Total Body Surface Area Adjusted Daily Diagnostic Blood Loss May Be Higher in Minor Burns—Are Our Patients the Victims of Daily Routine?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Collected Clinical Data
2.2. Estimation of Diagnostic Blood Loss
2.3. Collection of Transfusion Data
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Data
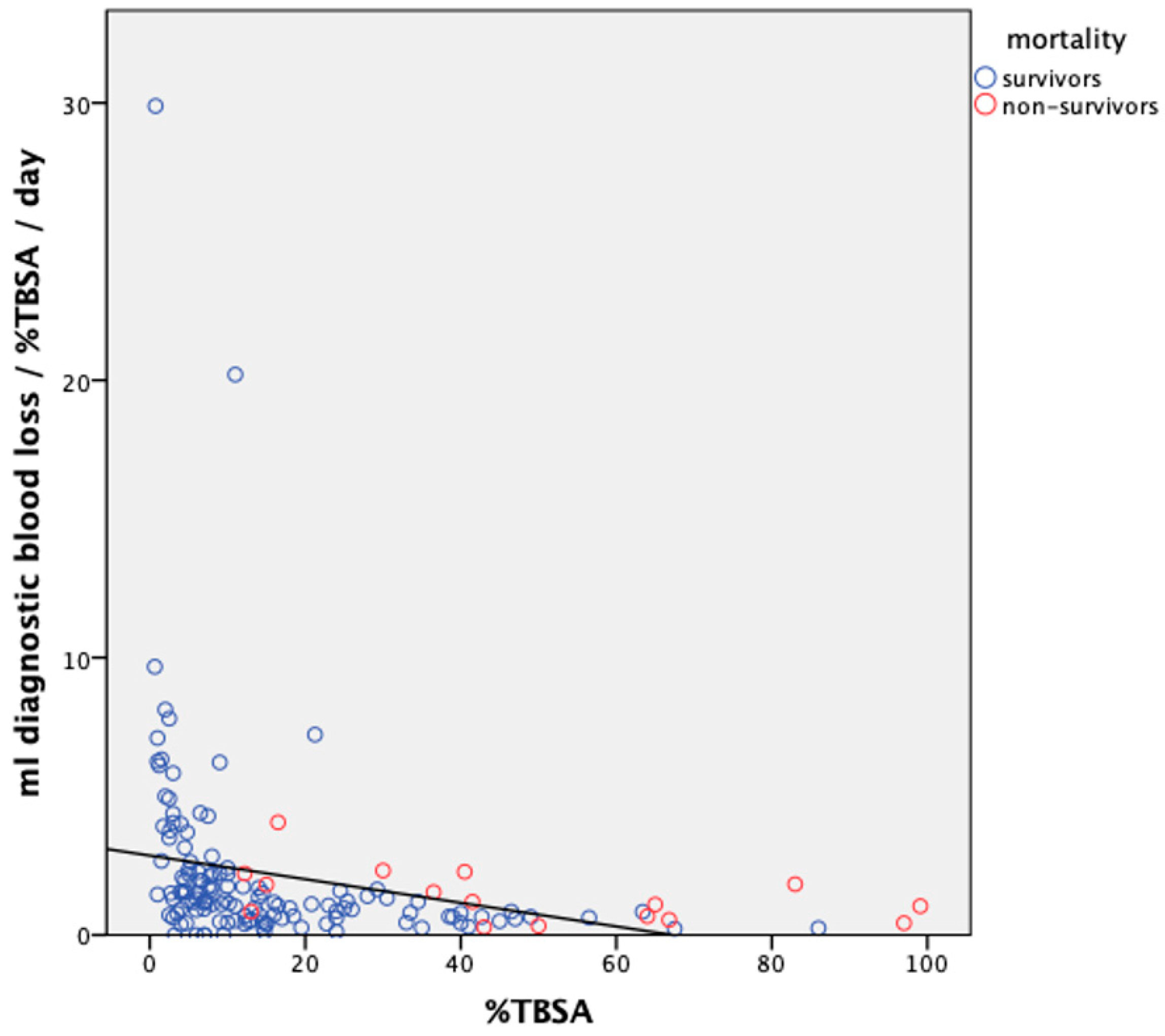
3.2. Diagnostic Blood Loss
3.3. Daily DBL Adapted for %TBSA
3.4. Transfusion Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamolz, L.P.; Sendlhofer, G.; Lumenta, D. Future burn care: Balancing the relationship between cost and quality. Wound Repair. Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair. Soc. 2014, 22, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns J. Int. Soc. Burn Inj. 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamoltz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns J. Int. Soc. Burn Inj. 2016, 43, 249–257. [Google Scholar] [CrossRef]
- Hasan, S.; Mosier, M.J.; Conrad, P.; Szilagyi, A.; Gamelli, R.L.; Muthumalaiappan, K. Terminal Maturation of Orthochromatic Erythroblasts Is Impaired in Burn Patients. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2018, 39, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Farny, B.; Fontaine, M.; Latarjet, J.; Poupelin, J.C.; Voulliaume, D.; Ravat, F. Estimation of blood loss during adult burn surgery. Burns J. Int. Soc. Burn Inj. 2018, 44, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Muniz Castro, J.; Burton, K.; Thurer, R.L.; Bernal, N.P. How does blood loss relate to the extent of surgical wound excision? Burns J. Int. Soc. Burn Inj. 2018, 44, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Fan, H.; Sun, W.; Peng, Y.; Chen, L.; Tao, J.; Li, J.; Yang, S.; Li, X.; Fitzgerald, M.; et al. Blood loss during extensive escharectomy and auto-microskin grafting in adult male major burn patients. Burns J. Int. Soc. Burn Inj. 2011, 37, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, T.L. Burn injury and blood transfusion. Curr. Opin. Anaesthesiol. 2019, 32, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Chernow, B.; Salem, M.; Stacey, J.; Baltimore, M.; Chernow, B.; Salem, M.; Stacey, J.; Baltimore, M. Blood conservation—A critical care imperative. Crit. Care Med. 1991, 3, 313. [Google Scholar]
- Nissenson, A.R.; Goodnough, L.T.; Dubois, R.W. Anemia: Not Just an Innocent Bystander? Arch. Intern. Med. 2003, 163, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.Q.; Wu, G.S.; Xu, L.; Alexander, K.P.; Masoudi, F.A.; Lai, S.-M.; Chan, S.P.; Bach, R.G.; Wang, T.Y.; Spertus, J.A.; et al. Diagnostic blood loss from phlebotomy and hospital acquired anemia in patients with severe burns. Burns J. Int. Soc. Burn Inj. 2019, 46, 579–588. [Google Scholar] [CrossRef]
- Palmieri, T.L.; Holmes, J.H.; Arnoldo, B.; Peck, M.; Potenza, B.; Cochran, A.; King, B.T.; Dominic, W.; Cartotto, R.; Bhavar, D.; et al. Transfusion Requirement in Burn Care Evaluation (TRIBE): A Multicenter Randomized Prospective Trial of Blood Transfusion in Major Burn Injury. Ann. Surg. 2017, 266, 595–602. [Google Scholar] [CrossRef] [PubMed]
- The American Burn Association Consensus Conference on Burn Sepsis and Infection Group; Greenhalgh, D.G.; Saffle, J.R.; Holmes, J.H., 4th; Gamelli, R.L.; Palmieri, T.L.; Horton, J.W.; Tompkins, R.G.; Traber, D.L.; Mozingo, D.W.; et al. American Burn Association Consensus Conference to Define Sepsis and Infection in Burns. J. Burn Care Res. 2007, 28, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188. [Google Scholar] [CrossRef] [PubMed]
- Chant, C.; Wilson, G.; Friedrich, J.O. Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: A cohort study. Crit. Care 2006, 10, R140. [Google Scholar] [CrossRef] [PubMed]
- Wisser, D.; van Ackern, K.; Knoll, E.; Wisser, H.; Bertsch, T. Blood Loss from Laboratory Tests. Clin. Chem. 2003, 49, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Jakacka, N.; Snarski, E.; Mekuria, S. Prevention of Iatrogenic Anemia in Critical and Neonatal Care. Adv. Clin. Exp. Med. 2016, 25, 191–197. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total | Female | Male | p-Value |
|---|---|---|---|---|
| n (%) | 166 (100) | 56 (33.7) | 110 (66.3) | - |
| Age (years), mean (±SD) | 53.2 (±20.1) | 57.6 (±21.1) | 51.0 (±19.3) | 0.046 * |
| LOS (days), mean (±SD) | 17.0 (±40.7) | 17.0 (±40.3) | 17.1 (±41.0) | 0.992 |
| Aetiology of burn, n (%) | ||||
| Flame | 127 (76.5) | 38 (67.9) | 89 (80.9) | 0.061 |
| Scald | 22 (13.3) | 15 (26.8) | 7 (6.4) | <0.001 * |
| Electric | 12 (7.2) | 1 (1.8) | 11 (10.0) | 0.053 |
| Contact | 3 (1.8) | 1 (1.8) | 2 (1.8) | 0.988 |
| Chemical | 2 (1.2) | 1 (1.8) | 1 (0.9) | 0.625 |
| %TBSA, mean (±SD) | 18.0 (±20.1) | 16.0 (±18.3) | 19.0 (±20.9) | 0.368 |
| n needing surgery (%) | 124 (74.7) | 40 (71.4) | 84 (76.4) | 0.489 |
| n of operations, mean (±SD) | 1.9 (±3.1) | 1.7 (±2.9) | 2.1 (±3.1) | 0.422 |
| n needing dialysis (%) | 6 (3.6) | 2 (3.6) | 4 (3.6) | 0.983 |
| n of days with CRRT, mean (±SD) | 0.6 (±7.1) | 0.1 (±0.7) | 0.9 (±8.8) | 0.506 |
| n needing mechanical ventilation (%) | 42 (25.3) | 12 (21.4) | 30 (27.3) | 0.413 |
| n of days in respirator, mean (±SD) | 1.4 (±3.6) | 1.2 (±4.0) | 1.5 (±3.5) | 0.665 |
| Mortality, n (%) | 20 (12.0) | 7 (12.5) | 13 (11.8) | 0.898 |
| Baux score, mean (±SD) | 71.2 (±27.0) | 73.6 (±29.4) | 70.0 (±25.8) | 0.419 |
| LOS per %TBSA, mean (±SD) | 1.9 (±4.0) | 2.2 (±3.6) | 1.7 (±4.2) | 0.538 |
| Category | Volume (mL) | Content |
|---|---|---|
| Blood culture | 10 | |
| Blood gas | 2 | pH, pCO2, pO2, O2sat, HCO3, BE, lactate, Na, K, iCa2+ activity, Cl−, EVF, Hb, glucose |
| CRRT post-filter ionised calcium (CRRT iCa2+) | 2 | |
| Complete blood count (CBC) | 4 | |
| Coagulation | 3.5 | APTT, fibrin, d-dimer, fibrinogen, PT (INR), antithrombin |
| Chemistry | 5 | CRP, ALAT, ASAT, ALP, bilirubin, bilirubin conjugated, calcium, GFR, phosphate, creatinine, magnesium, urea, sodium, potassium, LD, albumin, CKMB, CK, troponin I, myoglobin, triglycerides |
| Other | 5 | S-Osmolality, S-CDT |
| Test Category | Performed in n of Patients (%) | Number of Tests | Mean Estimated Blood Loss per Patient in mL (±SD) |
|---|---|---|---|
| Total | 166 (100) | 15,397 | 333.5 (±864.2) |
| CBC | 159 (95.8) | 2006 | 50.5 (±118.3) |
| Chemistry | 158 (95.2) | 1980 | 62.7 (±146.7) |
| Coagulation | 155 (93.4) | 1363 | 30.8 (±72.6) |
| Other | 146 (88.0) | 428 | 14.7 (±28.8) |
| Blood gas | 115 (69.3) | 8083 | 140.6 (±298.2) |
| Blood culture | 80 (48.2) | 1411 | 176.4 (±393.6) |
| CRRT iCa2+ | 9 (5.4) | 126 | 28.0 (±24.6) |
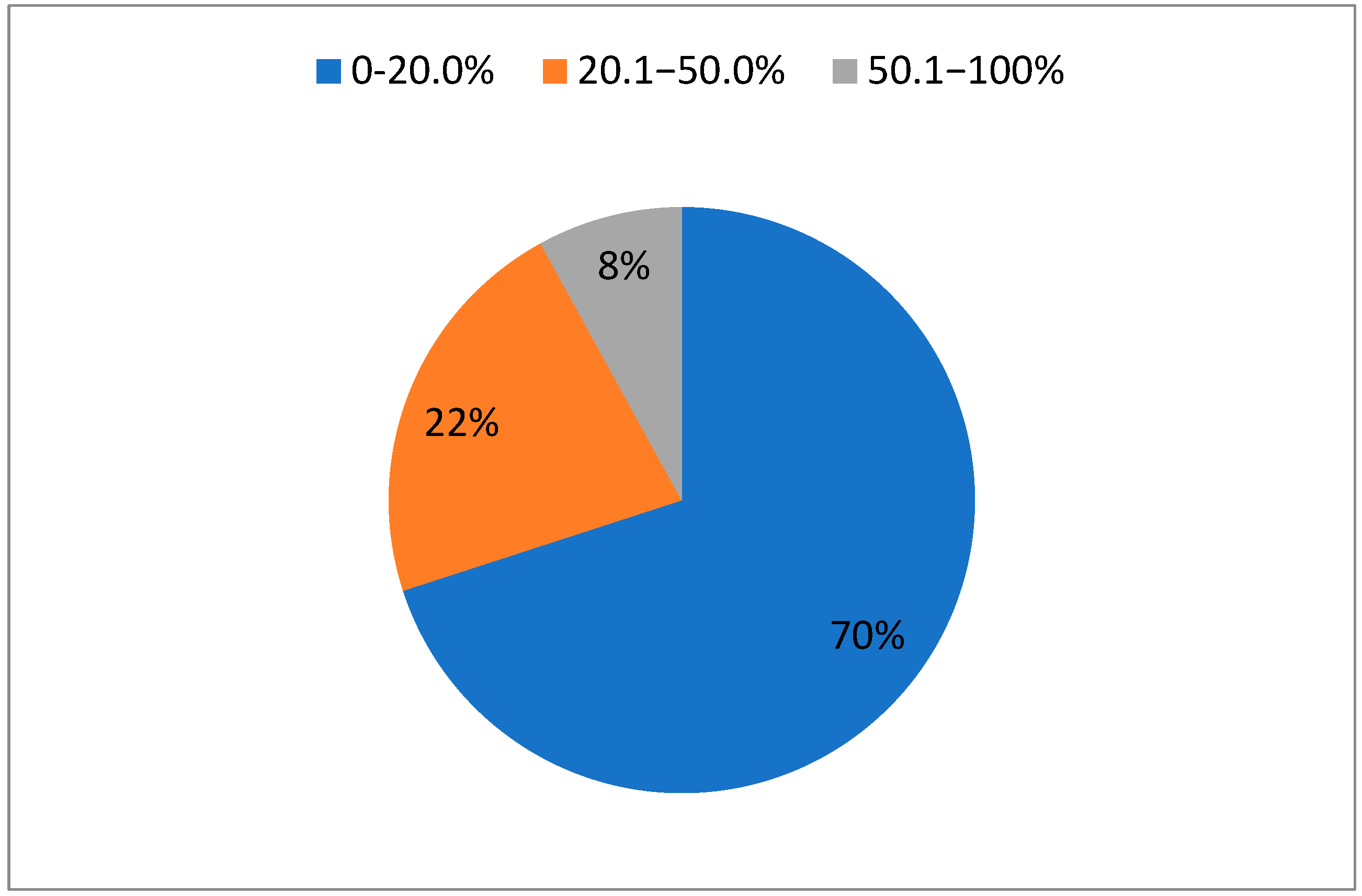
| %TBSA | Number of Patients | Median Total DBL/Patient in mL (IQR) | Median DBL/d/Patient in mL (IQR) |
|---|---|---|---|
| 0–20.0 | 117 | 48.5 (27.8, 134.3) | 10.1 (6.2, 17.5) |
| 20.1–50.0 | 36 | 523.5 (258.4, 1026.5) | 26.2 (16.6, 39.2) |
| 50.1–100 | 13 | 152.0 (36.3, 1683.0) | 36.5 (23.3, 62.2) |
| Parameter | Median volume in mL, (IQR) | ||
| Total DBL/patient | 87.5 (34.6, 304.8) | ||
| DBL/day/patient | 13.1 (7.0, 23.9) | ||
| DBL/%TBSA/patient | 10.8 (4.6, 27.8) | ||
| DBL/%TBSA/day/patient | 1.2 (0.7, 2.3) | ||
| Parameter | Total | Low DBL/day (<13.1 mL/d) | High DBL/day (≥13.1 mL/d) | p-Value |
|---|---|---|---|---|
| n (%) | 166 (100) | 56 (33.7) | 110 (66.3) | - |
| Age (years), mean (±SD) | 53.2 (±20.1) | 53.7 (±21.2) | 52.7 (±18.9) | 0.759 |
| Female gender, n (%) | 56 (33.7) | 33 (38.4) | 23 (28.8) | 0.190 |
| LOS (days), mean (±SD) | 17.0 (±40.7) | 10.1 (±9.7) | 24.5 (±56.9) | 0.029 * |
| Aetiology of burn, n (%) | ||||
| Flame | 127 (76.5) | 60 (69.8) | 67 (83.8) | 0.034 * |
| Scald | 22 (13.3) | 15 (17.4) | 7 (8.8) | 0.099 |
| Electric | 12 (7.2) | 6 (7.0) | 6 (7.5) | 0.897 |
| Contact | 3 (1.8) | 3 (3.5) | 0 (0) | 0.092 |
| Chemical | 2 (1.2) | 2 (2.3) | 0 (0) | 0.170 |
| %TBSA, mean (±SD) | 18.0 (±20.1) | 10.3 (±12.0) | 26.2 (±23.5) | <0.001 * |
| n needing surgery (%) | 124 (74.7) | 59 (68.6) | 65 (61.3) | 0.061 |
| n of operations, mean (±SD) | 1.9 (±3.1) | 1.1 (±1.0) | 2.9 (±4.1) | <0.001 * |
| n needing dialysis (%) | 6 (3.6) | 1 (1.2) | 5 (6.3) | 0.079 |
| n of days with CRRT, mean (±SD) | 0.6 (±7.1) | 0.0 (±0.3) | 1.3 (±10.3) | 0.275 |
| n needing mechanical ventilation (%) | 42 (25.3) | 8 (9.3) | 34 (42.5) | <0.001 * |
| n of days in respirator, mean (±SD) | 1.4 (±3.6) | 0.3 (±0.9) | 2.6 (±4.9) | <0.001 * |
| Mortality, n (%) | 20 (12.0) | 6 (7.0) | 14 (17.5) | 0.037 * |
| Baux score, mean (±SD) | 71.2 (±27.0) | 64.0 (±23.5) | 79.0 (±28.5) | <0.001 * |
| LOS per %TBSA, mean (±SD) | 1.9 (±4.0) | 2.2 (±3.4) | 1.6 (±4.5) | 0.290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolle, C.; Persson, A.A.E.; Lind, C.; Huss, F. Total Body Surface Area Adjusted Daily Diagnostic Blood Loss May Be Higher in Minor Burns—Are Our Patients the Victims of Daily Routine? Eur. Burn J. 2024, 5, 175-184. https://doi.org/10.3390/ebj5020016
Smolle C, Persson AAE, Lind C, Huss F. Total Body Surface Area Adjusted Daily Diagnostic Blood Loss May Be Higher in Minor Burns—Are Our Patients the Victims of Daily Routine? European Burn Journal. 2024; 5(2):175-184. https://doi.org/10.3390/ebj5020016
Chicago/Turabian StyleSmolle, Christian, Anna Alexandra Elisabeth Persson, Caroline Lind, and Fredrik Huss. 2024. "Total Body Surface Area Adjusted Daily Diagnostic Blood Loss May Be Higher in Minor Burns—Are Our Patients the Victims of Daily Routine?" European Burn Journal 5, no. 2: 175-184. https://doi.org/10.3390/ebj5020016
APA StyleSmolle, C., Persson, A. A. E., Lind, C., & Huss, F. (2024). Total Body Surface Area Adjusted Daily Diagnostic Blood Loss May Be Higher in Minor Burns—Are Our Patients the Victims of Daily Routine? European Burn Journal, 5(2), 175-184. https://doi.org/10.3390/ebj5020016








