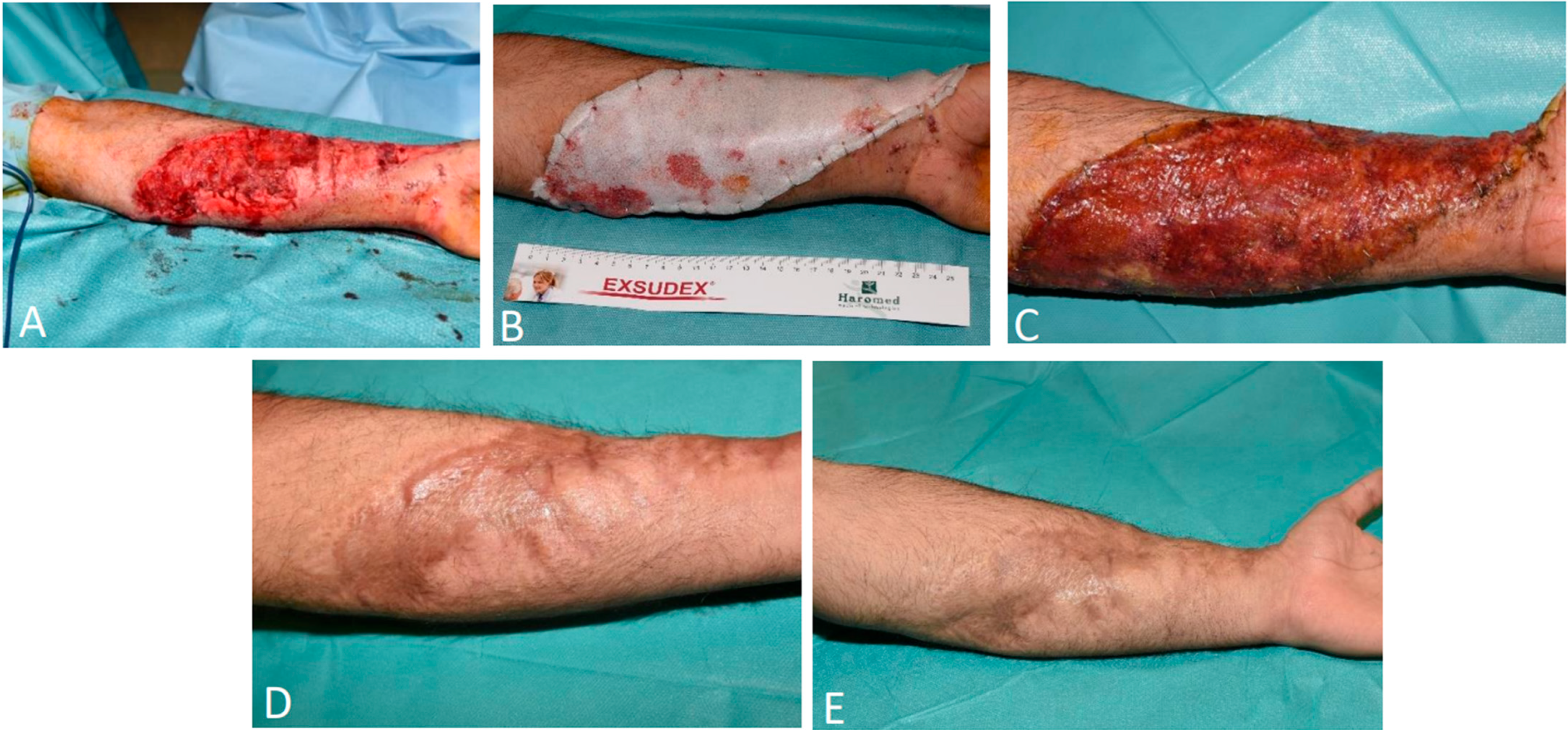
3.1.4. Patient 4

3.1.5. Patient 5
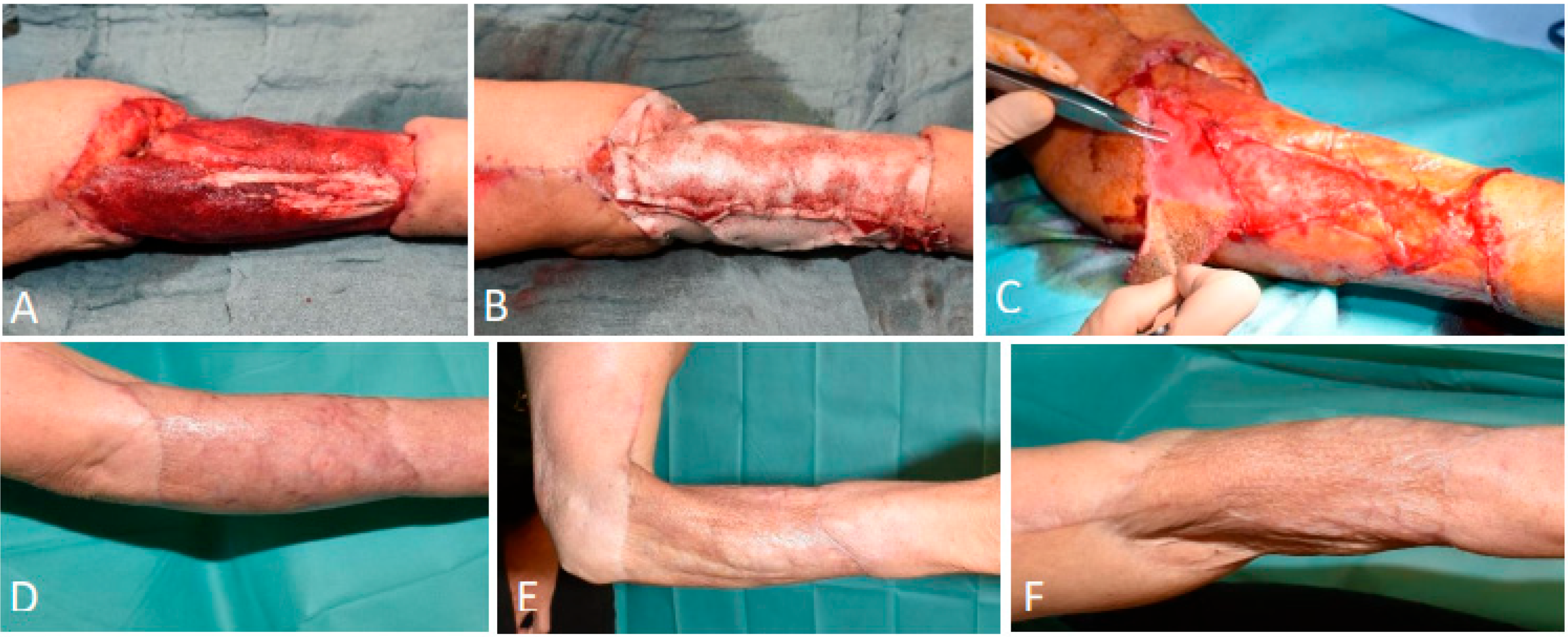
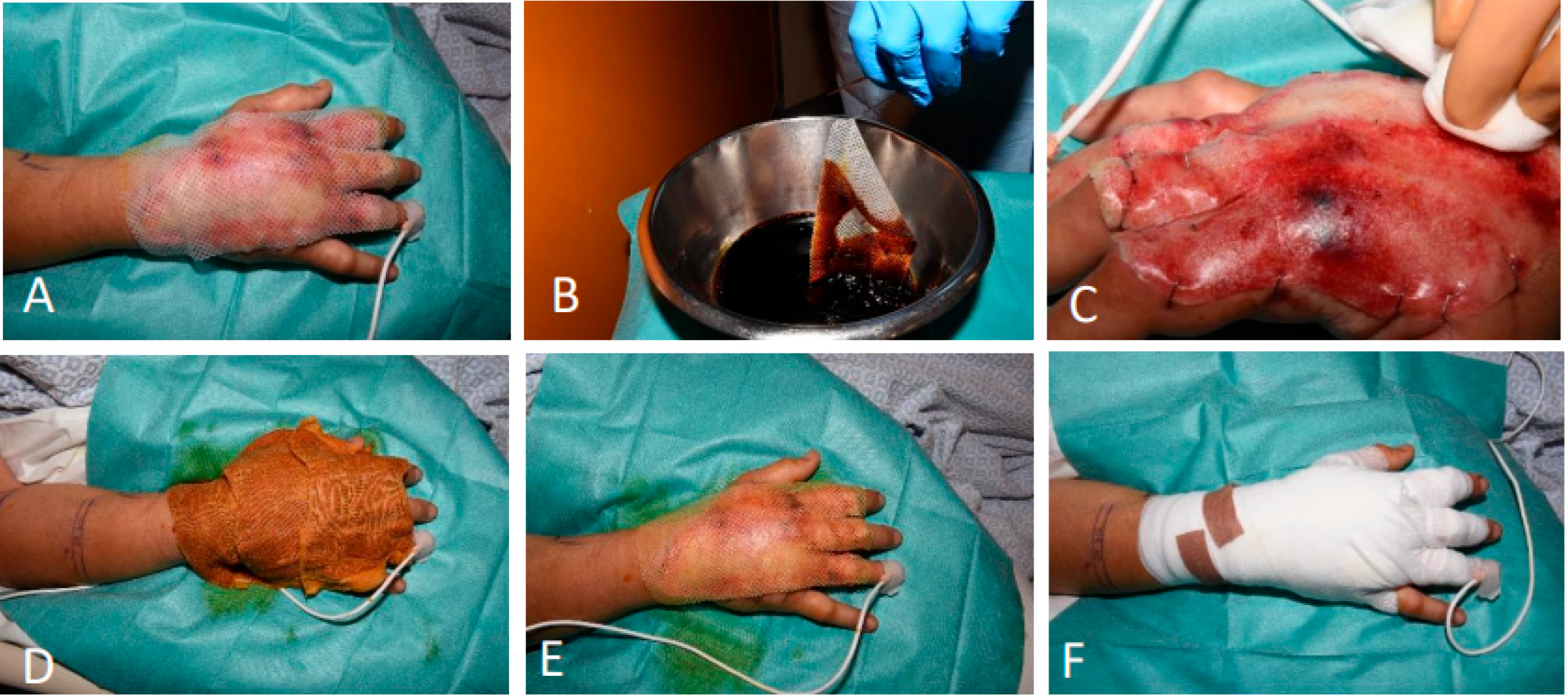
3.1.6. Patient 6

4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FTSDs | Full-thickness skin defects |
| BTM | Biodegradable temporising matrix |
| SOC | Standard of care |
| STSG | Split-thickness skin grafting |
| DRT | Dermal regeneration templates |
| NPWT | Negative pressure wound therapy |
| UV | Ultraviolet |
| PTA | Percutaneous transluminal angioplasty |
| ALT | Anterolateral thigh |
| LDI | Laser Doppler imaging |
| EDNX | Nexobrid |
References
- Kemp Bohan, P.M.; Cooper, L.E.; Fletcher, J.L.; Corkins, C.J.; Natesan, S.; Aden, J.K.; Carlsson, A.; Chan, R.K. Impact of dermal matrix thickness on split-thickness skin graft survival and wound contraction in a single-stage procedure. Int. Wound J. 2022, 19, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Obed, D.; Bingöl, A.S.; März, V.; Vogt, P.M.; Krezdorn, N. Treatment of Complex Wounds with NovoSorb® Biodegradable Temporising Matrix (BTM)—A Retrospective Analysis of Clinical Outcomes. J. Pers. Med. 2022, 12, 2002. [Google Scholar] [CrossRef]
- De Decker, I.; Szabó, A.; Hoeksema, H.; Speeckaert, M.; Delanghe, J.R.; Blondeel, P.; Van Vlierberghe, S.; Monstrey, S.; Claes, K.E. Treatment of hypertrophic scars with corticoid-embedded dissolving microneedles. J. Burn Care Res. 2022, 44, 158–169. [Google Scholar] [CrossRef]
- Pirayesh, A.; Decker, I.D.; Richters, C.D.; Paauw, N.J.; Hoeksema, H.; Hoekstra, M.J.; Claes, K.E.Y.; Van Der Lei, B.; Monstrey, S. Comparison of Glyaderm with different dermal substitute matrices in a porcine wound model. JPRAS Open 2022, 34, 257–267. [Google Scholar] [CrossRef]
- Claes, K.; Hoeksema, H.; Lafaire, C.; De Cuyper, L.; De Groote, K.; Vyncke, T.; De Deckera, I.; Verbelen, J.; De Coninck, P.; Depypereb, B.; et al. The process to obtain reimbursement for enzymatic debridement in clinically deep burns. Acta Chir. Belg. 2021, 123, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Rijpma, D.; Claes, K.; Hoeksema, H.; De Decker, I.; Verbelen, J.; Monstrey, S.; Pijpe, A.; van Zuijlen, P.; Meij-de Vries, A. The Meek micrograft technique for burns; review on its outcomes: Searching for the superior skin grafting technique. Burns 2022, 48, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- De Decker, I.; De Graeve, L.; Hoeksema, H.; Monstrey, S.; Verbelen, J.; De Coninck, P.; Ghen, E.; Claes, K.E. Enzymatic debridement: Past, present, and future. Acta Chir. Belg. 2022, 122, 279–295. [Google Scholar] [CrossRef]
- Pirayesh, A.; Hoeksema, H.; Richters, C.; Verbelen, J.; Monstrey, S. Glyaderm dermal substitute: Clinical application and long-term results in 55 patients. Burns 2014, 41, 132–144. [Google Scholar] [CrossRef]
- Verbelen, J.; Hoeksema, H.; Pirayesh, A.; Van Landuyt, K.; Monstrey, S. Exposed tibial bone after burns: Flap reconstruction versus dermal substitute. Burns 2016, 42, e31–e37. [Google Scholar] [CrossRef]
- Claes, K.E.; De Decker, I.; Monstrey, S.; Shoham, Y.; Vyncke, T.; Depypere, B.; De Wolf, E.; Decuypere, F.; Lannau, B.; Hoeksema, H. Helpful hints in deciding what and when to operate after enzymatic debridement. Burns 2023, 49, 80–90. [Google Scholar] [CrossRef]
- De Decker, I.; Hoeksema, H.; Vanlerberghe, E.; Beeckman, A.; Verbelen, J.; De Coninck, P.; Speeckaert, M.M.; Blondeel, P.; Monstrey, S.; Claes, K.E. Occlusion and hydration of scars: Moisturizers versus silicone gels. Burns 2023, 49, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Rijpma, D.; Pijpe, A.; Claes, K.; Hoeksema, H.; de Decker, I.; Verbelen, J.; van Zuijlen, P.; Monstrey, S.; Vries, A.M.-D. Outcomes of Meek micrografting versus mesh grafting on deep dermal and full thickness (burn) wounds: Study protocol for an intra-patient randomized controlled trial. PLoS ONE 2023, 18, e0281347. [Google Scholar] [CrossRef] [PubMed]
- Hierner, R.; Degreef, H.; Vranckx, J.J.; Garmyn, M.; Massagé, P.; van Brussel, M. Skin grafting and wound healing-the “dermato-plastic team approach”. Clin. Dermatol. 2005, 23, 343–352. [Google Scholar] [CrossRef]
- Mishra, A.; Rabiee, S.; Opel, S.; Jones, I. Applying the modified Meek technique to heal smaller burns: A retrospective review. Burn. Open 2022, 6, 120–124. [Google Scholar] [CrossRef]
- Asuku, M.; Yu, T.C.; Yan, Q.; Böing, E.; Hahn, H.; Hovland, S.; Donelan, M.B. Split-thickness skin graft donor-site morbidity: A systematic literature review. Burns 2021, 47, 1525–1546. [Google Scholar] [CrossRef]
- Tapking, C.; Thomas, B.F.; Hundeshagen, G.; Haug, V.F.M.; Gazyakan, E.; Bliesener, B.; Bigdeli, A.K.; Kneser, U.; Vollbach, F.H. NovoSorb® Biodegradable Temporising Matrix (BTM): What we learned from the first 300 consecutive cases. J. Plast. Reconstr. Aesthetic Surg. 2024, 92, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg.–Glob. Open 2015, 3, 63–72. [Google Scholar] [CrossRef]
- Li, H.; Lim, P.; Stanley, E.; Lee, G.; Lin, S.; Neoh, D.; Liew, J.; Ng, S.K. Experience with NovoSorb® Biodegradable Temporising Matrix in reconstruction of complex wounds. ANZ J. Surg. 2021, 91, 1744–1750. [Google Scholar] [CrossRef]
- Claes, K.E.Y.; Amar, S.; Hoeksema, H.; Kornhaber, R.; de Jong, A.; Monstrey, S.; Haik, J.; Biros, E.; Harats, M. Pain management during a bromelain-based selective enzymatic debridement in paediatric and adult burn patients. Burns 2022, 48, 555–567. [Google Scholar] [CrossRef]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef]
- Claes, K.E.Y.; De Decker, I.; Vyncke, T.; Verbelen, J.; Dhooghe, N.; Monstrey, S.; Hoeksema, H. Enzymatic Debridement with Nexobrid ® Reduces Surgery in Laser Doppler Imaging-Confirmed Deep Burns. Ann. Burn. Fire Disasters 2023, 36, 347. [Google Scholar]
- Egemen, O.; Ozkaya, O.; Ozturk, M.B.; Aksan, T.; Orman, Ç.; Akan, M. Effective use of negative pressure wound therapy provides quick wound-bed preparation and complete graft take in the management of chronic venous ulcers. Int. Wound J. 2012, 9, 199–205. [Google Scholar] [CrossRef]
- Snyder, R.J. Treatment of nonhealing ulcers with allografts. Clin. Dermatol. 2005, 23, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Struble, S.L.; Patel, N.K.; Graham, E.M.; Tipps, J.A.; Vaile, J.R.; Leeflang, E.J.; Goodwin, I.; Mendenhall, S.D. Outcomes of Biodegradable Temporizing Matrix for Soft Tissue Reconstruction of the Hand and Extremities. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5956. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, F.P.; Clark, R.A.; Miller, M.; Delaney, C.L. Overcoming Barriers to Wound Healing in a Neuropathic and Neuro-Ischaemic Diabetic Foot Cohort Using a Novel Bilayer Biodegradable Synthetic Matrix. Biomedicines 2023, 11, 721. [Google Scholar] [CrossRef]
- Concannon, E.; Coghlan, P.; Damkat Thomas, L.; Solanki, N.S.; Greenwood, J.E. Biodegradable temporizing matrix reconstruction of complex perineal burn wound: A case report. J. Burn Care Res. 2021, 42, 1038–1042. [Google Scholar] [CrossRef]
- Wagstaff, M.J.D.; Schmitt, B.J.; Coghlan, P.; Finkemeyer, J.P.; Caplash, Y.; Greenwood, J.E. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: A pilot study. Eplasty 2015, 15, e13. [Google Scholar]
- Wagstaff, M.J.; Caplash, Y.; Greenwood, J.E. Reconstruction of an Anterior Cervical Necrotizing Fasciitis Defect Using a Biodegradable Polyurethane Dermal Substitute. Eplasty 2017, 17, e3. [Google Scholar]
- Cheshire, P.A.; Herson, M.R.; Cleland, H.; Akbarzadeh, S. Artificial dermal templates: A comparative study of NovoSorbTM Biodegradable Temporising Matrix (BTM) and Integra® Dermal Regeneration Template (DRT). Burns 2016, 42, 1088–1096. [Google Scholar] [CrossRef]
- Greenwood, J.E.; Schmitt, B.J.; Wagstaff, M.J.D. Experience with a synthetic bilayer Biodegradable Temporising Matrix in significant burn injury. Burn. Open 2018, 2, 17–34. [Google Scholar] [CrossRef]
- Wagstaff, M.J.D.; Schmitt, B.J.; Caplash, Y.; Greenwood, J.E. Free Flap Donor Site Reconstruction: A Prospective Case Series Using an Optimized Polyurethane Biodegradable Temporizing Matrix. Eplasty 2015, 15, e27. [Google Scholar]
- Betar, N.; Maher, D.; Wheatley, L.; Barker, T.; Brown, J. Clinical outcomes and resource utilisation in patients with major burns treated with NovoSorb® BTM. Burns 2023, 49, 1663–1669. [Google Scholar] [CrossRef]
- Lo, C.H.; Brown, J.N.; Dantzer, E.J.G.; Maitz, P.K.M.; Vandervord, J.G.; Wagstaff, M.J.D.; Barker, T.M.; Cleland, H. Wound healing and dermal regeneration in severe burn patients treated with NovoSorb® Biodegradable Temporising Matrix: A prospective clinical study. Burns 2022, 48, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Tapking, C.; Panayi, A.C.; Hundeshagen, G.; Thomas, B.F.; Gazyakan, E.; Bliesener, B.; Bigdeli, A.K.; Kneser, U.; Vollbach, F.H. The Application of a Synthetic Biodegradable Temporizing Matrix in Extensive Burn Injury: A Unicenter Experience of 175 Cases. J. Clin. Med. 2024, 13, 2661. [Google Scholar] [CrossRef] [PubMed]
- De Decker, I.; Beeckman, A.; Hoeksema, H.; De Mey, K.; Verbelen, J.; De Coninck, P.; Blondeel, P.; Speeckaert, M.M.; Monstrey, S.; Claes, K.E. Pressure therapy for scars: Myth or reality? A systematic review. Burns 2023, 49, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Wagstaff, M.J.D.; Barker, T.M.; Damkat-Thomas, L.; Salerno, S.; Holden, D.; Concannon, E.; Heath, K.; Coghlan, P.; Cleland, H. Long-term scarring outcomes and safety of patients treated with NovoSorb® Biodegradable Temporizing Matrix (BTM): An observational cohort study. JPRAS Open 2023, 37, 42–51. [Google Scholar] [CrossRef]
- Solanki, N.S.; York, B.; Gao, Y.; Baker, P.; Wong She, R.B. A consecutive case series of defects reconstructed using NovoSorb® Biodegradable Temporising Matrix: Initial experience and early results. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1845–1853. [Google Scholar] [CrossRef]
- Damkat-Thomas, L.; Greenwood, J.E.; Wagstaff, M.J.D. A Synthetic Biodegradable Temporising Matrix in Degloving Lower Extremity Trauma Reconstruction: A Case Report. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2110. [Google Scholar] [CrossRef]
- Knightly, N.; de Blacam, C. NovoSorb Biodegradable Temporizing Matrix for Reconstruction of Multiplanar Degloving Injury of the Upper Limb. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4909. [Google Scholar] [CrossRef]
- Kuang, B.; Pena, G.; Cowled, P.; Fitridge, R.; Greenwood, J.; Wagstaff, M.; Dawson, J. Use of Biodegradable Temporising Matrix (BTM) in the reconstruction of diabetic foot wounds: A pilot study. Scars Burn. Health 2022, 8, 20595131221122272. [Google Scholar] [CrossRef]
- Concannon, E.; Damkat-Thomas, L.; Rose, E.; Coghlan, P.; Solanki, N.; Wagstaff, M. Use of a Synthetic Dermal Matrix for Reconstruction of 55 Patients with Nongraftable Wounds and Management of Complications. J. Burn Care Res. 2023, 44, 894–904. [Google Scholar] [CrossRef] [PubMed]
- NovoSorb® BTM|Synthetic Polymer for Traumatic Wound Treatment. Available online: https://polynovo.com/new-version-to-serve-de-in-de-for-novosorb-btm/ (accessed on 23 May 2023).
- Austin, C.L.; Draper, B.; Larson, K.W.; Thompson, S.J. Biodegradable temporising matrix: Use of negative pressure wound therapy shows a significantly higher success rate. J. Wound Care 2023, 32, 159–166. [Google Scholar] [CrossRef]
- Najem, S.; Fattouh, M.; Wintges, K.; Schoof, B.; Koerner, M.; Reinshagen, K.; Koenigs, I. NovoSorb® Biodegradable Temporizing Matrix: A novel approach for treatment of extremity avulsion injuries in children. Eur. J. Trauma Emerg. Surg. 2024, 50, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, W.; Tan, J.; Maitz, P.K.M. Efficacy of dermal substitute on deep dermal to full thickness burn injury: A systematic review. ANZ J. Surg. 2017, 87, 446–452. [Google Scholar] [CrossRef]
- van den Bosch, A.S.; Verwilligen, R.A.F.; Pijpe, A.; Bosma, E.; van der Vlies, C.H.; Lucas, Y.; Burchell, G.L.; van Zuijlen, P.P.M.; Middelkoop, E. Outcomes of dermal substitutes in burns and burn scar reconstruction: A systematic review and meta-analysis. Wound Repair Regen. 2024, 32, 960–978. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.E.; Dearman, B.L. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra® dermal regeneration template in a porcine wound model. J. Burn Care Res. 2012, 33, 163–173. [Google Scholar] [CrossRef]
- Jou, C.; Chepla, K.J. Reconstruction of Complex Upper Extremity Wounds with Novosorb Biodegradable Temporizing Matrix Versus Integra Collagen-Chondroitin Silicone: A Cost Analysis. Eplasty 2024, 24, e38. [Google Scholar]
- Dickson, K.; Lee, K.C.; Abdulsalam, A.; Amirize, E.; Kankam, H.K.N.; Ter Horst, B.; Gardiner, F.; Bamford, A.; Hejmadi, R.K.; Moiemen, N. A Histological and Clinical Study of MatriDerm® Use in Burn Reconstruction. J. Burn Care Res. 2023, 44, 1100–1109. [Google Scholar] [CrossRef]
- De Decker, I.; Hoeksema, H.; Verbelen, J.; De Coninck, P.; Speeckaert, M.; De Schepper, S.; Blondeel, P.; Pirayesh, A.; Monstrey, S.; Claes, K.E.Y. A single-stage bilayered skin reconstruction using Glyaderm® as an acellular dermal regeneration template results in improved scar quality: An intra-individual randomized controlled trial. Burn. Trauma 2023, 11, tkad015. [Google Scholar] [CrossRef]
- Wu-Fienberg, Y.; Wu, S.S.; Gatherwright, J.; Chepla, K.J. An Alternative Dermal Template for Reconstruction of Complex Upper Extremity Wounds. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3674. [Google Scholar] [CrossRef]
- Greenwood, J.E.; Dearman, B.L. Split skin graft application over an integrating, biodegradable temporizing polymer matrix: Immediate and delayed. J. Burn Care Res. 2012, 33, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dearman, B.L.; Crompton, K.E.; Moore, T.G.; Greenwood, J.E. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J. Burn Care Res. 2009, 30, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Carrière, M.E.; Tyack, Z.; Westerman, M.J.; Pleat, J.; Pijpe, A.; van Zuijlen, P.P.M.; de Vet, H.; Mokkink, L. From qualitative data to a measurement instrument: A clarification and elaboration of choices made in the development of the Patient Scale of the Patient and Observer Scar Assessment Scale (POSAS) 3.0. Burns 2023, 49, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.N.; Thraen, A.C.J.; Gohritz, A.; Rennekampff, H.O.; Vogt, P.M. Burn Scar Evaluation Using the Cutometer ® MPA 580 in Comparison to “patient and Observer Scar Assessment Scale” and “vancouver Scar Scale”. J. Burn Care Res. 2018, 39, 516–526. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adult patients with full-thickness skin defects Able to provide written informed consent | Children and adolescents (<18 y) Patients with superficial and partial thickness skin defects Inability to provide informed consent due to health condition or refusal to sign |
| Patient Demographics | Wound Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Age (y) | Gender | Localization | Etiology | Time to BTM (d) | Time to STSG (d) | Total BTM Integration Time (d) | Complications | NPWT After BTM Application |
| 1 | 40 | F | Upper leg Suprapubic Iliac region | Necrotizing fasciitis | 25 | 47 | 22 | None | Yes |
| 2 | 54 | M | Leg | Degloving | 23 | 46 | 23 | None | No |
| 3 | 18 | M | Forearm | Degloving | 4 | 26 | 22 | None | Yes |
| 4 | 63 | M | Ankle | Ulcerations | 28 | 51 | 23 | Hypergranulation with secondary infection | Yes |
| 5 | 63 | F | Forearm | Degloving | 32 | 53 | 21 | None | Yes |
| 6 | 42 | M | Hand | Burn | 2 | 15 | 13 | Partial integration | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Durme, J.; Dhont, T.; De Decker, I.; Van Waeyenberghe, M.; De Mey, K.; Hoeksema, H.; Verbelen, J.; De Coninck, P.; Roche, N.A.; Blondeel, P.; et al. The Reconstruction of Various Complex Full-Thickness Skin Defects with a Biodegradable Temporising Matrix: A Case Series. Eur. Burn J. 2025, 6, 24. https://doi.org/10.3390/ebj6020024
van Durme J, Dhont T, De Decker I, Van Waeyenberghe M, De Mey K, Hoeksema H, Verbelen J, De Coninck P, Roche NA, Blondeel P, et al. The Reconstruction of Various Complex Full-Thickness Skin Defects with a Biodegradable Temporising Matrix: A Case Series. European Burn Journal. 2025; 6(2):24. https://doi.org/10.3390/ebj6020024
Chicago/Turabian Stylevan Durme, Julie, Thibaut Dhont, Ignace De Decker, Michiel Van Waeyenberghe, Kimberly De Mey, Henk Hoeksema, Jozef Verbelen, Petra De Coninck, Nathalie A. Roche, Phillip Blondeel, and et al. 2025. "The Reconstruction of Various Complex Full-Thickness Skin Defects with a Biodegradable Temporising Matrix: A Case Series" European Burn Journal 6, no. 2: 24. https://doi.org/10.3390/ebj6020024
APA Stylevan Durme, J., Dhont, T., De Decker, I., Van Waeyenberghe, M., De Mey, K., Hoeksema, H., Verbelen, J., De Coninck, P., Roche, N. A., Blondeel, P., Monstrey, S., & Claes, K. E. Y. (2025). The Reconstruction of Various Complex Full-Thickness Skin Defects with a Biodegradable Temporising Matrix: A Case Series. European Burn Journal, 6(2), 24. https://doi.org/10.3390/ebj6020024






