High-Voltage Electrical Burn Requiring Urgent Scalp Reconstruction after Developing a Brain Abscess
Abstract
1. Introduction
2. Case Report
- Low BMI—exclusive fruitarian diet.
- Laparoscopic partial gastrectomy (7 September 2022)—T4N0R0 GIST—Adjuvant imatinib from October to December 2022 (patient self-ceased).
- Robotic left partial nephrectomy (13 February 2020)—Histopathology: pT1b type 1 papillary RCC.
3. Discussion
- ▪
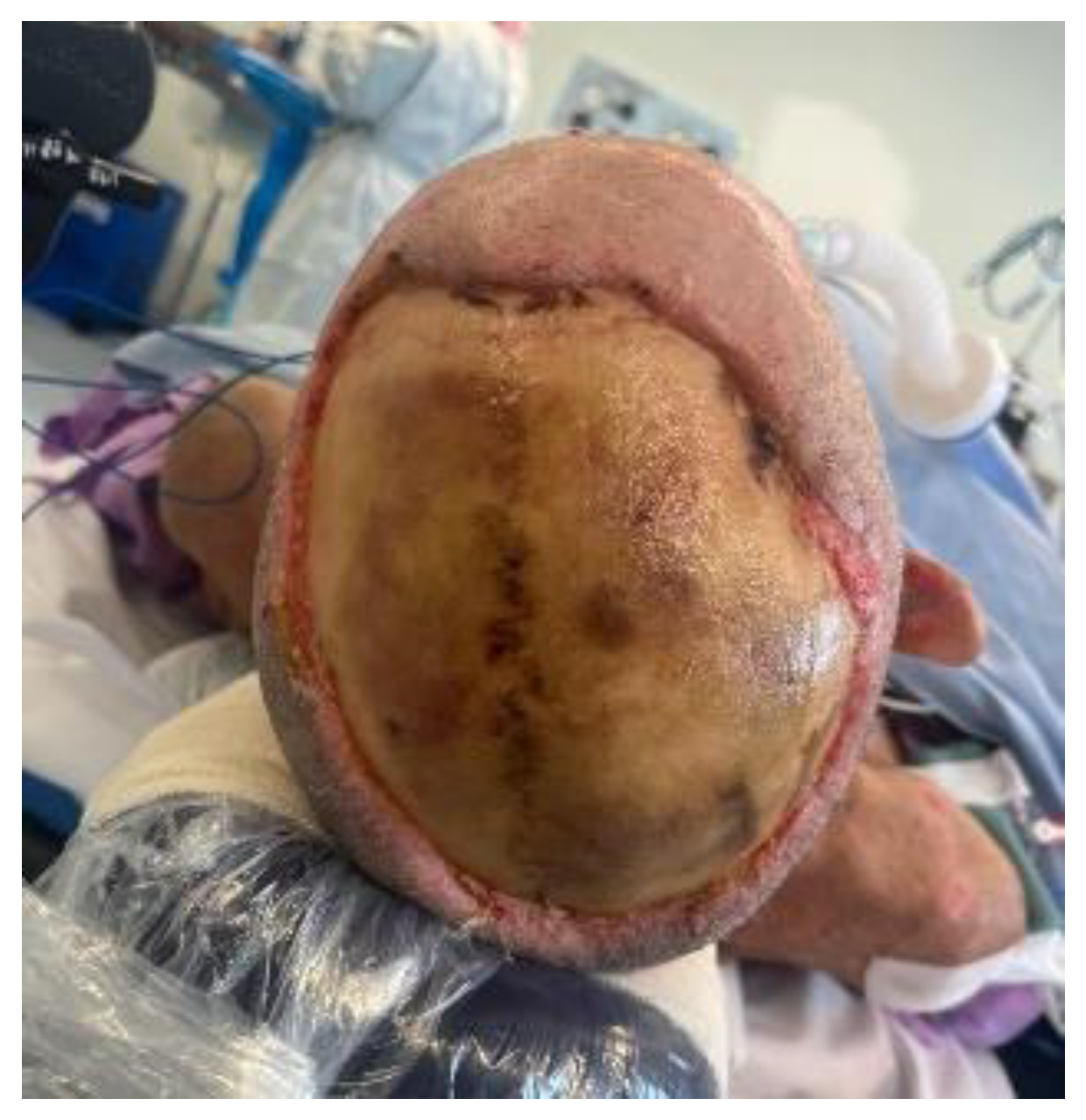
- Management of an extra-dural abscess in the context of a large scalp defect, with an intact calvarium exhibiting partial thickness loss of bone viability.
- ▪
- Challenges and surgical timing of tissue coverage given that definitive and urgent treatment of the abscess was required.
- ▪
- The neurological sequelae from his spinal cord injury as a result of the electrical burn.
- ▪
- Presence of an extensive full-thickness scalp defect and need for definitive coverage following the aspiration.
- ▪
- Porosity of the underlying bone affecting access and closure.
- ▪
- Perfusion of the calvarium.
- ▪
- Risk of intracranial re-accumulation post-graft and need for further surgery.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgadillo, D.; Chapman, S.; Fahrenkopf, M.P.; Martin, M.D. Acute-Onset Quadriplegia with Recovery after High-Voltage Electrical Injury. Ann. Plast. Surg. 2017, 79, e33–e36. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, S.S.; Benkova, E.G.; Chokoeva, A.A. High-voltage electrical injury: Modified surgical technique for optimal defect closuring of extra-large cranial defect. Dermatol. Ther. 2018, 31, e12581. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.A.; Ahmed, O.A.; Jibran Rabbani, M.; Saleem, M.; Amin, M.; Malik Mujahid, A.; Younas Mehrose, M.; Tarar, M.N.; Shahzad, F. An Algorithm for Reconstruction of Electrical Injuries of the Scalp. Plast. Reconstr. Surg. 2022, 150, 630e–638e. [Google Scholar] [CrossRef] [PubMed]
- Zemaitis, M.R.; Foris, L.A.; Lopez, R.A.; Huecker, M.R. Electrical Injuries [Internet]. Nih.gov. Publishing. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448087/ (accessed on 3 March 2024).
- Ko, S.H.; Chun, W.; Kim, H.C. Delayed spinal cord injury following electrical burns: A 7-year experience. Burns 2004, 30, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Kim, S.H.; Minn, Y.K. Electrodiagnostic Study of Peripheral Nerves in High-Voltage Electrical Injury. J. Burn. Care Res. 2014, 35, e230–e233. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.; Khan, M.M.; Dubepuria, R.; Cheruvu, V.P.R. Reconstruction of Scalp and Forehead Defects: Options and Strategies. Cureus 2023, 15, e41479. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.; Bhayana, S.; Nag, V. Cerebral Abscess: A Delayed Complication of Electrical Burns. Indian Pediatr. 2021, 58, 497. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, F.; Bloom, I.; Alexander, A.J. The Critical Role of Nutrition in Facial Plastic Surgery. Facial Plast. Surg. Clin. N. Am. 2019, 27, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.W.; Alem, S.E. Late onset quadriparesis in high voltage electrical burn—A case report. Burns Open 2022, 6, 169–172. [Google Scholar] [CrossRef]
- Koller, J.; Orságh, J. Delayed neurological sequelae of high-tension electrical burns. Burns 1989, 15, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, P.; Hussain, A.T.; Ahamed, A.R. Reconstruction of Extensive Post–Electric Burn Scalp Defects with Exposed Bones. Ann. Plast. Surg. 2018, 81, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.H. Exposure of the skull from burns. Br. J. Plast. Surg. 1951, 4, 279–292. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Jones, G.E.; Neligan, P.C.; Newman, M.I.; Phillips, B.T.; Sacks, J.M.; Zenn, M.R. Intraoperative laser angiography using the SPY system: Review of the literature and recommendations for use. Ann. Surg. Innov. Res. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]


| Time (Days) | Event |
|---|---|
| 0 | Initial injury |
| 2 | Patient presented to his local hospital and was subsequently transferred to a regional hospital. |
| 3 | Admitted to the Burns unit. |
| 4 | Initial neurological assessment in the Intensive Care Unit showed 4/5 power in bilateral limbs. |
| Normal MRI Brain and Spine. | |
| 5 | Tangential excision of the burn wound and application of BiobraneTM to the chest, abdomen, left forearm, and right flank. |
| 13 | Further debridement and application of allograft to the scalp, chest, right flank, and left forearm. |
| 16 | ASIA impairment scale completed. Total motor scores: upper extremity 37/50; lower extremity 7/25 Total sensory scores: light touch 72/112; pin prick 53/92 * |
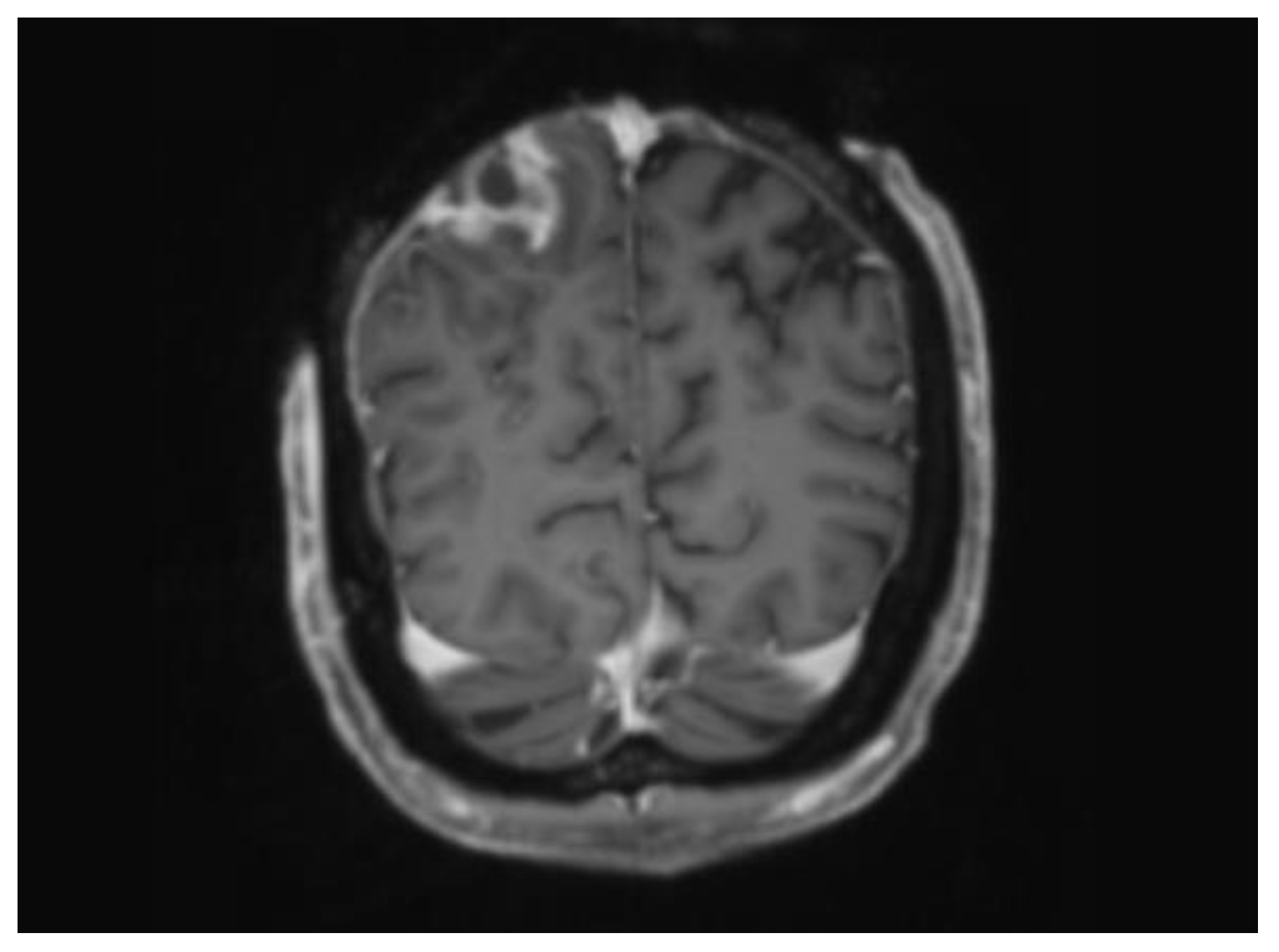
| 27 | CT Head demonstrating loss of bone marrow signal and a peripherally enhancing lesion highly suspicious for an underlying abscess. These findings were consistent with MRI imaging. |
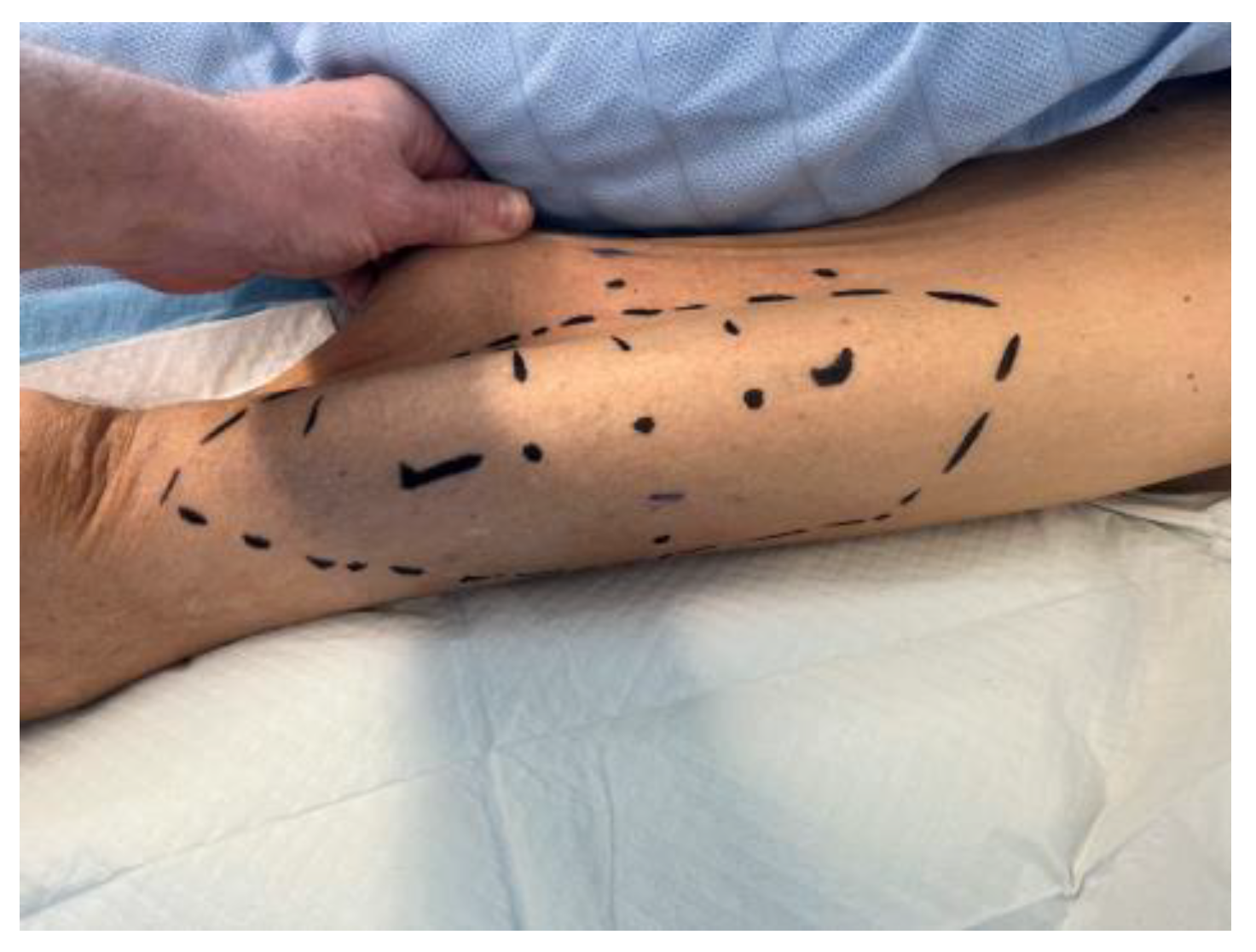
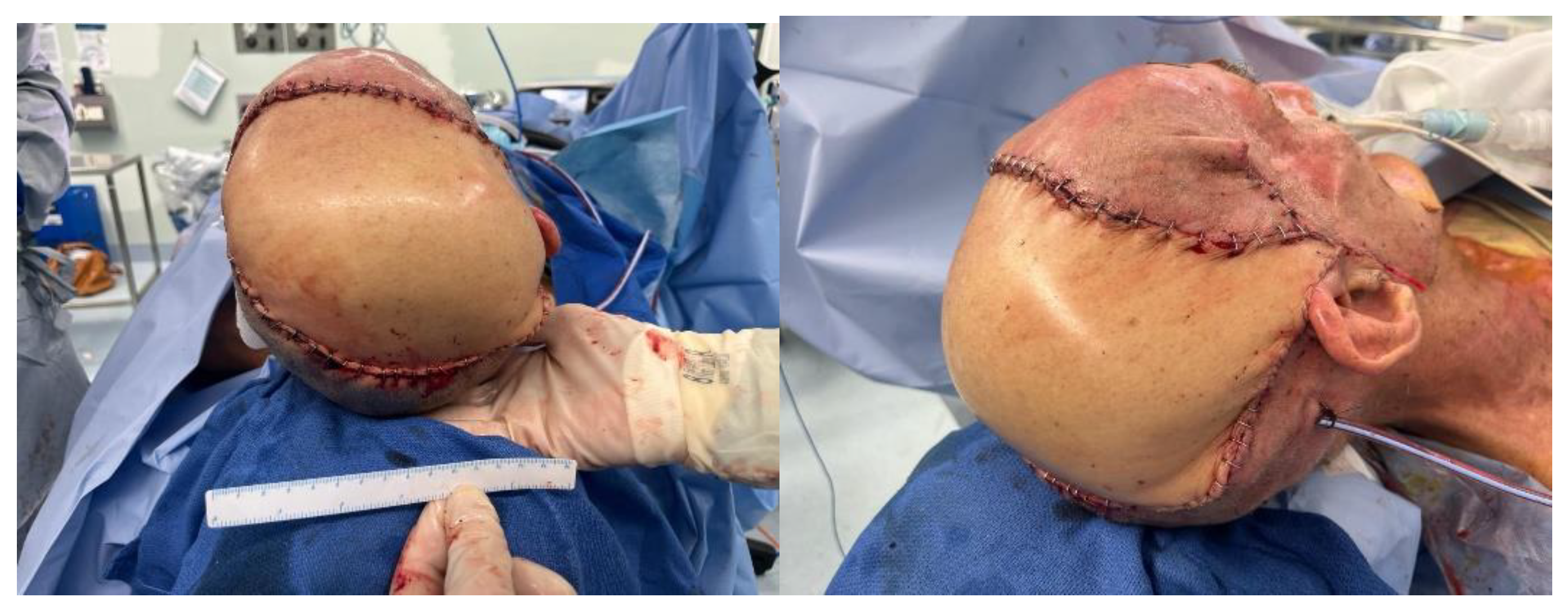
| 28 | Drainage of abscess and scalp reconstruction with a free myocutaneous flap from the anterolateral thigh. |
| 40 | MRI Brain revealing a superficial fluid filled collection in the subcutaneous tissue over the right parietal lobe. MRI spine showed symmetric T2 abnormalities within the cervical cord posterolaterally. |
| 42 | Debridement of remaining burn wound and split skin grafting to his chest, left forearm, and right back. |
| 43 | Ultrasound-guided drainage of subcutaneous collection. |
| 47 | MRI confirming interval reduction in size of the subcutaneous collection. No evidence of intracranial re-accumulation. |
| 53 | Repeat ASIA impairment scale: Total motor scores: upper extremity 36/50; lower extremity 13/25 Total sensory scores: light touch 62/72; pin prick 46/72 ** |
| 65 | Transferred to a regional hospital for rehabilitation. |
| 169 | Patient self-discharged from rehabilitation. He requires full-time carers and mobilises independently in a wheelchair. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blyth, E.; Vujcich, E.; Dunn, D. High-Voltage Electrical Burn Requiring Urgent Scalp Reconstruction after Developing a Brain Abscess. Eur. Burn J. 2024, 5, 288-295. https://doi.org/10.3390/ebj5030027
Blyth E, Vujcich E, Dunn D. High-Voltage Electrical Burn Requiring Urgent Scalp Reconstruction after Developing a Brain Abscess. European Burn Journal. 2024; 5(3):288-295. https://doi.org/10.3390/ebj5030027
Chicago/Turabian StyleBlyth, Elena, Elizabeth Vujcich, and Darryl Dunn. 2024. "High-Voltage Electrical Burn Requiring Urgent Scalp Reconstruction after Developing a Brain Abscess" European Burn Journal 5, no. 3: 288-295. https://doi.org/10.3390/ebj5030027
APA StyleBlyth, E., Vujcich, E., & Dunn, D. (2024). High-Voltage Electrical Burn Requiring Urgent Scalp Reconstruction after Developing a Brain Abscess. European Burn Journal, 5(3), 288-295. https://doi.org/10.3390/ebj5030027







