Operating Room Fomites as Potential Sources for Microbial Transmission in Burns Theatres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
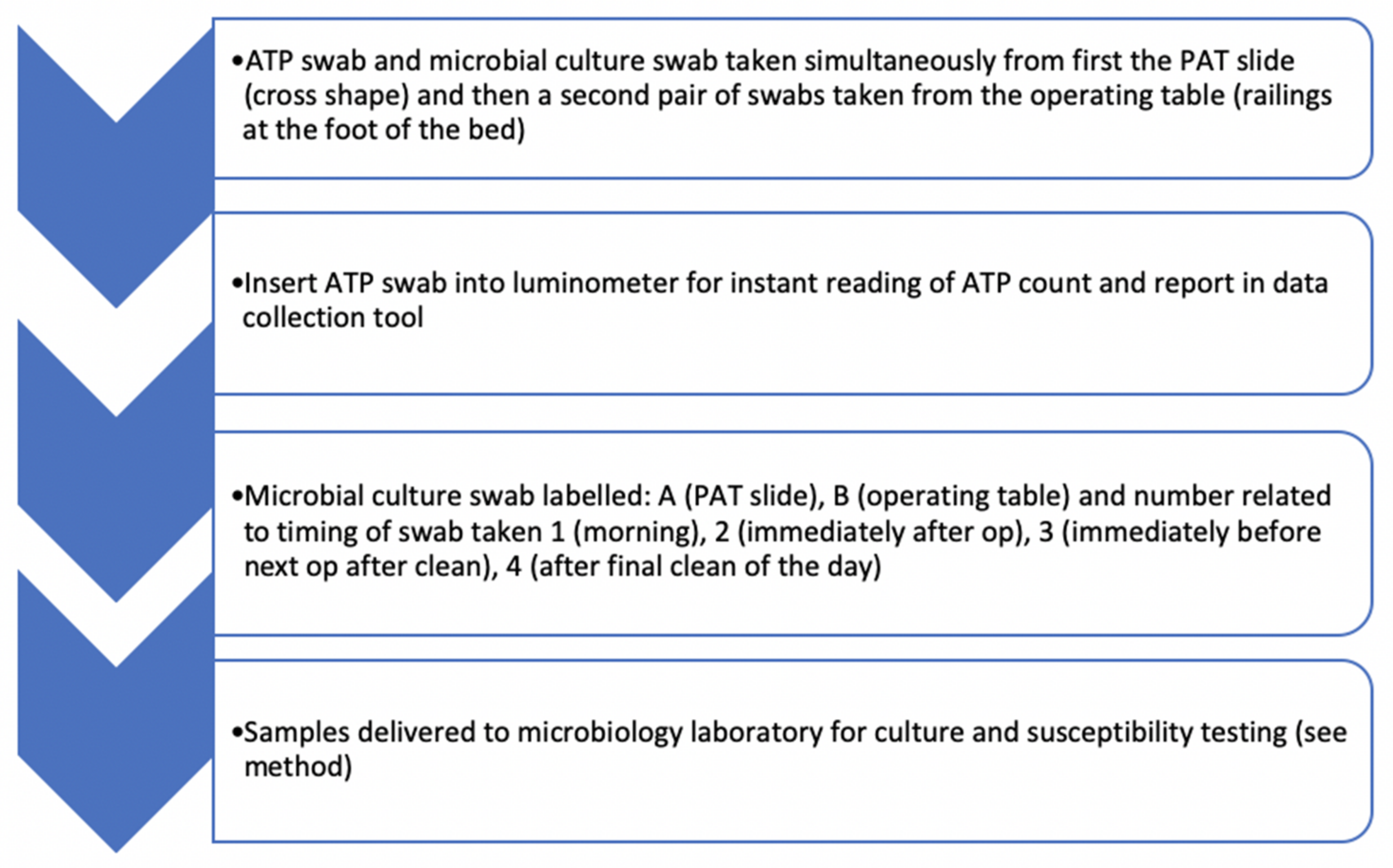
2.2. Sampling Protocol
2.3. Determining Biological Contamination of Operating Theatre Fomites
2.4. Determining Microbiological Contamination of Operating Theatre Fomites
2.5. Statistical Analysis
2.6. Study Approval
3. Results
3.1. Determining Biological Contamination of Operating Theatre Fomites through ATP Detection
3.2. Determining Microbiological Contamination of Operating Theatre Fomites through Microbiological Culture
3.3. Clinical Correlation between Patient Colonisation and Microbial Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent
Data Availability
References
- Baier, C.; Ipaktchi, R.; Ebadi, E.; Rennekampff, H.O.; Just, H.M.; Vogt, P.; Bange, F.C.; Suchodolski, K. Infection control in german-speaking burn centres: Results of an online survey. Ann. Burn. Fire Disasters 2018, 31, 189–193. [Google Scholar]
- Neely, C.J.; Kartchner, L.B.; Mendoza, A.E.; Linz, B.M.; Frelinger, J.A.; Wolfgang, M.C.; Maile, R.; Cairns, B.A. Flagellin Treatment Prevents Increased Susceptibility to Systemic Bacterial Infection after Injury by Inhibiting Anti-Inflammatory IL-10+ IL-12- Neutrophil Polarization. PLoS ONE 2014, 9, e85623. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, A.B.; Van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Korona-Glowniak, I.; Buszewicz, G.; Forma, A.; Sitarz, M.; Teresiński, G. Viral Infections in Burn Patients: A State-Of-The-Art Review. Viruses 2020, 12, 1315. [Google Scholar] [CrossRef] [PubMed]
- Rafla, K.; Tredget, E.E. Infection control in the burn unit. Burns 2011, 37, 5–15. [Google Scholar] [CrossRef]
- Bayat, A.; Shaaban, H.; Dodgson, A.; Dunn, K.W. Implications for Burns Unit design following outbreak of multi-resistant Acinetobacter infection in ICU and Burns Unit. Burns 2003, 29, 303–306. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Wagenvoort, J.H.T.; De Brauwer, E.; Penders, R.; Willems, R.; Top, J.; Bonten, M.J.M. Environmental survival of vancomycin-resistant Enterococcus faecium. J. Hosp. Infect. 2011, 77, 282–283. [Google Scholar] [CrossRef]
- Bache, S.E.; MacLean, M.; Gettinby, G.; Anderson, J.G.; MacGregor, S.J.; Taggart, I. Airborne bacterial dispersal during and after dressing and bed changes on burns patients. Burns 2015, 41, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Garvey, M.I.; Bradley, C.W.; Jumaa, P. Environmental decontamination following occupancy of a burns patient with multiple carbapenemase-producing organisms. J. Hosp. Infect. 2016, 93, 136–140. [Google Scholar] [CrossRef]
- Decraene, V.; Ghebrehewet, S.; Dardamissis, E.; Huyton, R.; Mortimer, K.; Wilkinson, D.; Shokrollahi, K.; Singleton, S.; Patel, B.; Turton, J.; et al. An outbreak of multidrug-resistant Pseudomonas aeruginosa in a burns service in the North of England: Challenges of infection prevention and control in a complex setting. J. Hosp. Infect. 2018, 100, e239–e245. [Google Scholar] [CrossRef] [PubMed]
- Chlor-Clean Tablets. Chlorine & Detergent Tablets. Available online: https://rmsupply.co.uk/chlor-clean-tablets/232-chlor-clean-tablets.html (accessed on 4 December 2020).
- Chlor-Clean Detergent Sanitiser Tablets–Guest Medical n.d. Available online: https://guest-medical.co.uk/chlor-clean/ (accessed on 4 December 2020).
- Zambrano, A.A.; Jones, A.; Otero, P.; Ajenjo, M.C.; Labarca, J. Assessment of hospital daily cleaning practices using ATP bioluminescence in a developing country. Braz. J. Infect. Dis. 2014, 18, 675–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, C.; Cooper, R.; Gilmore, J.; Davies, C.; Lewis, M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Infect. 2000, 45, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [Green Version]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect. Dis. 2018, 18, 583. [Google Scholar] [CrossRef]
- UK SMI B 11: Swabs from Skin and Superficial Soft Tissue Infections-GOV.UK n.d. Available online: https://www.gov.uk/government/publications/smi-b-11-investigation-of-skin-superficial-and-non-surgical-wound-swabs (accessed on 2 June 2020).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and Zone Diameters. Version 9.0 2019. Available online: http://www.eucast.org (accessed on 2 June 2020).
- Nante, N.; Ceriale, E.; Messina, G.; Lenzi, D.; Manzi, P. Effectiveness of ATP bioluminescence to assess hospital cleaning: A review. J. Prev. Med. Hyg. 2017, 58, E177–E183. [Google Scholar]
- Richard, R.D.; Bowen, T.R. What Orthopaedic Operating Room Surfaces Are Contaminated With Bioburden? A Study Using the ATP Bioluminescence Assay. Clin. Orthop. Relat. Res. 2017, 475, 1819–1824. [Google Scholar] [CrossRef] [Green Version]
- Ellis, O.; Godwin, H.A.; David, M.; Morse, D.J.; Humphries, R.; Uslan, D.Z. How to better monitor and clean irregular surfaces in operating rooms: Insights gained by using both ATP luminescence and RODAC assays. Am. J. Infect. Control. 2018, 46, 906–912. [Google Scholar] [CrossRef]
- Tissot, F.; Blanc, D.; Basset, P.; Zanetti, G.; Berger, M.; Que, Y.A.; Eggimann, P.; Senn, L. New genotyping method discovers sustained nosocomial Pseudomonas aeruginosa outbreak in an intensive care burn unit. J. Hosp. Infect. 2016, 94, 2–7. [Google Scholar] [CrossRef]
- Barbut, F.; Yezli, S.; Mimoun, M.; Pham, J.; Chaouat, M.; Otter, J.A. Reducing the spread of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus on a burns unit through the intervention of an infection control bundle. Burns 2013, 39, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P.; Greenwood, N. Understanding the Hawthorne effect. BMJ 2015, 351, h4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, J.M.; Havill, N.L.; Dumigan, D.G.; Golebiewski, M.; Balogun, O.; Rizvani, R. Monitoring the Effectiveness of Hospital Cleaning Practices by Use of an Adenosine Triphosphate Bioluminescence Assay. Infect. Control. Hosp. Epidemiol. 2009, 30, 678–684. [Google Scholar] [CrossRef] [PubMed]

| Day | Operating Theatre Fomite Sampled | Start of Day ATP Count (RLU) | Microbial Culture Results | After Case before Cleaning ATP Count (RLU) | Microbial Culture Results | Before Case after Cleaning ATP Count (RLU) | Microbial Culture Results | After Final Clean ATP Count (RLU) | Microbial Culture Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PAT slide | 0 | NSG | 0 | NSG | 2 | NSG | 0 | NSG |
| Operating table | 1 | NSG | 1 | NSG | 2 | NSG | 0 | NSG | |
| 2 | PAT slide | 0 | NSG | 1 | NSG | 0 | NSG | 0 | NSG |
| Operating table | 0 | NSG | 1 | NSG | 1 | NSG | 2 | NSG | |
| 3 | PAT slide | 0 | NSG | 16 * | MSSA * | N/A | N/A | 0 | NSG |
| Operating table | 6 | NSG | 0 | NSG | N/A | N/A | 0 | NSG | |
| 4 | PAT slide | 1 | NSG | 1 | NSG | 0 | NSG | 33 * | NSG |
| Operating table | 0 | NSG | 1 | NSG | 1 | NSG | 1 | NSG | |
| 5 | PAT slide | 0 | NSG | 1 | NSG | N/A | N/A | 0 | NSG |
| Operating table | 0 | NSG | 0 | NSG | N/A | N/A | 0 | NSG | |
| 6 | PAT slide | 0 | NSG | 0 | NSG | 0 | NSG | 0 | NSG |
| Operating table | 0 | NSG | 0 | NSG | 0 | NSG | 0 | NSG | |
| 7 | PAT slide | 3 | NSG | 14 * | NSG | 1 | NSG | 0 | NSG |
| Operating table | 0 | NSG | 2 | NSG | 1 | NSG | 1 | NSG | |
| 8 | PAT slide | 3 | NSG | 24 * | Enterobacter cloacae * | N/A | N/A | 36 * | Enterobacter cloacae * |
| Operating table | 0 | NSG | 2 | NSG | N/A | N/A | 0 | NSG | |
| 9 | PAT slide | 0 | NSG | 2 | NSG | 0 | NSG | 1 | NSG |
| Operating table | 0 | NSG | 0 | NSG | 0 | NSG | 1 | NSG | |
| 10 | PAT slide | 1 | NSG | 1 | NSG | 0 | NSG | 0 | NSG |
| Operating table | 1 | NSG | 0 | NSG | 1 | NSG | 0 | NSG | |
| 11 | PAT Slide | 0 | NSG | 149 * | Pseudomonas aeruginosa * | 102 * | NSG | 0 | NSG |
| Operating table | 0 | NSG | 61 * | NSG | 161 * | NSG | 2 | NSG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rela, M.; Opel, S.; Williams, S.; Collins, D.P.; Martin, K.; Mughal, N.; Moore, L.S.P. Operating Room Fomites as Potential Sources for Microbial Transmission in Burns Theatres. Eur. Burn J. 2021, 2, 1-8. https://doi.org/10.3390/ebj2010001
Rela M, Opel S, Williams S, Collins DP, Martin K, Mughal N, Moore LSP. Operating Room Fomites as Potential Sources for Microbial Transmission in Burns Theatres. European Burn Journal. 2021; 2(1):1-8. https://doi.org/10.3390/ebj2010001
Chicago/Turabian StyleRela, Mariam, Sophia Opel, Sarah Williams, Declan P. Collins, Kevin Martin, Nabeela Mughal, and Luke S. P. Moore. 2021. "Operating Room Fomites as Potential Sources for Microbial Transmission in Burns Theatres" European Burn Journal 2, no. 1: 1-8. https://doi.org/10.3390/ebj2010001
APA StyleRela, M., Opel, S., Williams, S., Collins, D. P., Martin, K., Mughal, N., & Moore, L. S. P. (2021). Operating Room Fomites as Potential Sources for Microbial Transmission in Burns Theatres. European Burn Journal, 2(1), 1-8. https://doi.org/10.3390/ebj2010001





