Assessment of Population Dynamics and Fishery Exploitation of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Iranian Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Length Frequency Distribution
2.2. Biometry
2.3. Growth Studies
2.4. Mortality Estimate
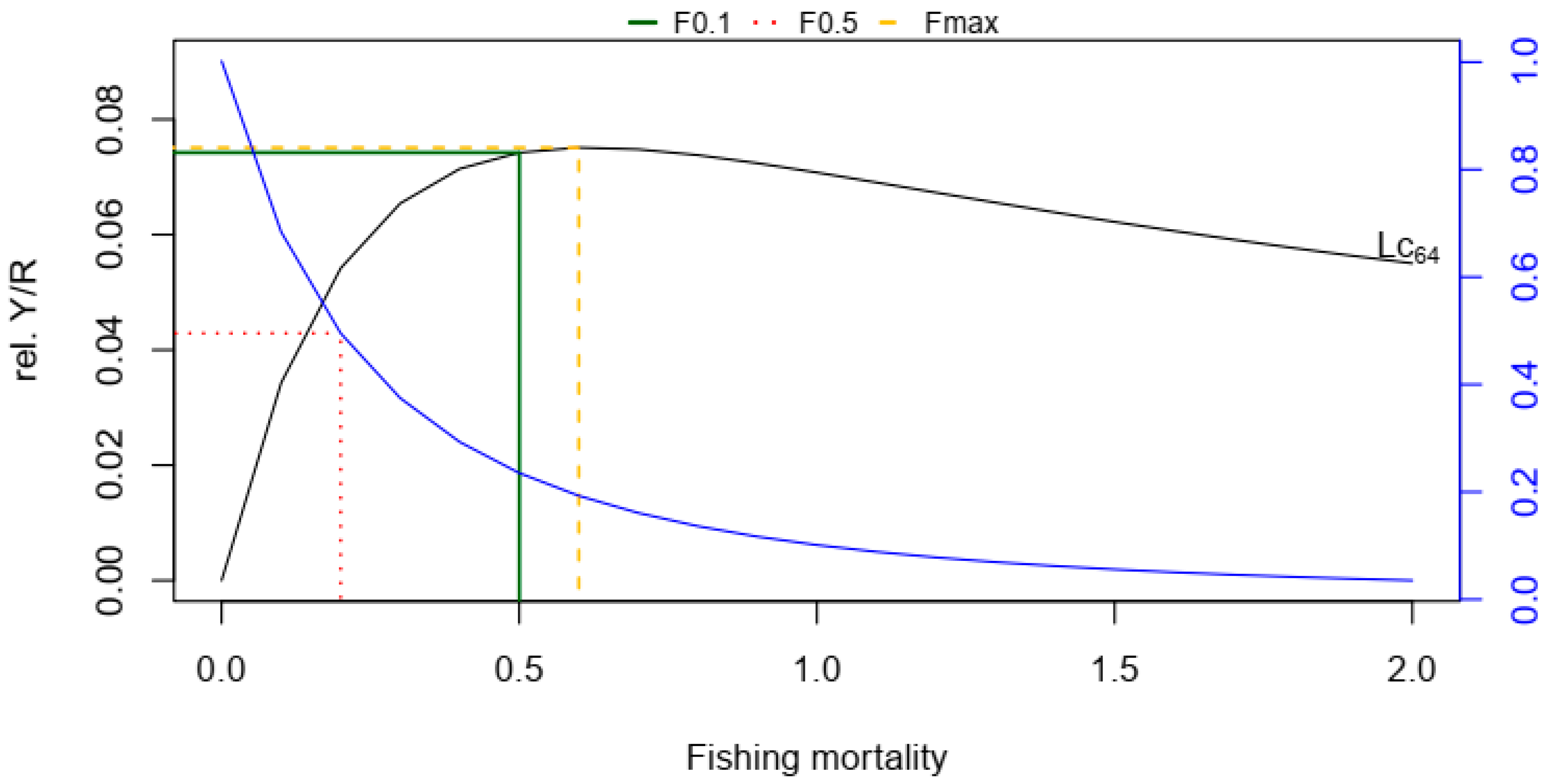
2.5. Length-Based Reference Point
2.6. Fishery Assessment
2.7. Yield per Recruit and Biomass per Recruit
2.8. Length-Based SPR Assessment Methodology (LB-SPR)
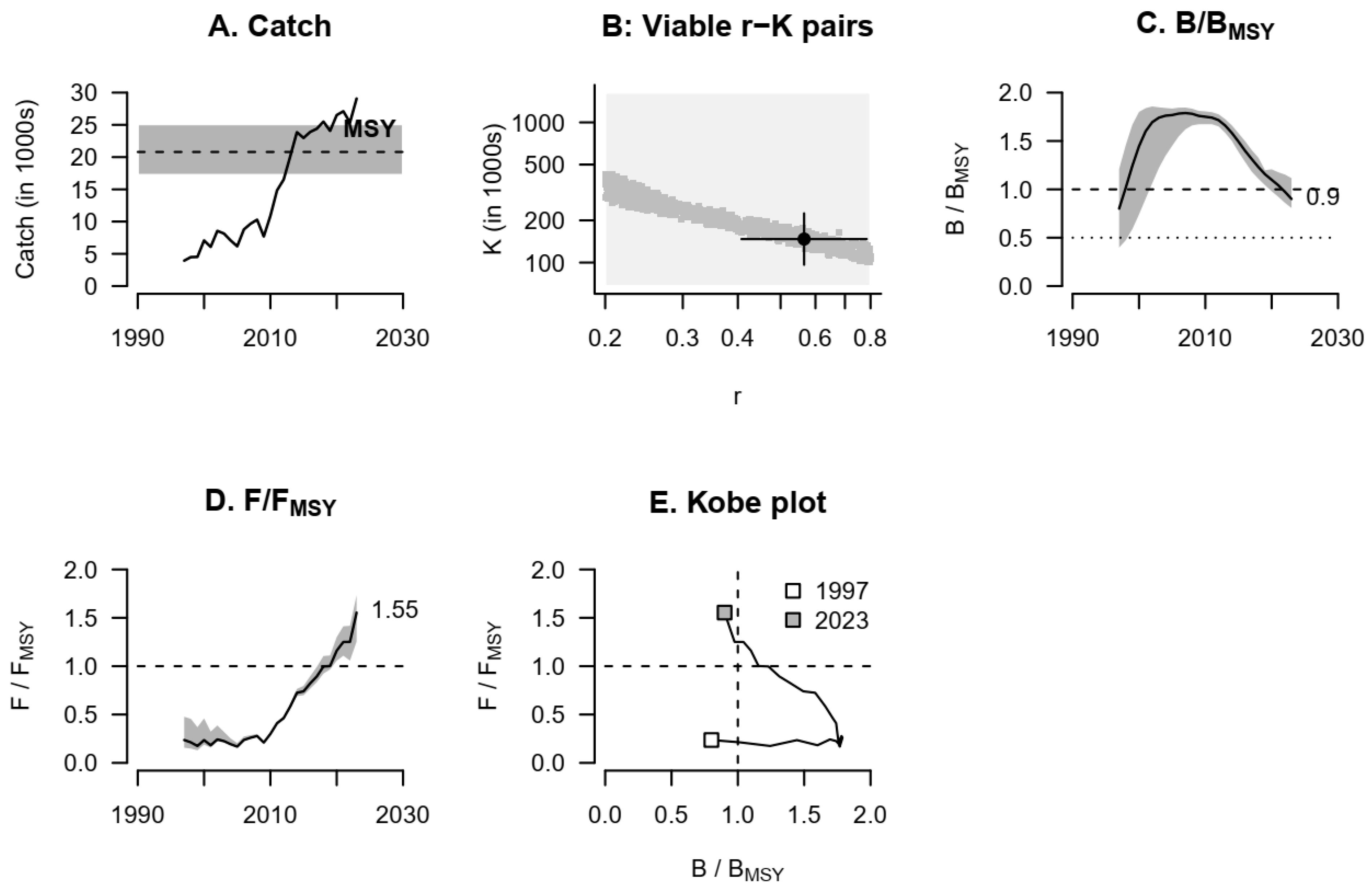
2.9. C-MSY (CMSY) Method (Monte Carlo Algorithm)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhariya, M.K.; Banerjee, A.; Meena, R.S. Importance of natural resources conservation: Moving toward the sustainable world. In Natural Resources Conservation and Advances for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–27. [Google Scholar]
- Alsaleh, M. The role of the fishery industry in the shift towards sustainable food security: A critical study of blue food. Environ. Sci. Pollut. Res. 2023, 30, 105575–105594. [Google Scholar] [CrossRef]
- Karp, M.A.; Peterson, J.; Lynch, P.D.; Griffis, R. (Eds.) Accounting for Shifting Distributions and Changing Productivity in the Fishery Management Process: From Detection to Management Action; NOAA Technical Memorandum NMFS-F/SPO-188; U.S. Department of Commerce, NOAA: Washington, DC, USA, 2018; 37p. [Google Scholar]
- Lynch, P.D.; Methot, R.D.; Link, J.S. Implementing a Next Generation Stock Assessment Enterprise: An Update to the NOAA Fisheries Stock Assessment Improvement Plan; NOAA technical memorandum NMFS-F/SPO; Department of Commerce, NOAA: Washington, DC, USA, 2018. [Google Scholar]
- Medeiros-Leal, W.; Santos, R.; Peixoto, U.I.; Casal-Ribeiro, M.; Novoa-Pabon, A.; Sigler, M.F.; Pinho, M. Performance of length-based assessment in predicting small-scale multispecies fishery sustainability. Rev. Fish Biol. Fish. 2023, 33, 819–852. [Google Scholar] [CrossRef]
- Pons, M.; Cope, J.M.; Kell, L.T. Comparing performance of catch-based and length-based stock assessment methods in data-limited fisheries. Can. J. Fish. Aquat. Sci. 2020, 77, 1026–1037. [Google Scholar] [CrossRef]
- Fitzgerald, C.J.; Droll, J.S.; Shephard, S.; Monk, C.T.; Rittweg, T.; Arlinghaus, R. Length-based assessment of an exploited coastal pike (Esox lucius) stock (Rügen, southern Baltic Sea) underscores the crucial relevance of growth and natural mortality for assessment outcomes. Fish. Res. 2023, 263, 106667. [Google Scholar] [CrossRef]
- Al-Shehhi, A.M.; Dutta, S.; Paul, S. Stock assessment of Scomberomorus commerson (Kingfish) fishery of Oman: Perspectives of sustainability. Reg. Stud. Mar. Sci. 2021, 47, 101970. [Google Scholar] [CrossRef]
- Collette, B.B.; Nauen, C.E. FAO species catalogue. Vol. 2. Scombrids of the world. An annotated and illustrated catalogue of tuna, mackerel’s bonitos and related species known to date. FAO Fish. Synop. 1983, 2, 137. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. 2023. Available online: www.fishbase.org (accessed on 16 November 2023).
- Kaymaram, F.; Gashemi, S.H.; Vahabnezhab, A.; Darvishi, M. Growth, mortality and exploitation rate of narrow-barred Spanish mackerel (Scomberomorus commerson) in the Persian Gulf and Oman Sea, Iran, Hormozgan’s waters. In Proceedings of the Third Working Party on neritic tuna in Bali Indonesia, Bali, Indonesia, 2–5 July 2013; IOTC-2013-WPNT03–29 Rev.1. pp. 1–7. [Google Scholar]
- Newman, S.J.; Mackie, M.C.; Lewis, P.D. Age-based demography and relative fisheries productivity of Spanish mackerel, Scomberomorus commerson (Lacepede) in Western Australia. Fish. Res. 2012, 129, 46–60. [Google Scholar] [CrossRef]
- Johnson, M.G.; Mgaya, Y.D.; Shaghude, Y.W. Growth, Mortality and Reproductive Biology of Narrow-Barred Spanish Mackerel, Scomberomorus commerson (Lacepede, 1800) Along the Northern Tanzanian Coastal Waters; IOTC-2014-WPNT02–29 Rev.1; FAO: Rome, Italy, 2014; pp. 1–17. [Google Scholar]
- Niamaimandi, N.; Kaymaram, F.; Hoolihan, J.P.; Mohammadi, G.H.; Fatemi, S.M.R. Population dynamic parameters of narrow-barred Spanish mackerel (Scomberomorus commerson) from commercial catch in the Northern Persian Gulf. Glob. Ecol. Conserv. 2015, 4, 666–672. [Google Scholar] [CrossRef]
- Lee, B.; Mann, B.Q. Age and growth of narrow-barred Spanish mackerel Scomberomorus commerson in the coastal waters of southern Mozambique and Kwa Zulu Natal South Africa. Afr. J. Mar. Sci. 2017, 39, 397–407. [Google Scholar] [CrossRef]
- Yuliana, E.; Nurhasanah, A. The King mackerel and frigate tuna rate exploitation in Karimunjawa Java Sea. J. Mat. Sains dan Teknol. 2017, 18, 44–55. (In Indonesian) [Google Scholar] [CrossRef][Green Version]
- Mallawa, A.; Amir, F. Population dynamics of narrow-barred Spanish mackerel Scomberomorus commerson (Lacepede, 1800) in Bone Bay waters, South Sulawesi, Indonesia. Aquac. Aquar. Conserv. Legis. 2019, 12, 908–917. [Google Scholar][Green Version]
- Ghodrati Shojaei, M.; Taghavi Motlagh, S.A.; Seyfabadi, S.J.; Abtahi, B.; Dehghani, R. Age, Growth and Mortality Rate of the Narrow-Barred Spanish Mackerel (Scomberomerus commerson Lacepède, 1800) in Coastal Waters of Iran from Length Frequency Data. Turk. J. Fish. Aquat. Sci. 2007, 7, 115–121. [Google Scholar][Green Version]
- Taghavi Motlagh, S.A.; Ghodrati Shojaei, M.; Seyfabadi, S.J.; Abtahi, B.; Dehghani, R. Population dynamics of narrow barred Spanish mackerel (Scomberomorus commerson) in coastal waters of Oman sea (Iran). J. Iran. Sci. Fish. 2008, 7, 257–270. [Google Scholar][Green Version]
- Darvishi, M.; Kaymaram, F.; Parafkandeh, F.; Salarpouri, A. Estimating growth and mortality parameters of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in the Iranian waters of the Persian Gulf and Oman Sea. J. Persian Gulf 2012, 3, 57–62. [Google Scholar][Green Version]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 2010; p. 662. [Google Scholar]
- Mildenberger, T.; Taylor, M.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Pauly, D. Theory and management of tropical multispecies stocks: A review with emphasis on the Southeast Asian demersal fisheries. ICLARM Stud. Rev. 1979, 1, 35. [Google Scholar]
- Then, A.Y.; Hoenig, J.M.; Hall, N.G.; Hewitt, D.A. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Pauly, D. Some simple methods for the assessment of tropical fish stocks. FAO Fish. Tech. Pap. 1983, 234, 52. [Google Scholar]
- Hosseini, S.A.; Keymaram, F.; Alasvandi, F.; Mohammadkhani, H. Investigation of the status of tuna fish stocks in the Oman Sea. In Chabahar Offshore Fisheries Research Center (Chabahar); Iranian Fisheries Science and Research Institute Publication: Tehran, Iran, 2009; 96p. [Google Scholar]
- Beverton, R.J.H. Patterns of reproductive strategy parameters in some marine teleost fishes. J. Fish Biol. 1992, 41, 137–160. [Google Scholar] [CrossRef]
- IFO. Bureau of Statistics. In Yearbook of Fisheries Statistics; Iran Fisheries Organization (IFO): Tehran, Iran, 2024; pp. 1–25. [Google Scholar]
- Gayanilo, F.; Sparre, P.; Pauly, D. 2003. FAO-ICLARM Stock Assessment Tools (FiSAT) user’s Guide. In FAO Computerised Information Series (Fisheries); FAO: Rome, Italy, 2003; pp. 1–266. [Google Scholar]
- Hordyk, A.; Ono, K.; Valencia, S.; Loneragan, N.; Prince, J. A novel length-based empirical estimation method of spawning potential ratio (SPR), and tests of its performance, for small-scale, data-poor fisheries. ICES J. Mar. Sci. 2015, 72, 217–231. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 17 November 2023).
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2017, 18, 506–526. [Google Scholar] [CrossRef]
- Martell, S.; Froese, R. A simple method for estimating MSY from catch and resilience. Fish Fish. 2013, 14, 504–514. [Google Scholar] [CrossRef]
- Biswas, S.P. Manuel of Methods in Fish Biology, Fish Biology & Ecology Laboratory; Dibruyarh University: Dibrugarh, India, 1993; pp. 1–157. [Google Scholar]
- Dash, G.; Sen, S.; Pradhan, R.K.; Ghosh, S.; Josileen, J.; Jayasankar, J. Modeling framework for establishing the power law between length and weight of fishes and a meta-analysis for validation of LWRs for six commercially important marine fishes from the northwestern Bay of Bengal. Fish. Res. 2023, 257, 106496. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment and Management; Wiley: Hoboken, NJ, USA, 2007; pp. 1–340. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Lin, D. Sexual maturation, reproductive habits, and fecundity of fish. In Biology of Fishery Resources; Springer: Singapore, 2022; pp. 113–142. [Google Scholar]
- Al Alawi, Y.; Dutta, S. Assessment of seabream fisheries stock of oman using the monte carlo catch maximum sustainable yield and the bayesian schaefer model methods. Sustainability 2023, 15, 15692. [Google Scholar] [CrossRef]
- Mateus, A.; Estupina, B. Fish stock assessment of piraputanga Brycon microlepis in the Cuiabá River basin, Pantanal of Mato Grosso, Brazil. Braz. J. Biol. 2002, 1, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.N.; Libecap, G.D. Contracting problems and regulation: The case of the fishery. In Fisheries Economics, Volume I; Routledge: Oxfordshire, UK, 2019; pp. 345–362. [Google Scholar]
- Cheung, W.; Pitcher, T.; Pauly, D. A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol. Conserv. 2004, 124, 97–111. [Google Scholar] [CrossRef]
- Haruna, A.; Mallawa, A.; Musbir, M.; Zainuddin, M. Population dynamic indicator of the yellowfin tuna Thunnus albacares and its stock condition in the Banda Sea, Indonesia. AACL Bioflux. 2018, 11, 1323–1333. [Google Scholar]
- Mallawa, A.; Amir, F.; MusbirSusianti, W. Assessment of Katsuwonus pelamis conditions in Flores Sea waters, South Sulawesi. In Proceedings of the National Symposium on Marine and Fisheries II; Hasanuddin University Press: Makassar, Indonesia, 2015; pp. 299–307. (In Indonesian). [Google Scholar]
- Cope, J.M.; Punt, A.E. Drawing the lines: Resolving fishery management units with simple fisheries data. Can. J. Fish. Aquat. Sci. 2009, 66, 1256–1273. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.; Dimarchopoulou, D. Status and rebuilding of European fisheries. Mar. Policy 2018, 93, 159–170. [Google Scholar] [CrossRef]
- Zhai, L.; Liang, C.; Pauly, D. Assessments of 16 Exploited Fish Stocks in Chinese Waters Using the CMSY and BSM Methods. Front. Mar. Sci. 2020, 7, 483–993. [Google Scholar] [CrossRef]
- Hashemi, S.; Doustdar, M.; Ghasemzade, J.; Gholampor, A. Length-based fishery status of skipjack tuna, Katsuwonus pelamis (Linnaeus, 1758) (Teleostei: Scombridae: Scombrinae) in the northern waters of the Oman Sea (Iran). Ichthyol. J. 2021, 8, 160–169. [Google Scholar]
- Hashemi, S.; Doustdar, M. Stock Assessment of Indo-Pacific King Mackerel, Scomberomorus guttatus (Bloch & Schneider, 1801) in the Persian Gulf and Oman Sea, southern Iranian waters, using CMSY and DBSRA. J. Int. Biol. Aquat. 2022, 10, 11–20. [Google Scholar]





| References | Region | L∞ (cm) | K (yr −1) | to | Φ′ | M | F | Z | E |
|---|---|---|---|---|---|---|---|---|---|
| Ghodrati Shojaei et al., [18] | Persian Gulf and Sea of Oman (Iran) | 140 | 0.42 | −0.26 | 3.91 | 0.49 | 0.98 | 1.47 | 0.66 |
| Taghavi Motlagh et al., [18] | Sea of Oman (Iran) | 170 | 0.24 | −0.36 | 3.94 | 0.36 | 0.59 | 0.95 | 0.62 |
| Darvishi et al., [20] | Persian Gulf and Sea of Oman (Iran) | 175 | 0.45 | − | 4.10 | 0.5 | 1.48 | 1.98 | 0.74 |
| Newman et al., [12] | Australia | 122 (M) 133 (F) | 0.33 0.38 | −1.83 −1.49 | 8.51 8.77 | - | - | - | - |
| Kaymaram et al., [11] | Persian Gulf and Sea of Oman (Iran) | 151 | 0.46 | − | 4.02 | 0.54 | 1.39 | 1.93 | 0.72 |
| Johnson et al., [13] | Tanzania | 122 | 0.68 | −0.17 | - | 0.43 | 1.01 | 1.44 | 0.7 |
| Niamaimandi et al., [14] | Persian Gulf (Iran) | 148 | 0.5 | − | - | 0.56 | 0.41 | 0.97 | 0.42 |
| Lee and Mann, [15] | South Africa | 119 (M) 130 (F) | 0.28 0.31 | −1.85 −1.35 | 8.28 8.57 | - | - | - | - |
| Yuliana and Nurhasanah, [16] | Indonesia | 97 | 0.45 | −0.26 | - | 0.79 | 0.33 | 1.12 | 0.29 |
| Present study | Persian Gulf and Sea of Oman (Iran) | 173 | 0.5 | −0.2 | 4.17 | 0.47 | 0.95 | 1.42 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, S.A.; Doustdar, M.; Al Kindi, A.; Dutta, S. Assessment of Population Dynamics and Fishery Exploitation of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Iranian Waters. Oceans 2025, 6, 55. https://doi.org/10.3390/oceans6030055
Hashemi SA, Doustdar M, Al Kindi A, Dutta S. Assessment of Population Dynamics and Fishery Exploitation of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Iranian Waters. Oceans. 2025; 6(3):55. https://doi.org/10.3390/oceans6030055
Chicago/Turabian StyleHashemi, Seyed Ahmadreza, Mastooreh Doustdar, Abdullah Al Kindi, and Sachinandan Dutta. 2025. "Assessment of Population Dynamics and Fishery Exploitation of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Iranian Waters" Oceans 6, no. 3: 55. https://doi.org/10.3390/oceans6030055
APA StyleHashemi, S. A., Doustdar, M., Al Kindi, A., & Dutta, S. (2025). Assessment of Population Dynamics and Fishery Exploitation of Narrow-Barred Spanish Mackerel (Scomberomorus commerson) in Iranian Waters. Oceans, 6(3), 55. https://doi.org/10.3390/oceans6030055






