Mechanomodulation: Physical Treatment Modalities Employ Mechanotransduction to Improve Scarring
Abstract
:1. Introduction
2. Mechanotransduction in Scarred Skin
3. Matrix Stiffness
4. Mechanomodulation of Matrix Stiffness
4.1. Physical Scar Management
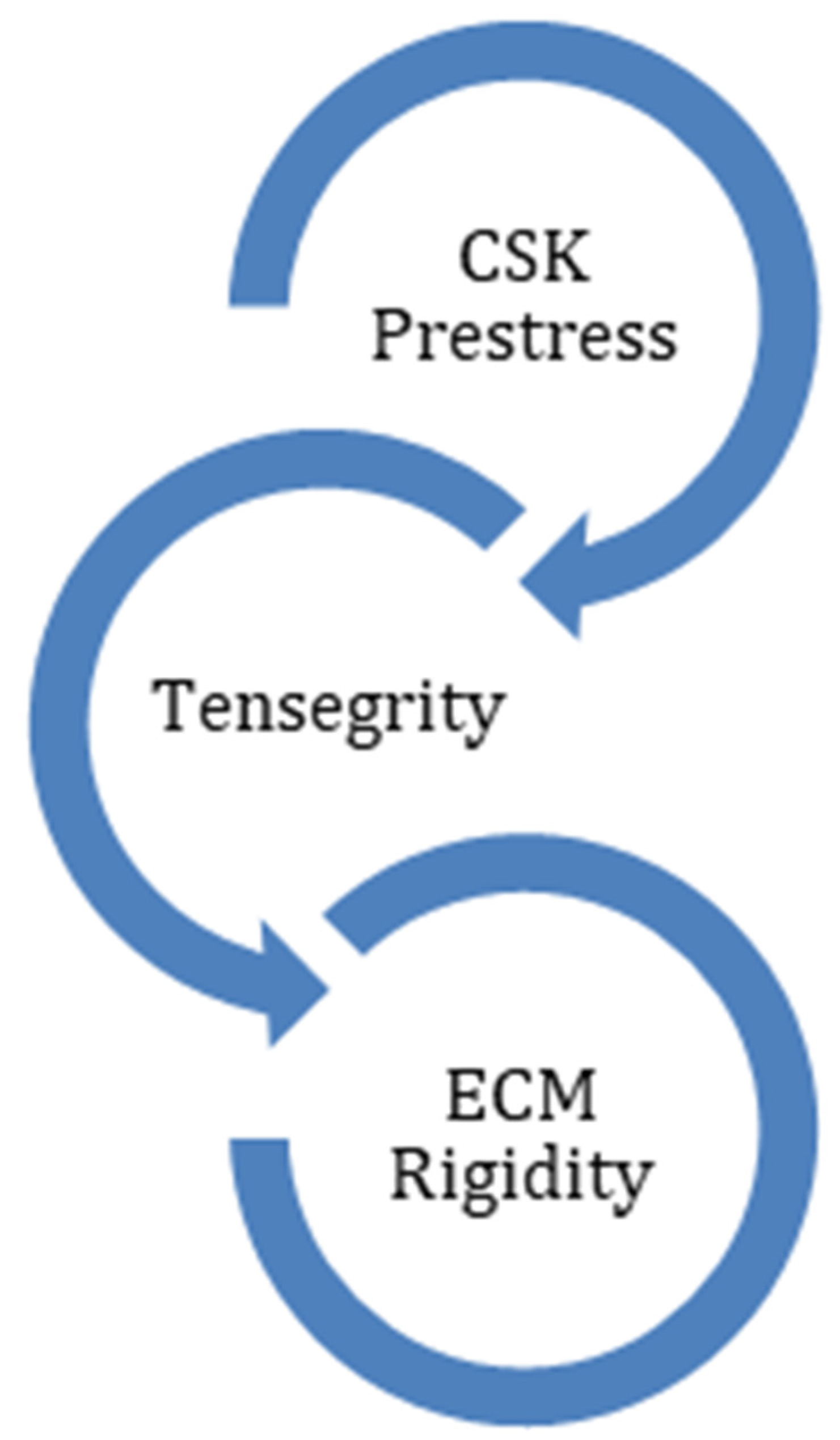
4.2. Interactions between Extracellular Matrix and Cytoskeleton
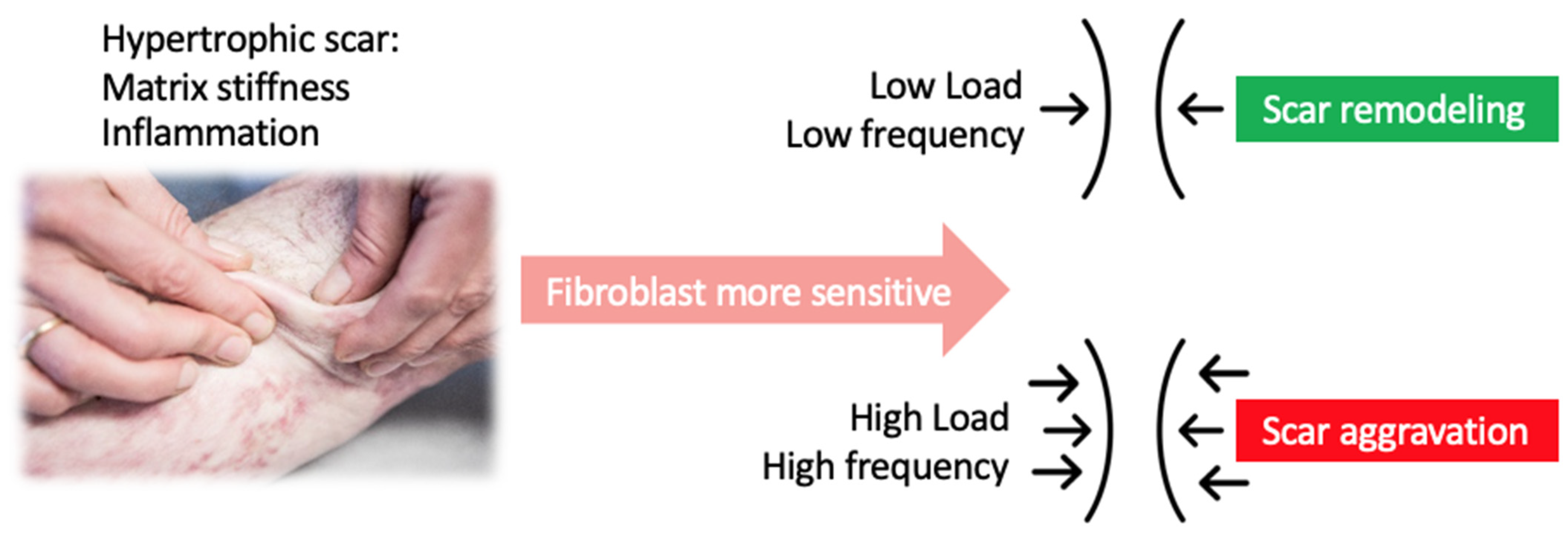
5. Dose Dependency of Applied Forces during Physical Scar Management
6. Physical Modalities That Improve Tissue Stiffness
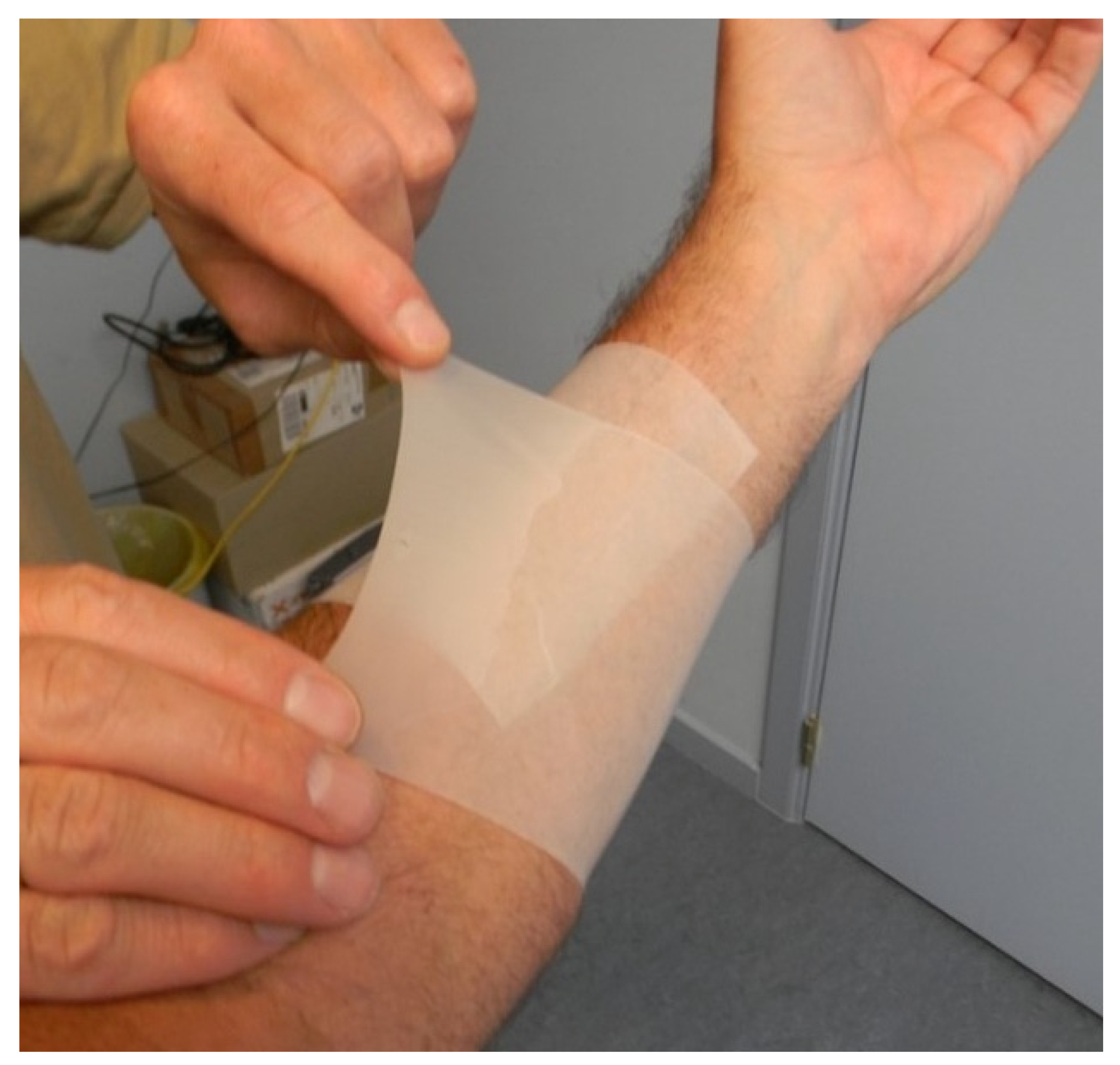
6.1. Silicone Therapy
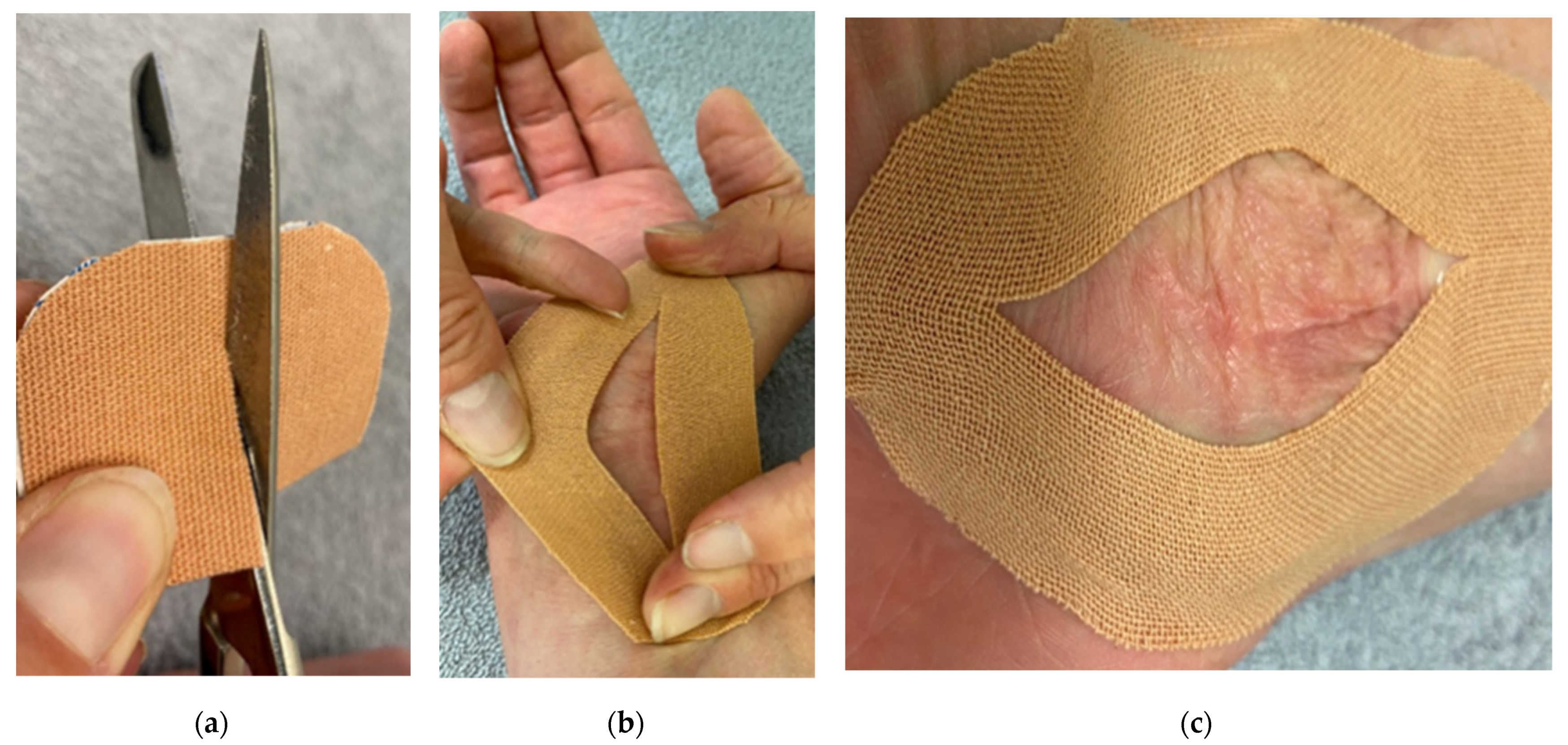
6.2. Scar Taping
6.3. Shockwave Therapy
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bayat, A.; McGrouther, D.; Ferguson, M. Skin scarring. BMJ 2003, 326, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Meirte, J. The ICF as a Framework for Post Burn Dysfunctioning: Evaluation, Quality of Life and Vacuum Massage in Patients with Hypertrophic Burn Scars; Universiteit Antwerpen: Antwerpen, Belgium, 2016. [Google Scholar]
- Lee, H.J.; Jang, Y. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedstat, J.S.; Hultman, C.S. Hypertrophic Burn Scar Management. What Does the Evidence Show? A Systematic Review of Randomized Controlled Trials. Ann. Plast. Surg. 2014, 72, S198–S201. [Google Scholar] [CrossRef] [PubMed]
- Woolard, A.; Hill, N.T.M.; McQueen, M.; Martin, L.; Milroy, H.; Wood, F.M.; Bullman, I.; Lin, A. The psychological impact of paediatric burn injuries: A systematic review. BMC Public Health 2021, 21, 2281. [Google Scholar] [CrossRef]
- Mascharak, S.; Desjardins-Park, M.H.E.; Davitt, M.F.; Guardino, N.J.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. Modulating Cellular Responses to Mechanical Forces to Promote Wound Regeneration. Adv. Wound Care 2021. [Google Scholar] [CrossRef]
- Anthonissen, M.; Daly, D.; Janssens, T.; Kerckhove, E.V.D. The effects of conservative treatments on burn scars: A systematic review. Burns 2016, 42, 508–518. [Google Scholar] [CrossRef]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and hypertrophic scars: Pathophysiology, classification, and treatment. Dermatol. Surg. 2017, 43, S3–S18. [Google Scholar] [CrossRef]
- Ault, P.; Plaza, A.; Paratz, J. Scar massage for hypertrophic burns scarring—A systematic review. Burns 2017, 44, 24–38. [Google Scholar] [CrossRef]
- Macintyre, L.; Baird, M. Pressure garments for use in the treatment of hypertrophic scars—A review of the problems associated with their use. Burns 2006, 32, 10–15. [Google Scholar] [CrossRef]
- Deflorin, C.; Hohenauer, E.; Stoop, R.; Van Daele, U.; Clijsen, R.; Taeymans, J. Physical Management of Scar Tissue: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 2020, 26, 854–865. [Google Scholar] [CrossRef]
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Holfeld, J.; Schaden, W.; Orgill, D.; Ogawa, R. Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol. Med. 2013, 19, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Maan, Z.; Wong, V.W.; Rennert, R.C.; Januszyk, M.; Rodrigues, M.; Hu, M.; Whitmore, A.J.; Whittam, A.J.; Longaker, M.T.; et al. Mechanotransduction and fibrosis. J. Biomech. 2014, 47, 1997–2005. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.; Paterno, J.; Sorkin, M.; Glotzbach, J.P.; Levi, K.; Januszyk, M.; Rustad, K.C.; Longaker, M.T.; Gurtner, G.C. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011, 25, 4498–4510. [Google Scholar] [CrossRef]
- Fu, S.; Panayi, A.; Fan, J.; Mayer, H.F.; Daya, M.; Khouri, R.K.; Gurtner, G.C.; Ogawa, R.; Orgill, D.P. Mechanotransduction in Wound Healing: From the Cellular and Molecular Level to the Clinic. Adv. Ski. Wound Care 2021, 34, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Murphy, W.L.; McDevitt, T.C.; Engler, A. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Derderian, C.A.; Bastidas, N.; Lerman, O.Z.; Bhatt, K.A.; Lin, S.-E.; Voss, J.; Holmes, J.; Levine, J.P.; Gurtner, G.C. Mechanical Strain Alters Gene Expression in an in Vitro Model of Hypertrophic Scarring. Ann. Plast. Surg. 2005, 55, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Lagares, D. Matrix Stiffness: The Conductor of Organ Fibrosis. Curr. Rheumatol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Chiquet, M.; Gelman, L.; Lutz, R.; Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta 2009, 1793, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2016, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Akaishi, S.; Longaker, M.T.; Gurtner, G.C. Pushing Back: Wound Mechanotransduction in Repair and Regeneration. J. Investig. Dermatol. 2011, 131, 2186–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrazy, V.; Billet, F.; Micallef, L.; Coulomb, B.; Desmoulière, A. Mechanisms of pathological scarring: Role of myofibroblasts and current developments. Wound Repair Regen. 2011, 19 (Suppl. S1), s10–s15. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Akaishi, S.; Ogawa, R. Mechanosignaling pathways in cutaneous scarring. Arch. Dermatol. Res. 2012, 304, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Tissue stiffness, latent TGF-β1 Activation, and mechanical signal transduction: Implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 2009, 11, 120–126. [Google Scholar] [CrossRef]
- Goffin, J.M.; Pittet, P.; Csucs, G.; Lussi, J.W.; Meister, J.J.; Hinz, B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.; Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.-Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef] [Green Version]
- Ko, G.Y.P.; Ko, M.L.; Dryer, S.E. Circadian Regulation of cGMP-Gated Cationic Channels of Chick Retinal Cones: Erk MAP Kinase and Ca2+/Calmodulin-Dependent Protein Kinase II. Neuron 2001, 29, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, Y.; Ishijima, M.; Kaneko, H.; Kurebayashi, N.; Ichikawa, N.; Futami, I.; Kurosawa, H.; Arikawa-Hirasawa, E. Distinct mechanosensitive Ca2+influx mechanisms in human primary synovial fibroblasts. J. Orthop. Res. 2010, 28, 859–864. [Google Scholar] [CrossRef]
- Nishimura, K.; Blume, P.; Ohgi, S.; Sumpio, B.E. Effect of different frequencies of tensile strain on human dermal fibroblast proliferation and survival. Wound Repair Regen. 2007, 15, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.S.; Cheah, A.Y.L.; Turley, S.; Nadesan, P.; Poon, R.; Clevers, H.; Alman, B.A. β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. USA 2002, 99, 6973–6978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-J.; Byun, M.R.; Furutani-Seiki, M.; Hong, J.-H.; Jung, H.-S. YAP and TAZ Regulate Skin Wound Healing. J. Investig. Dermatol. 2014, 134, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tendegrity: The architectural basis of cellular mechanotransduction. Annurev. Physiol. 2003, 59, 575–599. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Suo, Z. Long-distance propagation of forces in a cell. Biochem. Biophys. Res. Commun. 2005, 328, 1133–1138. [Google Scholar] [CrossRef]
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 2005, 15, 223–230. [Google Scholar] [CrossRef]
- Berta, L.; Fazzari, A.; Ficco, A.M.; Enrica, P.M.; Catalano, M.G.; Frairia, R. Extracorporeal shock waves enhance normal fibroblast proliferation in vitro and activate mRNA expression for TGF-β1 and for collagen types I and III. Acta Orthop. 2009, 80, 612–617. [Google Scholar] [CrossRef]
- Sukubo, N.G.; Tibalt, E.; Respizzi, S.; Locati, M.; D’Agostino, M. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int. J. Surg. 2015, 24, 124–130. [Google Scholar] [CrossRef]
- Cai, Z.; Falkensammer, F.; Andrukhov, O.; Chen, J.; Mittermayr, R.; Rausch-Fan, X. Effects of Shock Waves on Expression of IL-6, IL-8, MCP-1, and TNF-α Expression by Human Periodontal Ligament Fibroblasts: An In Vitro Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, C.L.; Daugherty, M.B.; Warden, G.D. Serial casting as a technique to correct burn scar contractures. A case report. J. Burn. Care Rehabil. 1991, 12, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Grover, L.M.; Lewis, M.P.; Liu, Y. PDGF is a potent initiator of bone formation in a tissue engineered model of pathological ossification. J. Tissue Eng. Regen. Med. 2016, 12, e355–e367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohari, S.P.; Grover, L.; Hukins, D.W. Pulsed low-intensity ultrasound increases proliferation and extracelluar matrix production by human dermal fibroblasts in three-dimensional culture. J. Tissue Eng. 2015, 6, 2041731415615777. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.D.; Ng, W.P.; Fletcher, D.A. Tensional Homeostasis in Single Fibroblasts. Biophys. J. 2014, 107, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungbauer, S.; Gao, H.; Spatz, J.P.; Kemkemer, R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 2008, 95, 3470–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, U.; Hampe, N.; Rubner, W.; Kirchgeßner, N.; Safran, S.; Hoffmann, B.; Merkel, R. Cyclic Stress at mHz Frequencies Aligns Fibroblasts in Direction of Zero Strain. PLoS ONE 2011, 6, e28963. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Blume, P.A.; Sumpio, B.E. Role of integrins and focal adhesion kinase in the orientation of dermal fibroblasts exposed to cyclic strain. Int. Wound J. 2009, 6, 149–158. [Google Scholar] [CrossRef]
- Powell, H.M.; McFarland, K.L.; Butler, D.L.; Supp, D.M.; Boyce, S.T. Uniaxial Strain Regulates Morphogenesis, Gene Expression, and Tissue Strength in Engineered Skin. Tissue Eng. Part A 2009, 16, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Kuang, R.; Wang, Z.; Xu, Q.; Cai, X.; Liu, T. Exposure to Varying Strain Magnitudes Influences the Conversion of Normal Skin Fibroblasts Into Hypertrophic Scar Cells. Ann. Plast. Surg. 2016, 76, 388–393. [Google Scholar] [CrossRef]
- Bouffard, N.A.; Cutroneo, K.R.; Badger, G.J.; White, S.L.; Buttolph, T.R.; Ehrlich, H.P.; Stevens-Tuttle, D.; Langevin, H.M. Tissue stretch decreases soluble TGF-β1 and type-1 procollagen in mouse subcutaneous connective tissue: Evidence from ex vivo and in vivo models. J. Cell. Physiol. 2007, 214, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Perkins, K.; Davey, R.; Wallis, K. Silicone gel: A new treatment for burn scars and contractures. Burns 1983, 9, 201–204. [Google Scholar] [CrossRef]
- Quinn, K.J. Silicone gel in scar treatment. Burn. Incl. Therm. Inj. 1987, 13, S33–S40. [Google Scholar] [CrossRef]
- Ahn, S.T.; Monafo, W.W.; Mustoe, T.A. Topical Silicone Gel for the Prevention and Treatment of Hypertrophic Scar. Arch. Surg. 1991, 126, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Li-Tsang, C.W.; Lau, J.C.; Choi, J.; Chan, C.C.; Jianan, L. A prospective randomized clinical trial to investigate the effect of silicone gel sheeting (Cica-Care) on post-traumatic hypertrophic scar among the Chinese population. Burns 2006, 32, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H. A controlled clinical trial of topical silicone gel sheeting in the treatment of hypertrophic scars and keloids. J. Am. Acad. Dermatol. 1994, 30, 506–507. [Google Scholar] [CrossRef]
- Carney, S.; Cason, C.; Gowar, J.; Stevenson, J.; McNee, J.; Groves, A.; Thomas, S.; Hart, N.; Auclair, P. Cica-care gel sheeting in the management of hypertrophic scarring. Burns 1994, 20, 163–167. [Google Scholar] [CrossRef]
- Cruz-Korchin, N.I. Effectiveness of Silicone Sheets in the Prevention of Hypertrophic Breast Scars. Ann. Plast. Surg. 1996, 37, 345–348. [Google Scholar] [CrossRef]
- Sakuraba, M.; Takahashi, N.; Akahoshi, T.; Miyasaka, Y.; Suzuki, K. Use of silicone gel sheets for prevention of keloid scars after median sternotomy. Surg. Today 2011, 41, 496–499. [Google Scholar] [CrossRef]
- Maján, J.C. Evaluation of a self-adherent soft silicone dressing for the treatment of hypertrophic postoperative scars. J. Wound Care 2006, 15, 193–196. [Google Scholar] [CrossRef]
- Mustoe, T.A. Evolution of Silicone Therapy and Mechanism of Action in Scar Management. Aesthetic Plast. Surg. 2007, 32, 82–92. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.H.; Park, S.W.; Chang, H. Regulation of Transforming Growth Factor β1, Platelet-Derived Growth Factor, and Basic Fibroblast Growth Factor by Silicone Gel Sheeting in Early-Stage Scarring. Arch. Plast. Surg. 2015, 42, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, M.A.; Moffit, M.R.; Smith, P.D.; Lyle, W.G.; Ko, F.; Meltzer, D.D.; Robson, M.C. Silicone sheeting decreases fibroblast activity and downregulates TGFbeta2 in hypertrophic scar model. Int. J. Surg. Investig. 2001, 2, 467–474. [Google Scholar] [PubMed]
- Kikuchi, R.; Khalil, A.J.; Zoumalan, C.I. Gene expression analysis in scars treated with silicone cream: A case series. Scars Burn. Heal. 2019, 5, 2059513119868345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Wal, M.; Van Zuijlen, P.; Van De Ven, P.; Middelkoop, E. Topical Silicone Gel versus Placebo in Promoting the Maturation of Burn Scars: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2010, 126, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Ting, P.S.; Hsu, K.C. Does the form of dressings matter? A comparison of the efficacy in the management of postoperative scars between silicone sheets and silicone gel, a randomized controlled trial. Medicine 2018, 97, e11767. [Google Scholar] [CrossRef]
- Akaishi, S.; Akimoto, M.; Hyakusoku, H.; Ogawa, R. The Tensile Reduction Effects of Silicone Gel Sheeting. Plast. Reconstr. Surg. 2010, 126, 109e–111e. [Google Scholar] [CrossRef]
- Kerckhove, E.V.D.; Stappaerts, K.; Boeckx, W.; Hof, B.V.D.; Monstrey, S.; Van der Kelen, A.; De Cubber, J. Silicones in the rehabilitation of burns: A review and overview. Burns 2001, 27, 205–214. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Flak, E.; Witte, B.; Ring, A.; Tilkorn, D.; Hauser, J.; Langer, S.; Steinau, H.-S.; Al-Benna, S. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: Randomized controlled trial with intraindividual comparison. Plast. Reconstr. Surg. 2011, 128, 306e–313e. [Google Scholar]
- Li-Tsang, C.W.P.; Zheng, Y.P.; Lau, J.C.M. A Randomized Clinical Trial to Study the Effect of Silicone Gel Dressing and Pressure Therapy on Posttraumatic Hypertrophic Scars. J. Burn Care Res. 2010, 31, 448–457. [Google Scholar] [CrossRef]
- Wiseman, J.; Simons, M.; Kimble, R.; Ware, R.S.; McPhail, S.M.; Tyack, Z. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children 12-months postburn: A parallel group randomised controlled trial. Clin. Rehabil. 2021, 35, 1126–1141. [Google Scholar] [CrossRef]
- Moortgat, P.; Meirte, J.; Maertens, K.; Lafaire, C.; De Cuyper, L.; Anthonissen, M. Can a Cohesive Silicone Bandage Outperform an Adhesive Silicone Gel Sheet in the Treatment of Scars? A Randomized Comparative Trial. Plast. Reconstr. Surg. 2019, 143, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Chait, L.A.; Stals, R.; Stals, P.J. New Innovations in Scar Management. Aesthetic Plast. Surg. 2000, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Chait, L.A.; Stals, P.J.; Stals, R.; Candy, G. Multimodality Scar Management Program. Aesthetic Plast. Surg. 2008, 33, 533–543. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Crofton, E.; Brown, J.; Strong, J.; Ziviani, J. Use of tape for the management of hypertrophic scar development: A comprehensive review. Scars Burn. Heal. 2021, 7, 20595131211029206. [Google Scholar] [CrossRef] [PubMed]
- Daya, M. Abnormal scar modulation with the use of micropore tape. Eur. J. Plast. Surg. 2010, 34, 45–51. [Google Scholar] [CrossRef]
- Reiffel, R.S. Prevention of Hypertrophic Scars by Long-Term Paper Tape Application. Plast. Reconstr. Surg. 1995, 96, 1715–1718. [Google Scholar] [CrossRef]
- Atkinson, J.-A.M.; McKenna, K.T.; Barnett, A.G.; McGrath, D.J.; Rudd, M. A Randomized, Controlled Trial to Determine the Efficacy of Paper Tape in Preventing Hypertrophic Scar Formation in Surgical Incisions that Traverse Langers Skin Tension Lines. Plast. Reconstr. Surg. 2005, 116, 1648–1656. [Google Scholar] [CrossRef]
- Rosengren, H.; Askew, D.A.; Heal, C.; Buettner, P.G.; Humphreys, W.O.; Semmens, L.A. Does taping torso scars following dermatologic surgery improve scar appearance? Dermatol. Pract. Concept. 2013, 3, 75. [Google Scholar] [CrossRef]
- Moortgat, P.; Van Daele, U.; Anthonissen, M.; Meirte, J.; Lafaire, C.; De Cuyper, L.; Maertens, K. Tension Reducing Taping as a Mechanotherapy for Hypertrophic Burn Scars: A Proof of Concept. Ann. Burn. Fire Disasters 2015, XXVIII. Available online: http://www.medbc.com/annals/review/vol_28/num_3b/text/vol28n3bp300.pdf (accessed on 8 February 2022).
- d’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef]
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Teot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef]
- Lee, F.Y.; Zhen, Y.Y.; Yuen, C.M.; Fan, R.; Chen, Y.T.; Sheu, J.J.; Chen, Y.L.; Wang, C.J.; Sun, C.K.; Yip, H.K. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res. 2017, 9, 1603–1617. [Google Scholar] [PubMed]
- Zhao, J.-C.; Zhang, B.-R.; Shi, K.; Wang, J.; Yu, Q.-H.; Yu, J.-A. Lower energy radial shock wave therapy improves characteristics of hypertrophic scar in a rabbit ear model. Exp. Ther. Med. 2018, 15, 933–939. [Google Scholar] [CrossRef]
- Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Seo, C.H. Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study. J. Clin. Med. 2020, 9, 1376. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.H.; Suh, H.S.; Choi, Y.S. Benefits of extracorporeal shock waves for keloid treatment: A pilot study. Dermatol. Ther. 2020, 33, e13653. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Sáez, J.; Dos Santos, B.P.; Serracanta, J.; Monte-Soldado, A.; Bosacoma, P.; Rivas-Nicolls, D.; Barret, J.P. The effect of Extracorporeal Shock Wave Therapy in the treatment of burn scars: A prospective, randomized, controlled trial. Burns 2021, in press. [Google Scholar] [CrossRef]
- Moortgat, P.; Anthonissen, M.; Van Daele, U.; Vanhullebusch, T.; Maertens, K.; De Cuyper, L.; Lafaire, C.; Meirte, J. The effects of shock wave therapy applied on hypertrophic burn scars: A randomised controlled trial. Scars Burn. Heal. 2020, 6, 2059513120975624. [Google Scholar] [CrossRef] [PubMed]
| Determining Factors in Physical Scar Management | Scar Facts | Therapy Goal | Modulation | Clinical Application |
|---|---|---|---|---|
| Internal Forces | ||||
| ECM stiffness | Fibrosis (alignment collagen) increases ECM stiffness [11,14]. | Decrease ECM stiffness (and decrease fibroblast sensitivity). | Slow mechanical load rate -> decrease ECM stiffness [40,41,42,43,44,45,46]. Fast mechanical load rate -> increase ECM stiffness [40,41,42,43,44,45,46]. | Vacuum massage, manual skin techniques |
| CSK prestress | Inflammation and ECM stiffness increases CSK prestress and fibroblast sensitivity [11,15]. | Decrease CSK prestress. | Balance between internal and external forces -> induce CSK prestress [31]. Increased external forces -> increase CSK prestress [31]. | Tape application |
| External Forces | ||||
| Tensile force | External forces at the epidermis are shear forces due to friction, tensile forces and compression forces. These forces will increase tension in the dermis. | Balance between internal and external forces. | Intensity/amplitude: <2% no effect [41] 10-20% significant effect [43,44,45] Frequency: low frequency, cyclic strain [40,43] Duration: moderate (no sustained signals) [46] | Vacuum massage, manual skinfold technique |
| Compressive force | Compressure garments, silicones, shockwave | |||
| Shear force | Manual gliding and splitting-up technique | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Daele, U.; Meirte, J.; Anthonissen, M.; Vanhullebusch, T.; Maertens, K.; Demuynck, L.; Moortgat, P. Mechanomodulation: Physical Treatment Modalities Employ Mechanotransduction to Improve Scarring. Eur. Burn J. 2022, 3, 241-255. https://doi.org/10.3390/ebj3020021
Van Daele U, Meirte J, Anthonissen M, Vanhullebusch T, Maertens K, Demuynck L, Moortgat P. Mechanomodulation: Physical Treatment Modalities Employ Mechanotransduction to Improve Scarring. European Burn Journal. 2022; 3(2):241-255. https://doi.org/10.3390/ebj3020021
Chicago/Turabian StyleVan Daele, Ulrike, Jill Meirte, Mieke Anthonissen, Tine Vanhullebusch, Koen Maertens, Lot Demuynck, and Peter Moortgat. 2022. "Mechanomodulation: Physical Treatment Modalities Employ Mechanotransduction to Improve Scarring" European Burn Journal 3, no. 2: 241-255. https://doi.org/10.3390/ebj3020021
APA StyleVan Daele, U., Meirte, J., Anthonissen, M., Vanhullebusch, T., Maertens, K., Demuynck, L., & Moortgat, P. (2022). Mechanomodulation: Physical Treatment Modalities Employ Mechanotransduction to Improve Scarring. European Burn Journal, 3(2), 241-255. https://doi.org/10.3390/ebj3020021









