Novel Techniques in Fractional Skin Replacement
Abstract
1. Introduction
2. Principles of Fractional Skin Replacement
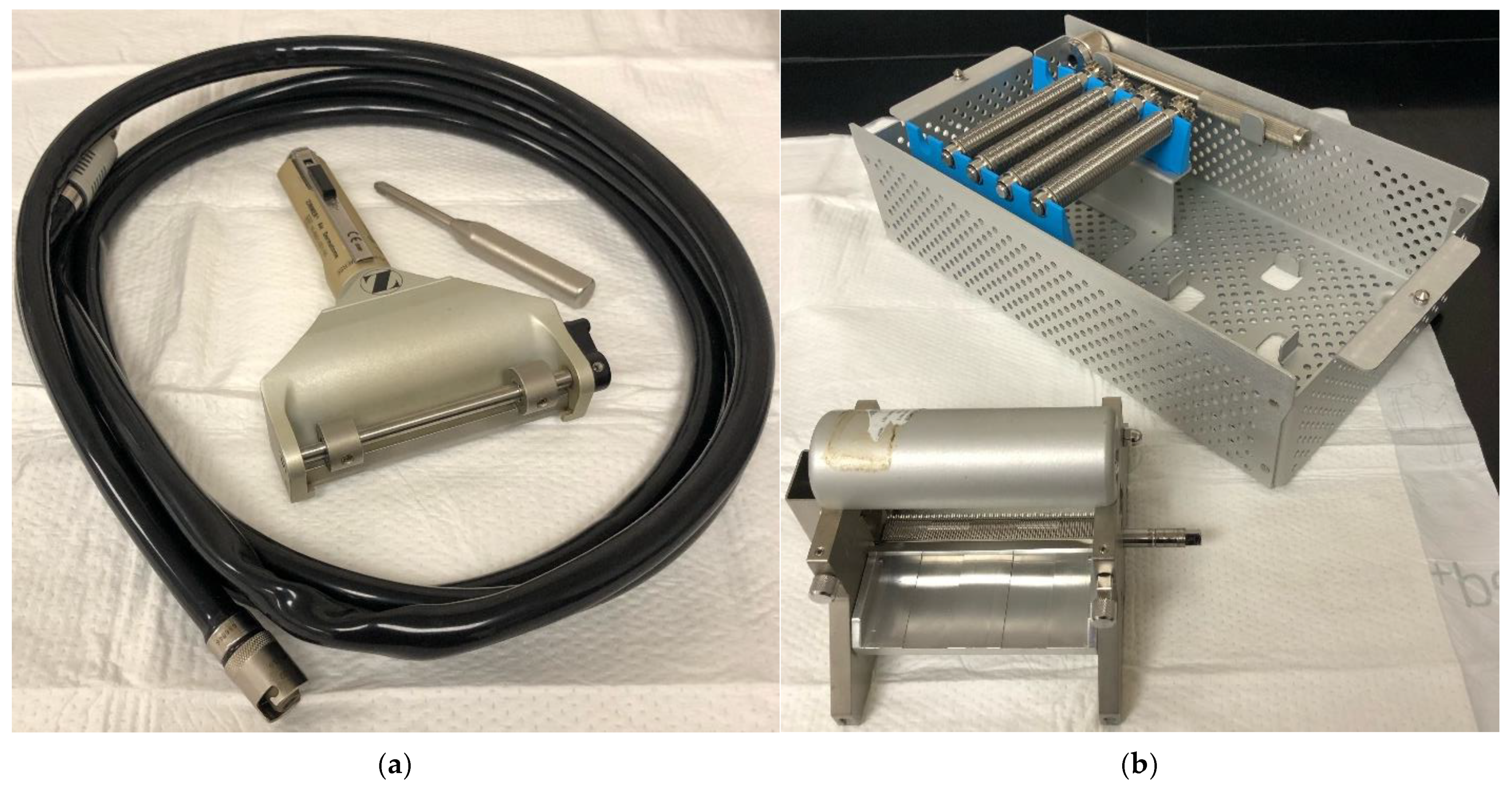
2.1. Xpansion Micrografting and Pixel Grafting
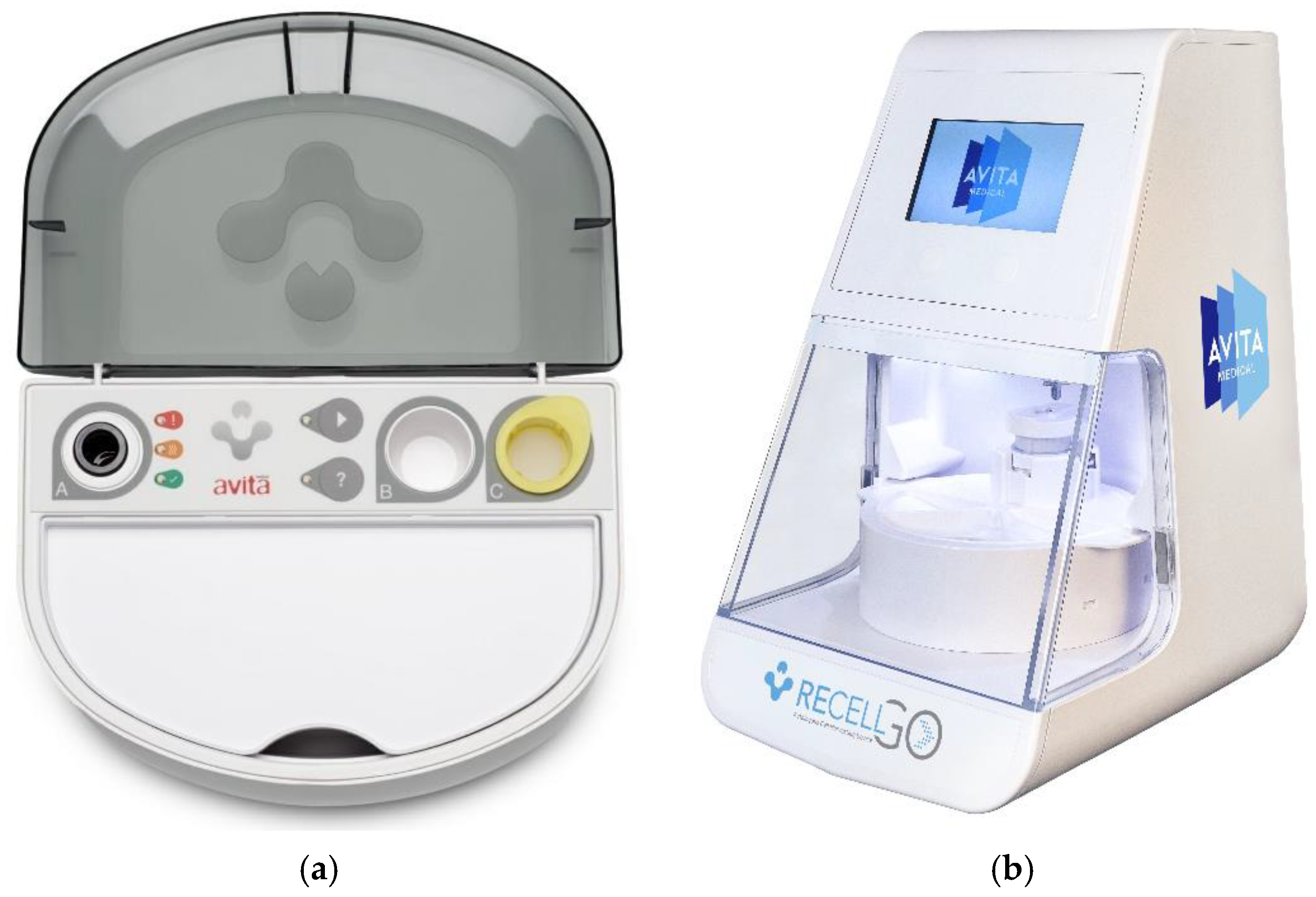
2.2. RECELL®
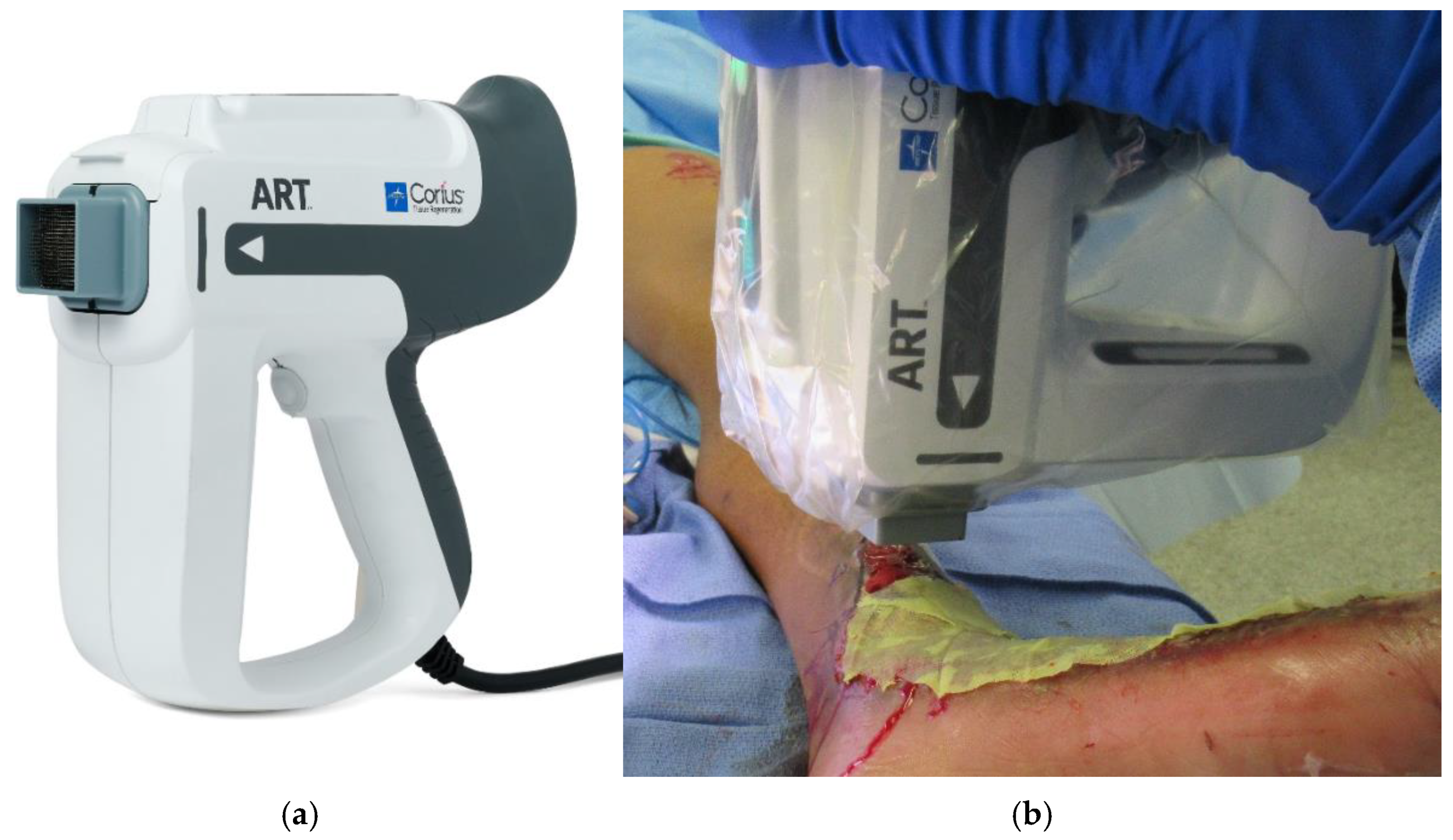
2.3. Full Thickness Skin Columns
2.4. Full Thickness Skin Columns in Development
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, D.C.; Ramsey, M.L. Grafts in Dermatologic Surgery: Review and Update on Full- and Split-Thickness Skin Grafts, Free Cartilage Grafts, and Composite Grafts. Dermatol. Surg. 2005, 31, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.A.; Carter, J.E.; Wilde, S.; Chamberlain, A.; Walsh, T.P.; Sparks, J.A. Autologous Skin Cell Suspension for Full-Thickness Skin Defect Reconstruction: Current Evidence and Health Economic Expectations. Adv. Ther. 2024, 41, 891–900. [Google Scholar] [CrossRef]
- Holmes, J.H.; Molnar, J.A.; Shupp, J.W.; Hickerson, W.L.; King, B.T.; Foster, K.N.; Cairns, B.A.; Carter, J.E. Demonstration of the Safety and Effectiveness of the RECELL® System Combined with Split-Thickness Meshed Autografts for the Reduction of Donor Skin to Treat Mixed-Depth Burn Injuries. Burns 2019, 45, 772–782. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Sanders, E.A.; Tomich, A.; Habershaw, G. Pinch Grafting: An Alternative Skin Grafting Technique. Foot Ankle Surg. Tech. Rep. Cases 2022, 2, 100214. [Google Scholar] [CrossRef]
- Kadam, D. Novel Expansion Techniques for Skin Grafts. Indian J. Plast. Surg. 2016, 49, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Nuutila, K.; Kruse, C.; Robson, M.C.; Caterson, E.; Eriksson, E. Challenging the Conventional Therapy: Emerging Skin Graft Techniques for Wound Healing. Plast. Reconstr. Surg. 2015, 136, 524e–530e. [Google Scholar] [CrossRef]
- Nuutila, K.; Mistry, R.; Broomhead, M.; Eriksson, E. Split-Thickness Skin and Dermal Pixel Grafts Can Be Expanded up to 500 Times to Re-Epithelialize a Full-Thickness Burn Wound. Adv. Wound Care 2024, 13, 176–186. [Google Scholar] [CrossRef]
- Sanches-Pinto, D.C.; Eriksson, E.; Gomez, D.S.; Nunes, M.P.T.; Gemperli, R.; Soriano, F.G. Minced Skin Grafts for Chronic Wounds Compared to Conventional Mesh Grafts. Health Sci. Rep. 2023, 6, e1353. [Google Scholar] [CrossRef]
- Laubach, H.-J.; Tannous, Z.; Anderson, R.R.; Manstein, D. Skin Responses to Fractional Photothermolysis. Lasers Surg. Med. 2006, 38, 142–149. [Google Scholar] [CrossRef]
- Tam, J.; Wang, Y.; Farinelli, W.A.; Jiménez-Lozano, J.; Franco, W.; Sakamoto, F.H.; Cheung, E.J.; Purschke, M.; Doukas, A.G.; Anderson, R.R. Fractional Skin Harvesting: Autologous Skin Grafting without Donor-Site Morbidity. Plast. Reconstr. Surg. Glob. Open 2013, 1, e47. [Google Scholar] [CrossRef]
- Fernandes, J.R.; Samayoa, J.C.; Broelsch, G.F.; McCormack, M.C.; Nicholls, A.M.; Randolph, M.A.; Mihm, M.C.; Austen, W.G. Micro-Mechanical Fractional Skin Rejuvenation. Plast. Reconstr. Surg. 2013, 131, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Wang, Y.; Vuong, L.N.; Fisher, J.M.; Farinelli, W.A.; Anderson, R.R. Reconstitution of Full-Thickness Skin by Microcolumn Grafting. J. Tissue Eng. Regen. Med. 2017, 11, 2796–2805. [Google Scholar] [CrossRef]
- Broccoli, N.; Rechtin, M.; Krishnan, D.G.; Phero, J.A. Step-by-Step: Skin Grafting. J. Oral Maxillofac. Surg. 2020, 78, e6–e10. [Google Scholar] [CrossRef] [PubMed]
- Kohlhauser, M.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P. Historical Evolution of Skin Grafting-A Journey through Time. Medicina 2021, 57, 348. [Google Scholar] [CrossRef]
- Ozhathil, D.K.; Tay, M.W.; Wolf, S.E.; Branski, L.K. A Narrative Review of the History of Skin Grafting in Burn Care. Medicina 2021, 57, 380. [Google Scholar] [CrossRef]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ramsey, M.L.; Walker, B.; Patel, B.C. Full-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shin, S.E.; Spoer, D.; Franzoni, G.; Berger, L.; Hill, A.; Sayyed, A.A.; Noe, N.; Steinberg, J.S.; Attinger, C.E.; Evans, K.K. To Mesh or Not to Mesh: What Is the Ideal Meshing Ratio for Split Thickness Skin Grafting of the Lower Extremity? J. Foot Ankle Surg. 2024, 63, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Pripotnev, S.; Papp, A. Split Thickness Skin Graft Meshing Ratio Indications and Common Practices. Burns 2017, 43, 1775–1781. [Google Scholar] [CrossRef]
- Akita, S.; Hayashida, K.; Yoshimoto, H.; Fujioka, M.; Senju, C.; Morooka, S.; Nishimura, G.; Mukae, N.; Kobayashi, K.; Anraku, K.; et al. Novel Application of Cultured Epithelial Autografts (CEA) with Expanded Mesh Skin Grafting Over an Artificial Dermis or Dermal Wound Bed Preparation. Int. J. Mol. Sci. 2017, 19, 57. [Google Scholar] [CrossRef]
- Simman, R.; Phavixay, L. Split-Thickness Skin Grafts Remain the Gold Standard for the Closure of Large Acute and Chronic Wounds. J. Am. Coll. Certif. Wound Spec. 2011, 3, 55–59. [Google Scholar] [CrossRef][Green Version]
- Isaac, A.L.; Rose, J.; Armstrong, D.G. Mechanically Powered Negative Pressure Wound Therapy as a Bolster for Skin Grafting. Plast. Reconstr. Surg. Glob. Open 2014, 2, e103. [Google Scholar] [CrossRef]
- Asuku, M.; Yu, T.-C.; Yan, Q.; Böing, E.; Hahn, H.; Hovland, S.; Donelan, M.B. Split-Thickness Skin Graft Donor-Site Morbidity: A Systematic Literature Review. Burns 2021, 47, 1525–1546. [Google Scholar] [CrossRef]
- Rettinger, C.L.; Fletcher, J.L.; Carlsson, A.H.; Chan, R.K. Accelerated Epithelialization and Improved Wound Healing Metrics in Porcine Full-Thickness Wounds Transplanted with Full-Thickness Skin Micrografts. Wound Repair Regen. 2017, 25, 816–827. [Google Scholar] [CrossRef]
- Sharma, K.; Bullock, A.; Ralston, D.; MacNeil, S. Development of a One-Step Approach for the Reconstruction of Full Thickness Skin Defects Using Minced Split Thickness Skin Grafts and Biodegradable Synthetic Scaffolds as a Dermal Substitute. Burns 2014, 40, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bharara, M.; Hurst, C.; Armstrong, D.G.; Rilo, H. The Micrograft Concept for Wound Healing: Strategies and Applications. J. Diabetes Sci. Technol. 2010, 4, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Successful Microdermagrafting Using the Meek-Wall Microdermatome. Available online: https://colab.ws/articles/10.1016%2F0002-9610%2858%2990975-9 (accessed on 11 February 2025).
- Kreis, R.W.; Mackie, D.P.; Vloemans, A.W.; Hermans, R.P.; Hoekstra, M.J. Widely Expanded Postage Stamp Skin Grafts Using a Modified Meek Technique in Combination with an Allograft Overlay. Burns 1993, 19, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Pham, L.; Wang, Y.; Farinelli, W.A.; Anderson, R.R.; Tam, J. MagneTEskin-Reconstructing Skin by Magnetically Induced Assembly of Autologous Microtissue Cores. Sci. Adv. 2021, 7, eabj0864. [Google Scholar] [CrossRef]
- Hackl, F.; Bergmann, J.; Granter, S.R.; Koyama, T.; Kiwanuka, E.; Zuhaili, B.; Pomahac, B.; Caterson, E.J.; Junker, J.P.E.; Eriksson, E. Epidermal Regeneration by Micrograft Transplantation with Immediate 100-Fold Expansion. Plast. Reconstr. Surg. 2012, 129, 443e–452e. [Google Scholar] [CrossRef]
- Svensjö, T.; Pomahac, B.; Yao, F.; Slama, J.; Wasif, N.; Eriksson, E. Autologous Skin Transplantation: Comparison of Minced Skin to Other Techniques. J. Surg. Res. 2002, 103, 19–29. [Google Scholar] [CrossRef]
- Chavan, V.; Chittoria, R.; Elankumar, S.; Reddy, K.S.; Aggarwal, A.; Gupta, S.; Reddy, C.L.; Mohan, P.L.B. Pixel Grafting: A Novel Skin Graft Expansion Technique. J. Cutan. Aesthetic Surg. 2021, 14, 229–232. [Google Scholar] [CrossRef]
- Singh, M.; Nuutila, K.; Kruse, C.; Dermietzel, A.; Caterson, E.J.; Eriksson, E. Pixel Grafting: An Evolution of Mincing for Transplantation of Full-Thickness Wounds. Plast. Reconstr. Surg. 2016, 137, 92e–99e. [Google Scholar] [CrossRef]
- Holmes, J.H. A Brief History of RECELL® and Its Current Indications. J. Burn Care Res. 2023, 44, S48–S49. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.C.; Carolan-Rees, G. ReCell® Spray-On Skin System for Treating Skin Loss, Scarring and Depigmentation after Burn Injury: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy. 2019, 17, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Holmes Iv, J.H.; Molnar, J.A.; Carter, J.E.; Hwang, J.; Cairns, B.A.; King, B.T.; Smith, D.J.; Cruse, C.W.; Foster, K.N.; Peck, M.D.; et al. A Comparative Study of the ReCell® Device and Autologous Spit-Thickness Meshed Skin Graft in the Treatment of Acute Burn Injuries. J. Burn Care Res. 2018, 39, 694–702. [Google Scholar] [CrossRef]
- Cooper, L.; Gronet, E.; Carlsson, A.; Chan, R. Full-Thickness Skin Micro-Columns within a Dermal Matrix: A Novel Method for “Donor-Free” Skin Replacement. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3304. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.S.; Cooper, L.; Nuutila, K.; Chapa, J.; Christy, S.; Chan, R.K.; Carlsson, A.H. Full-Thickness Skin Columns: A Method to Reduce Healing Time and Donor Site Morbidity in Deep Partial-Thickness Burns. Wound Repair Regen. 2023, 31, 586–596. [Google Scholar] [CrossRef]
- Jaller, J.A.; Herskovitz, I.; Borda, L.J.; Mervis, J.; Darwin, E.; Hirt, P.A.; Lev-Tov, H.; Kirsner, R.S. Evaluation of Donor Site Pain After Fractional Autologous Full-Thickness Skin Grafting. Adv. Wound Care 2018, 7, 309–314. [Google Scholar] [CrossRef]
- Smith, S.; Curtis, B.; Nicholson, L.; Koshy, T.; Max, T.; Prevish, B.; Goedegebuure, M.; Manista, G.; Tam, J. Application of a Minimally Invasive Full-thickness Autologous Microcolumn Skin Harvesting Device for Donor Site Tissue Collection and Augmenting Wound Healing in a Porcine Wound Model. Int. Wound J. 2024, 21, e70094. [Google Scholar] [CrossRef]

| Technique | Wound Depth | Harvest Size | Expansion | Donor site Morbidity | Reepithelization/Graft Take | Common Uses |
|---|---|---|---|---|---|---|
| Traditional skin grafting | ||||||
| FTSG | Full | Limited by closure | 1:1 | Primary closure | 7–14 days | Smaller wounds In esthetic zone At articulating joints |
| STSG | Full | Limited by available skin | 1:1–1:9 | Significant | 7–14 days | Large wound |
| Fractional skin grafting | ||||||
| Meek Micrograft | Partial | 18 cm2 as 5 × 5 mm each * | 1:2–1:4 | Moderate | 14–21 days | Large wound |
| Xpansion Micrograft | Partial | 4 cm2 as 0.8 × 0.8 mm each * | Up to 1:100 | Moderate | 14–21 days | Large wound |
| Pixel Micrograft | Partial | 4 cm2 as 0.3 × 0.3 mm each * | Up to 1:400 | Minimal | 14–30 days | Large wound |
| RECELL | Partial | 24 cm2 * | 1:80 | Minimal | 30 days | Vitiligo, wounds, donor sites |
| FTSC | Full | 15.5 cm2 * | 1:10–1:50 | Negligible | 14 days 60 days (normal skin) | Vitiligo, wounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, C.; Chan, R.K.; Carlsson, A.H. Novel Techniques in Fractional Skin Replacement. Eur. Burn J. 2025, 6, 13. https://doi.org/10.3390/ebj6010013
Kelly C, Chan RK, Carlsson AH. Novel Techniques in Fractional Skin Replacement. European Burn Journal. 2025; 6(1):13. https://doi.org/10.3390/ebj6010013
Chicago/Turabian StyleKelly, Courtney, Rodney K. Chan, and Anders H. Carlsson. 2025. "Novel Techniques in Fractional Skin Replacement" European Burn Journal 6, no. 1: 13. https://doi.org/10.3390/ebj6010013
APA StyleKelly, C., Chan, R. K., & Carlsson, A. H. (2025). Novel Techniques in Fractional Skin Replacement. European Burn Journal, 6(1), 13. https://doi.org/10.3390/ebj6010013






