1. Introduction
The prevention and treatment of hypertrophic scars (HSc) is still one of the most important issues in burn rehabilitation [
1] and has been described as “the greatest unmet challenge following burn injury” [
2]. This is the case, because HSc formation is associated with contracture development, severe pruritus, persisting functional limitations and psychological distress, decreased quality of life [
3], and delayed reintegration into society [
2]. In the literature, between 33 and 91% of burn survivors are expected to develop HSc, depending on various patient and injury characteristics [
4,
5,
6,
7,
8].
Very few objective scar evaluations have been conducted with the burn survivor population, which limits our knowledge of the clinical recovery profile of HSc, thereby causing an impact on rehabilitation intervention and treatment prioritization. However, scar quantification in burn survivors using objective instrumentation has been investigated at 3, 6, and 12 months post-burn [
9]. Nonetheless, the data before 3 months and between 3 and 6 and 6 and 12 months have not been produced, which could allow for a better understanding of the early recovery profile of HSc and donor site scars of the burn survivor population. Thus, objective scar evaluations would be valuable, since it would provide clinicians with data to establish HSc and donor site scar treatment priorities and objectives.
Furthermore, little is known about the similarities or differences in the recovery profile of post-burn scars at specific anatomical locations. However, the normative data for pliability, erythema, melanin, and thickness specific to 16 different anatomical locations were reported using the DermaScan C (Cortex Technology, Handsund, Denmark), Cutometer
®(MPA 580, Courage & Khazaka Electronic GmbH, Koln, Germany) and Mexameter
® (MX18, Courage & Khazaka Electronic GmbH, Koln, Germany) [
10]. This study provided objective measurements that showed how skin characteristics differed by their specific anatomical locations. Therefore, it is now possible to compare HSc and donor site skin characteristics to normative data for specific anatomical locations and better understand the effect of anatomic location on the development and resolution of HSc and normal scars.
Therefore, the objective of this study was to prospectively quantify the thickness, pliability, erythema, and pigmentation of post-burn HSc, donor sites (normal scar), and normal skin in different anatomical locations between 2 and 7 months post-burn using objective instrumentation.
2. Materials and Methods
A prospective, observational time-series study was conducted at the Montreal Burn Center. This study was approved by the McGill Faculty of Medicine Institutional Review Board, the ethics and scientific committees of the Centre de Recherche Centre Hospitalier de l’Université de Montréal (CRCHUM), and the Villa Medica Rehabilitation Hospital Research Approval Committee.
2.1. Study Population
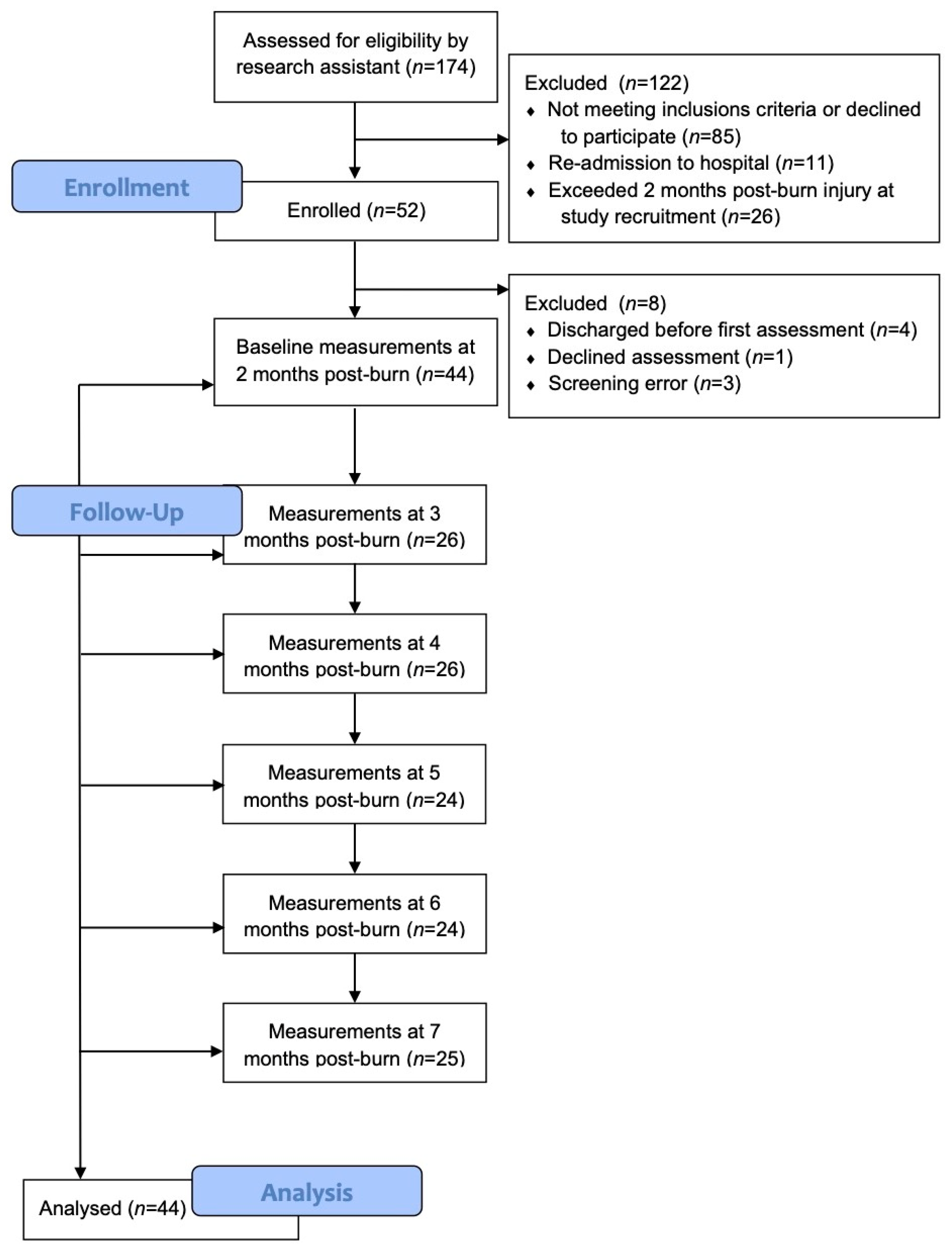
The participants were recruited from the Villa Medica Rehabilitation Hospital, where all evaluations and follow-ups were performed. Subjects who had developed HSc were recruited for this study according to the following inclusion criteria: (1) ≥18 years old, (2) admitted to Villa Medica Rehabilitation Hospital of the treatment of a burn injury, and (3) informed consent was obtained. The exclusion criteria included: (1) Was unable to understand French or English, (2) had a suspected or known allergy to ultrasound gel, (3) had dermatological conditions, which may interfere with the study results, such as psoriasis, eczema, etc., (4) had refused to give informed consent, or (5) had a psychiatric illness that impaired their ability to participate in the study or provide informed consent. Burn survivors who met the eligibility criteria were identified and were approached by one of their treating clinicians. These clinicians briefly described the study to the patients, and if they were interested in participating, their names were provided to the research assistant. The research assistant approached potential participants regarding the study, provided them with additional information, and gave them a copy of the informed consent form. Those who provided written consent were screened and examined to ascertain study eligibility. Eligible participants were identified by their initials and a preset 3-digit code to ensure confidentiality.
2.2. Measurement Procedures
Up to five sites were identified and selected for evaluation of the same participant if they were located in one of these three general anatomical locations: (1) upper extremity; (2) lower extremity; or (3) trunk. If no burn injury was sustained at each of these locations, the scar measurement was only performed at the locations that had sustained a burn injury. Within these site locations, the most severe scar was selected for evaluation if it had been rated as >2.034 mm thick, as measured by high-frequency ultrasound, and was hyperemic, defined as a Mexameter erythema index of >300.
If a grafting procedure was performed, a representative donor site was selected. A standard normal skin site was evaluated on the medial surface of the upper arm, if available. When unavailable, the closest unburned site to the medial surface of the upper arm was selected, and sites with substantial sun exposure were avoided.
Measurements were conducted in a temperature-controlled room (22 ± 1 °C), with the participant in the same position for each consecutive measurement (generally, sitting comfortably in a chair, with their arms resting at their sides, unless otherwise specified). If the participants were wearing pressure garments, they were asked to remove them 15 min prior to the scar assessment. A flexible, transparent film (Cling Vinyl Grafix, Creative-Coldsnow Artist Materials, Overland Park, KS, USA) was applied to the measurement site, the scar was traced, and a hole was cut over the exact site to be measured. Photographs of the hypertrophic scar sites were obtained to facilitate relocation for serial measurements, but not for evaluation purposes.
All patients were evaluated at baseline (admission to Villa Medica Rehabilitation Hospital) and re-evaluated monthly from 2 up to 7 months post-burn by the same evaluator. The thickness, pliability, erythema, and melanin were evaluated using several instruments: the DermaScan C (Cortex Technology, Handsund, Denmark) to measure thickness, the Cutometer
® (MPA 580, Courage & Khazaka Electronic GmbH, Koln, Germany) to measure skin pliability, and the Mexameter
® (MX18, Courage & Khazaka Electronic GmbH, Koln, Germany) to measure skin erythema and melanin. The participant’s current level of pain and itch were evaluated at each follow-up visit. Pain and itch have been previously assessed with the burn population and are clinically important when investigating HSc post-burn [
11,
12].
2.3. Measurement Tools
Scar thickness was measured using the DermaScan C (Cortex Technology, Handsund, Denmark), a high-frequency (20 MHz) ultrasound scanner with image-processing software (Dermavision 2D, Dermascan C v. 3, Cortex Technology, Handsund, Denmark), which captures and reproduces high-resolution images that allow skin thickness measurements to be generated. A medium-focus transducer with a 12 mm wide viewing field that was able to penetrate to 15 mm below the skin surface was used for this study. Prior to each measurement, a thin layer of conducting ultrasound gel (EcoGel 100 Imaging Ultrasound Gel, Eco-med Pharmaceutical Inc., Mississauga, ON, Canada) was applied to the transducer to provide contact between the clear plastic diaphragm and the skin surface. The transducer was held perpendicular to the site, while the echographic image was recorded. The research assistant, using the dedicated computer software, later generated the thickness measurements. The mean of three evenly spaced measures of the distance between the outer surface of the echogenic stratum corneum and the inner surface of the dermis, which is the boundary of the hypoechogenic subcutaneous fat, was recorded as the total skin thickness in millimeters. All measurements were performed with the ultrasound velocity set at 1580 m/s. High-frequency ultrasound has been shown to be a reliable and valid assessment of post-burn scar thickness in both adults [
13,
14] and children [
15].
Skin elasticity was measured with the Cutometer
® (MPA 580, Courage & Khazaka Electronic GmbH, Koln, Germany), which is based on the suction and elongation measuring principle. The device generates negative pressure, which draws the skin into a hollow aperture in the center of the probe and estimates the skin penetration depth with an optical measuring system. Four different measurement modes feature pre-programmed sequences of “on/off” pressure cycles. For this study, mode 1 was chosen, as it delivers three cycles of negative air pressure (450 mbar) for 2 s, followed by 2 s of no pressure. The results are expressed as the mean of three measurement cycles. The probe with a 6 mm hollow aperture was used for this study. Prior to the measurement cycle, the probe is applied lightly to the skin, without the outer ring contacting the skin surface, at a perpendicular axis to the skin. During the measurement cycle, it is important that neither the subject nor the probe move. Skin pliability parameters are traditionally expressed as either absolute parameters (Ua, Ue, Uf, Ur, Uv) or relative parameters (R-parameters). However, since the R0 = Uf parameter (which represents the maximum deformation or total distention of the skin) has previously been shown to provide the most reliable measurement of post-burn scar tissue when using the Cutometer [
14,
16,
17], only this measurement was used for the analysis in this study. The Cutometer was cleaned after every use and calibrated biweekly, as per the manufacturer’s specifications. The Cutometer has been shown to be a reliable and valid assessment of post-burn scar pliability [
14,
16,
17].
Erythema and melanin were evaluated with the Mexameter
® (MX18, Courage & Khazaka Electronic GmbH, Koln, Germany), which is based on the tissue’s narrow wavelength light absorption. The probe has 16 light-emitting diodes that send three defined wavelengths of light (568, 660, and 880 nm). A receiver then measures the light reflected by the skin. Since the quantity of emitted light is known, the absorption rate of defined wavelengths can be ascertained, which are selectively absorbed by melanin (660 nm) pigments or hemoglobin (568 nm). For each measurement, the probe was held perpendicular to the skin. It was lightly applied to the skin surface, without the outer ring making contact, thus activating the light emitter. The reflected light was measured by the receiver, and the erythema and melanin index (range 1–1000) was immediately displayed on the console; thus, the probe only remains in contact with the skin for a few seconds. The Mexameter probe was cleaned and the accuracy checked monthly, as per the manufacturer’s specifications. The Mexameter has been shown to be a reliable and valid assessment of post-burn erythema and melanin [
13,
14,
18].
The level of pain and itch were measured with a visual analogue scale (VAS). The participants were presented with a 10 cm line, anchored with no pain (itch) to the worse pain (itch) imaginable. The participants were asked to mark their current level of pain and itch on the line, and once selected, the measure in centimeters was recorded. The duration of the itch was also recorded using the following scale: 0 =no itch, 1 ≤ 5 min, 2 ≥ 5 min < 30 min, 3 ≥ 30 min < 60 min, and 4 ≥ 60 min. The weekly frequency of itch was recorded with a 0–7 day scale, and the daily frequency was recorded with an approximate number of times per day.
2.4. Statistical Analyses
Descriptive statistics for categorical variables are reported as frequency counts and percentages. For continuous variables, we report the means and standard deviations (SD) if there was evidence that the distribution of values followed a normal distribution, and medians and inter-quartile ranges (IQR: 25th percentile–75th percentile) otherwise.
Differences in the means between scar sites at each time point, as well as differences in the means between times at each scar site, were investigated for each skin characteristic separately with a mixed model two-way analysis of variance (ANOVA) model, taking into account two sources of within-subject correlation: one due to measurements of intra-individual scars and the other due to repeated measurements over time. The
p-values were not adjusted for multiple testing in order to avoid being overly conservative and subsequently missing important treatment effects [
19,
20].
All hypothesis tests were two-sided and were performed at the 0.05 level of significance. All data analyses were generated using the SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
4. Discussion
Thickness, pliability, erythema, and pigmentation of HSc, donor sites, and normal skin have previously been measured over time in burn survivors using objective instrumentation [
9], but the time spread between measures was at least three months. To the best of our knowledge, this is the first time that HSc characteristics have been compared over time on a monthly basis between 2 and 7 months post-burn. These data provide a better understanding of the recovery profile of HSc sites, relative to normal skin and donor sites in the same individual, and expands our understanding of specific anatomical site differences.
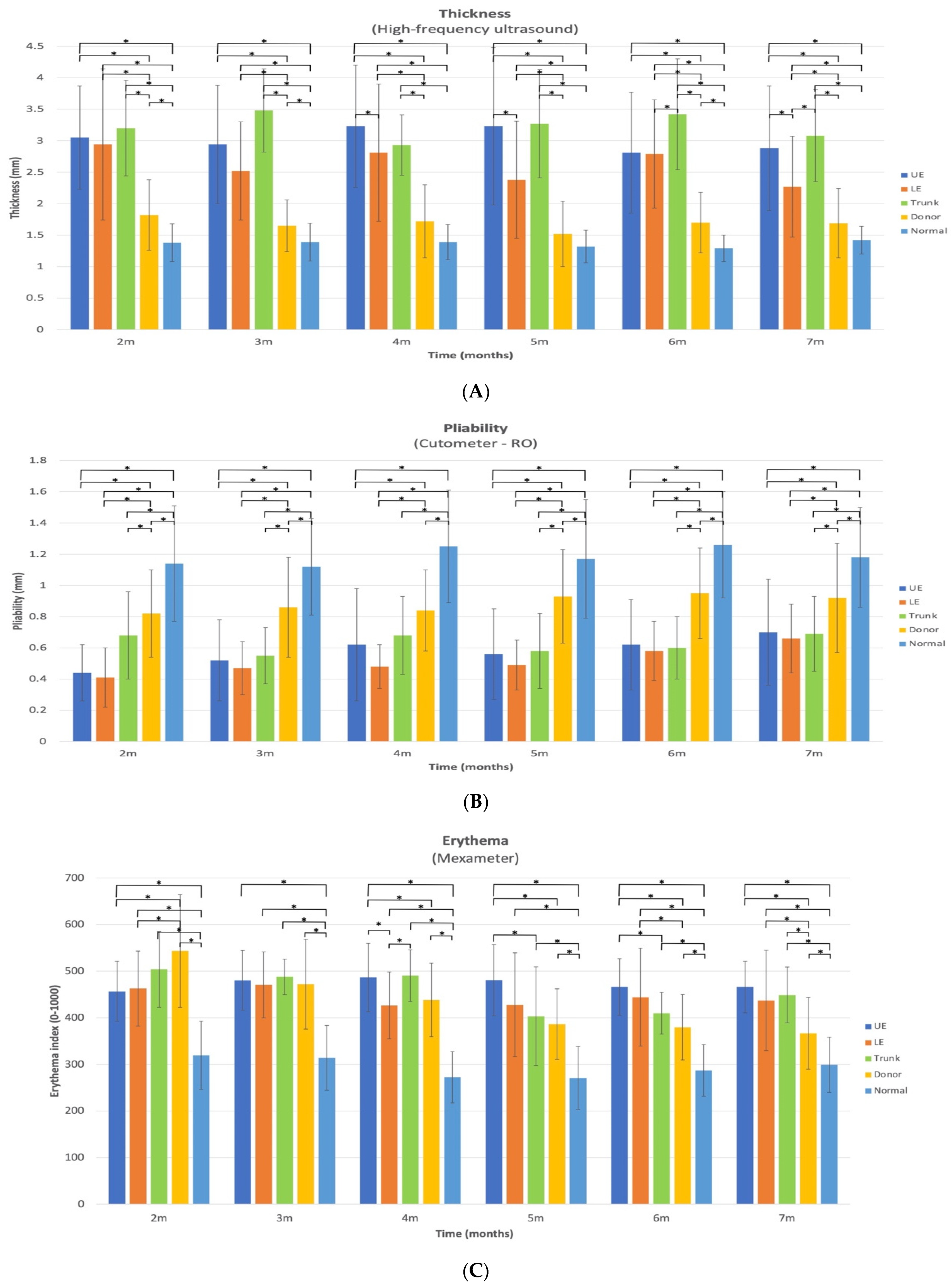
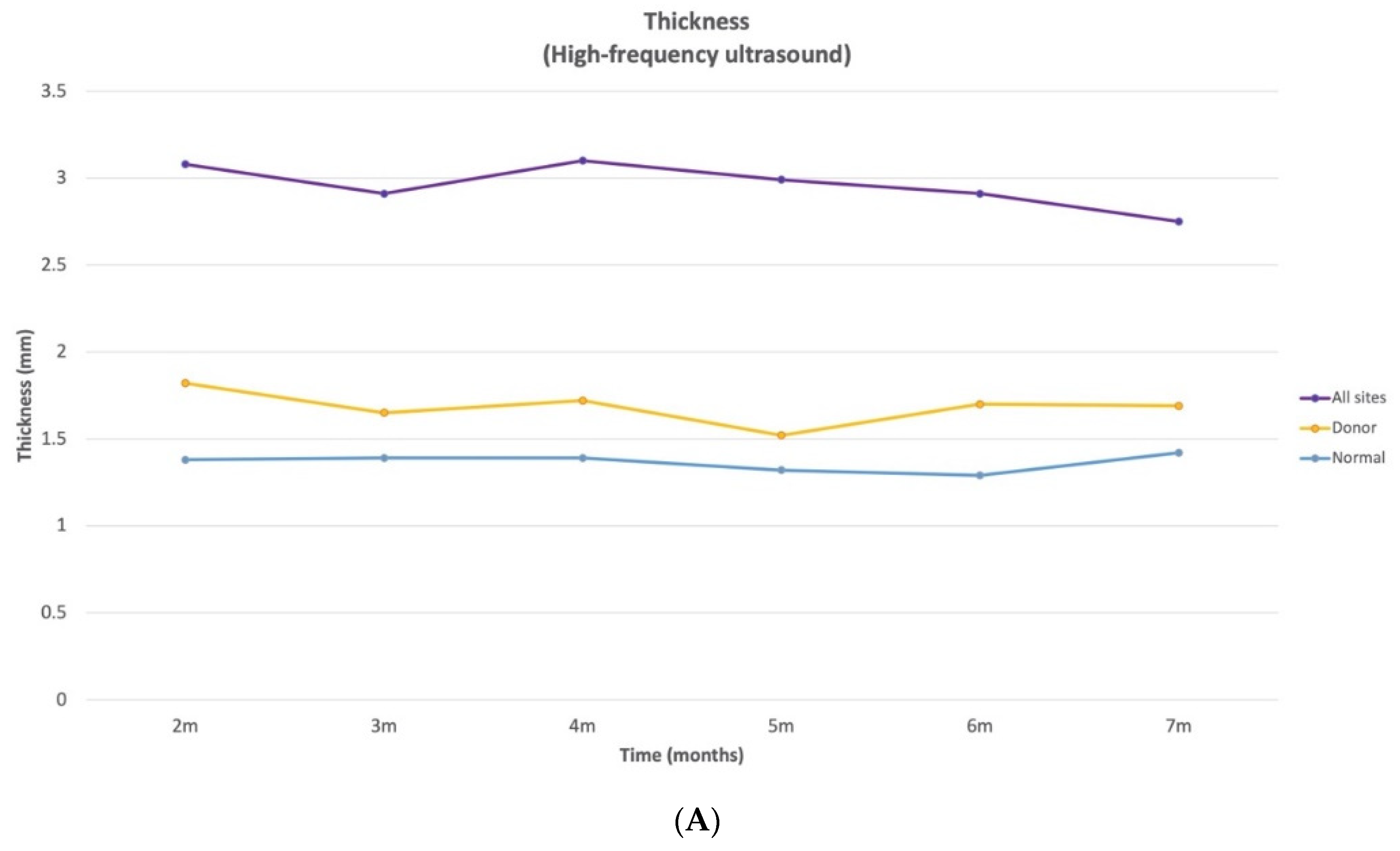
Scar thickness demonstrated notable site-specific changes with time that have important clinical implications. Between 2 and 7 months post-burn, the thickness of HSc in the combined anatomical sites (UE, LE, and trunk) was approximately 2 times thicker than normal skin and 1.6 times thicker than donor site scars. When examining specific anatomical locations, the three sites (UE, LE, and trunk) demonstrated both increases and decreases in thickness over time. However, in all 3 anatomical locations, there was a significant decrease in thickness from 3 to 7 months. The thickness of UE HSc sites were at their peak at 4 months post-burn and decreased by 10.8% between 4 and 7 months post-burn. For the HSc in the LE site, they were at their peak of thickness at 2 months and decreased by 22.8% between 2 and 7 months post-burn. The thickness of the HSc in the trunk sites were at their peak at 3 months and demonstrated an improvement of 11.5% between 3 and 7 months. Thus, scars appear to increase in thickness for a maximum of 4 months, then plateau or reduce across time.
While HSc thickness appears to vary by anatomical location, for example, the trunk sites were slightly but not significantly thicker than the UE and were significantly thicker than the LE at 7 months, this may be related to pre-burn thickness. The normative data for the trunk thickness of normal skin is approximately 0.73 mm and 0.65 mm thicker than normal UE and LE skin, respectively, for 20–59 year-old males and females [
10]. Therefore, the pre-burn difference may account for some of the site-specific differences in HSc thickness.
The comparison of different anatomical HSc sites to donor scars at 7 months post-burn (LE HSc 1.3 times thicker, UE 1.7 times thicker, and trunk 1.8 times thicker) suggests that the LE HSc improved at a faster rate. Nonetheless, at 7 months LE, the HSc thickness remained statistically different from the donor site scars, meaning its thickness did not return to a normal scar thickness at 7 months post-burn.
The donor site thickness was significantly thicker than the normal skin at all time points, except at 4, 5, and 7 months. This is consistent with a previous study, where the donor sites were not significantly thicker than the normal sites, starting at 3 months [
10]. Of all the skin characteristics that were evaluated in this study, thickness most clearly distinguished normal scar (donor site) and normal skin from HSc at all time points. Interestingly, in a clinical study where the Vancouver scar scale (VSS) (a subjective scar assessment) was used to evaluate HSc, the height (thickness) subscale was the most specific (85.9%) and sensitive (99.5%) skin characteristic for the diagnosis of HSc [
22].
Since usual care was continued during the data collection, the impact of pressure therapy on the scar thickness measures must be considered. Pressure garments have been shown to improve the thickness of HSc in burn survivors and compression garment wear is now considered a gold standard treatment for pathological scarring, like HSc [
23,
24]. However, studies have shown that pressure garments should be monitored regularly, since optimal pressure may not be achieved [
25,
26], and pressure may reduce with time [
25] or at different anatomical sites, partially due to the fact that pressure loss correlates to the radius of the curvature of that anatomical region [
27,
28,
29,
30].
Another factor that could have an impact on the variability of pressure benefits, depending on the anatomical location, is patient adherence to this treatment. A systematic review and meta-ethnography of the non-adherence with compression garment wear in adult burn patients revealed six themes impacting adherence: sensory factors (such as itch, pain, sensitivity, or body temperature), psychological state, impact of the garment on the patient’s function, availability of social support, degree of choice, and education provided to patients by their therapists [
31]. Since the UE is the anatomical site most solicited during functional activities, and the ribcage expands when breathing, perceived tightness may specifically limit patients adherence to UE and trunk garment wear. In fact, verbal reports from clinicians confirmed that they rarely aim to ensure adequate pressure for chest and trunk garments, because patients simply do not tolerate them. Thus, trunk HSc may take longer to improve because of this lack of adequate pressure dosage applied to this area. However, to the best of our knowledge, no study has systematically investigated the different factors associated with the anatomical variation of pressure tolerance, treatment adherence, or therapeutic benefit.
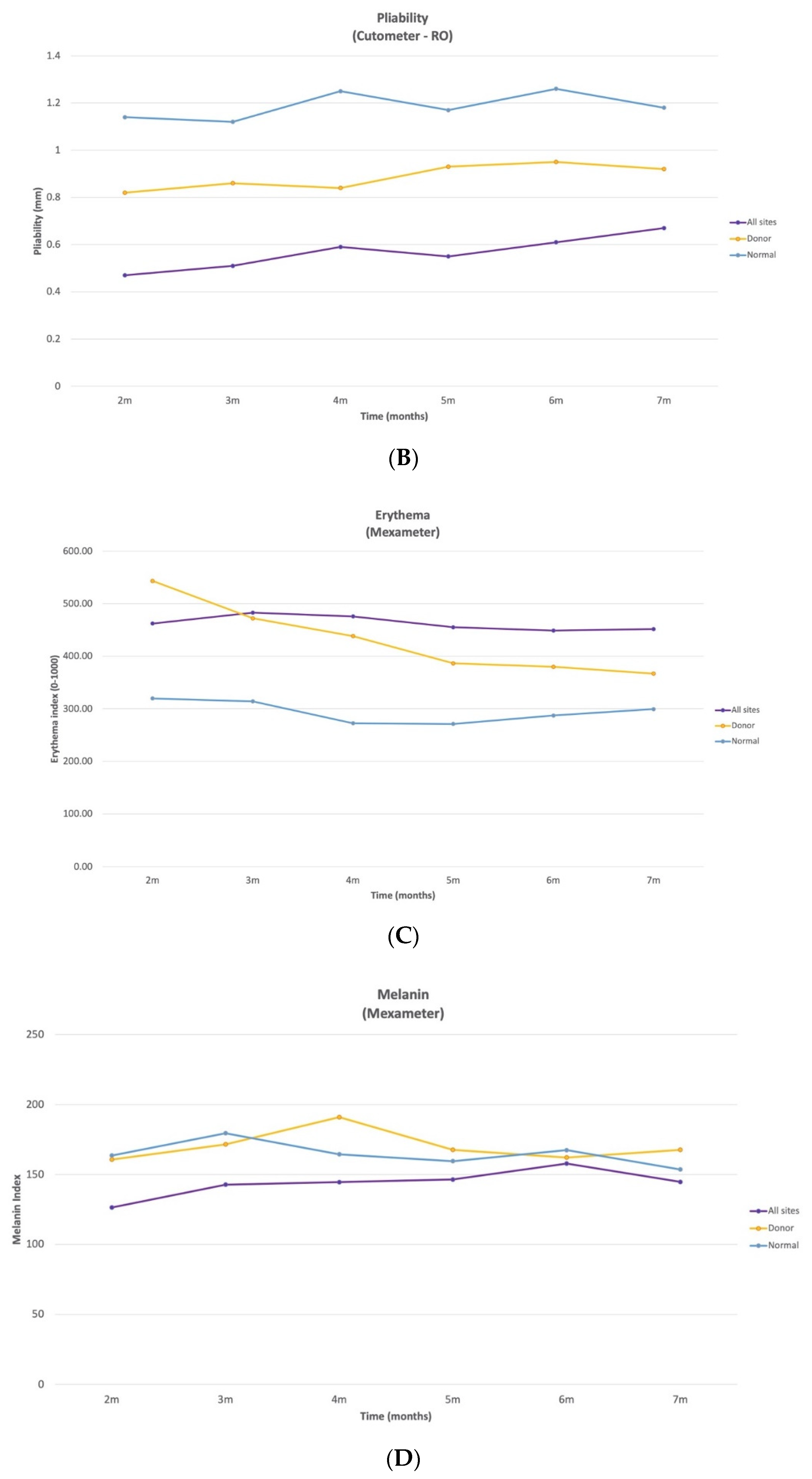
The pliability of HSc in the UE and LE and normal scar sites significantly increased between 2 and 7 months by 59%, 61%, and 12%, respectively. To the best of our knowledge, this study is the first to demonstrate an improvement in pliability for HSc and donor site scars as early as 4 months post-burn. On the other hand, the pliability for the trunk HSc did not demonstrate a significant change between at any time point and only increased by 1.5% between 2 and 7 months, which could demonstrate a lack of spontaneous resolution of pliability for this anatomical site and/or a lack of response to pressure therapy.
In this study, even though the donor site scar pliability significantly improved between 2 and 7 months, it remained significantly less pliable than normal skin. Thus, although a donor site scar is considered a normal scar, it does not return to a normal pliability by 7 months post-burn.
There were significant changes over time in the erythema index for all measured sites, including the normal skin. Between 2 and 7 months, the HSc redness in the UE was the only anatomical location where it significantly increased and peaked at 4 months post-burn. There was a significant decrease in UE HSc erythema between 3 and 7 months, but it remained the most erythematous at 7 months. Trunk HSc redness peaked at 2 months post-burn and significantly decreased from 2 to 7 months. LE HSc peaked at 3 months post-burn and significantly decreased from 3 to 4 months only. Conversely, donor site erythema decreased significantly (32.5%) between 2 and 7 months and almost returned to normal skin levels (ration 1.2:1), which has previously been reported [
9]. Surprisingly, at 2 months, donor site scars were redder than all HSc sites, making it difficult to distinguish donor from HSc based on erythema, until 5 months post burn. Similarly, clinicians found that vascularity (erythema) was very difficult to judge in subjective scar assessments, like the VSS [
22]. Thus, erythema alone should not be used to distinguish HSc from normal scars in the first months post-burn; thickness is a more reliable indicator.
To the best of our knowledge, this is the first time that normal skin erythema has been reported to vary significantly with time, where it peaked at 2 months and significantly decreased from 2 to 5 and 3 to 6 months. Since 61% of the normal skin measurements were taken on the UE, 34% on the LE, and 5% on the trunk, we compared the erythema index of normal skin at 7 months to the normative data [
10] for each of these anatomical locations for 20–59-year-old male and females. Normal skin at 7 months was 10%, 55%, and 12% more erythematous than the average normative data in the UE, LE, and trunk, respectively. Therefore, normal skin in this cohort did not return to normative data redness at 7 months post-burn. Further research is needed to determine why burn survivors demonstrate elevated levels of normal skin erythema and when it returns to normal. To the best of our knowledge, there are no other longitudinal studies that examined erythema in burn survivors using the Mexameter; therefore, it is not possible to make further comparisons.
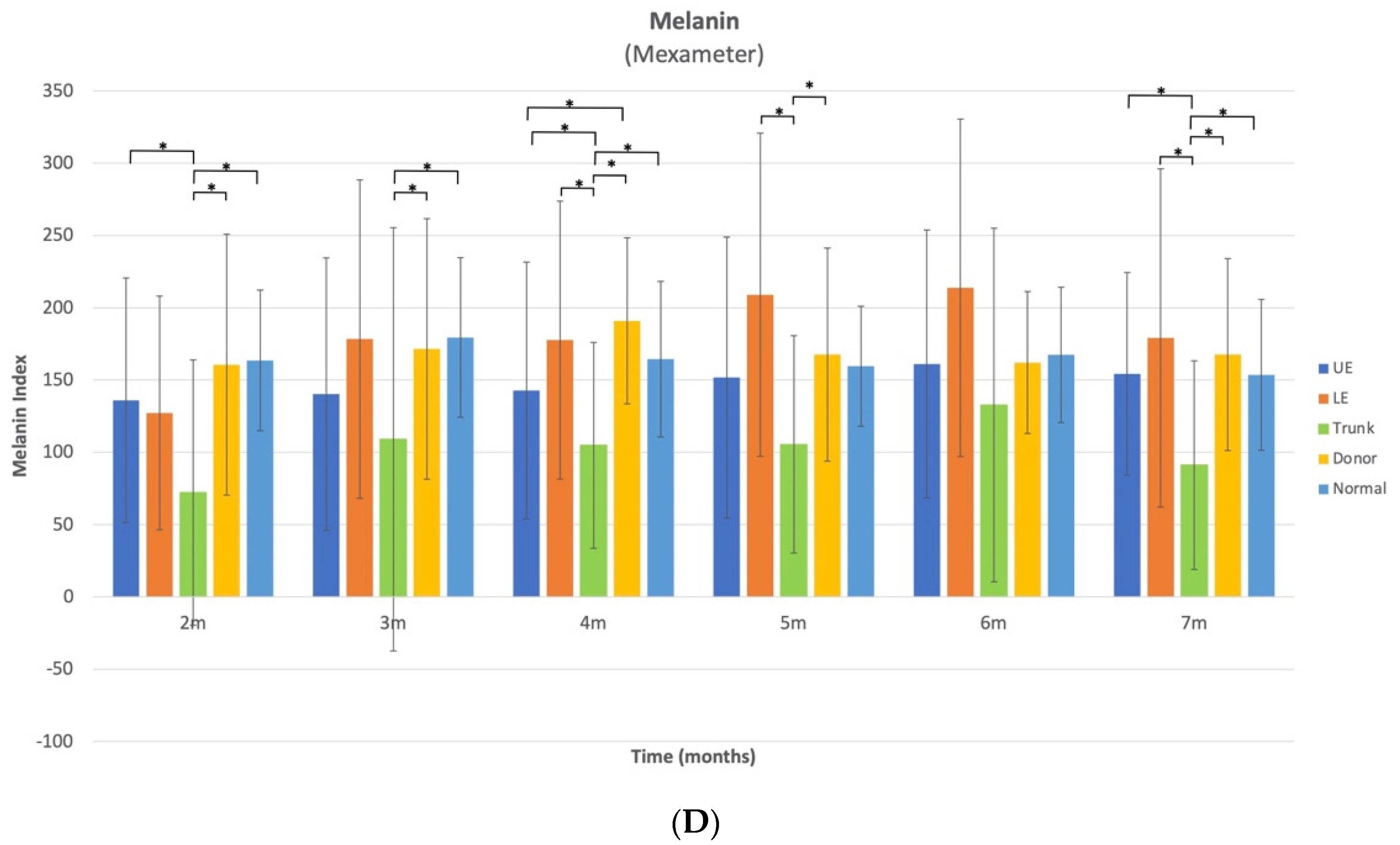
The melanin index of trunk HSc was lower than that of all other sites at all time points with sporadic significant variation across time. However, it is important to consider normative anatomical data, since melanin varies greatly based upon sun exposure, and the UE and LE are more likely to be exposed to the sun, compared to the trunk. When comparing to normative data, trunk melanin in normal skin for 20–59-year-old Caucasian male and females, it is approximately 5.4% and 7% less pigmented than normal skin in the UE and LE, respectively [
10]. However, this difference cannot be entirely responsible for the fact that the melanin index of HSc in the trunk site at 7 months was approximately 61% lower than UE HSc and 65% lower than LE HSc sites. Therefore, for this cohort, the HSc on the trunk were significantly less pigmented than the HSc on the UE at 2, 4, and 7 months post-burn and significantly less pigmented than the HSc on the LE sites at 4, 5, and 7 months, and normal anatomic variation did not entirely account for this difference. When examining the combined anatomical HSc results, the melanin index is quite similar for HSc, donor scar, and normal skin at 6 months post-burn. To the best of our knowledge, no other reports have used the Mexameter to examine melanin in burn survivors; thus, further comparisons are not possible.
Since only 11 of the 44 participants reported itch at baseline, it was generally episodic, and there was substantial variability in the location and itch intensity across time, there did not appear to be any discernable patterns, nor a persistence problem at specific locations. This may be related to the fact that our research group has been very involved in a number of research projects examining itch evaluation and treatment; therefore, clinicians may be attentive to addressing any issues that do arise.
Study Limitations
As with any study, the limitations associated with this study have to be considered when interpreting the results. All participants were recruited from a single burn center in North America, so these results are not generalizable to other burn centers. Since the participants predominantly fell into category II or III of the Fitzpatrick scale, these data may not be generalizable to populations in higher categories. Unfortunately, the sample size in the different categories does not allow us to make comparisons across the Fitzpatrick scale. As with any longitudinal study with burn survivors, it is challenging to obtain consistent data at set time points. Since the participants’ physical presence is required for the data reported in this study, and many participants live far away from the burn center, the extent of missing data is higher than what we would have hoped for, in spite of the fact that the data collection was coordinated with patients during follow-up clinic appointments. Future studies should monitor different treatment benefits and objectively measure their effects on HSc and normal scar characteristics for the burn population at specific anatomical locations. Larger samples from multiple burn centers and for longer periods of time should also be investigated.