Glyphosate: A Terrestrial Threat to Marine Plants? A Study on the Seagrass Zostera marina
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Water Physical–Chemical Parameters
2.3. Sampling Procedures
2.4. Growth Rate
2.5. Photosynthetic Efficiency
2.6. Photosynthesis-Irradiance (P-I) Curves and Dark Respiration Rate
2.7. Photosynthetic Pigments
2.8. Adenylate Compounds (ATP, ADP and AMP) and Energy Charge (AEC)
2.9. Data Analysis
3. Results
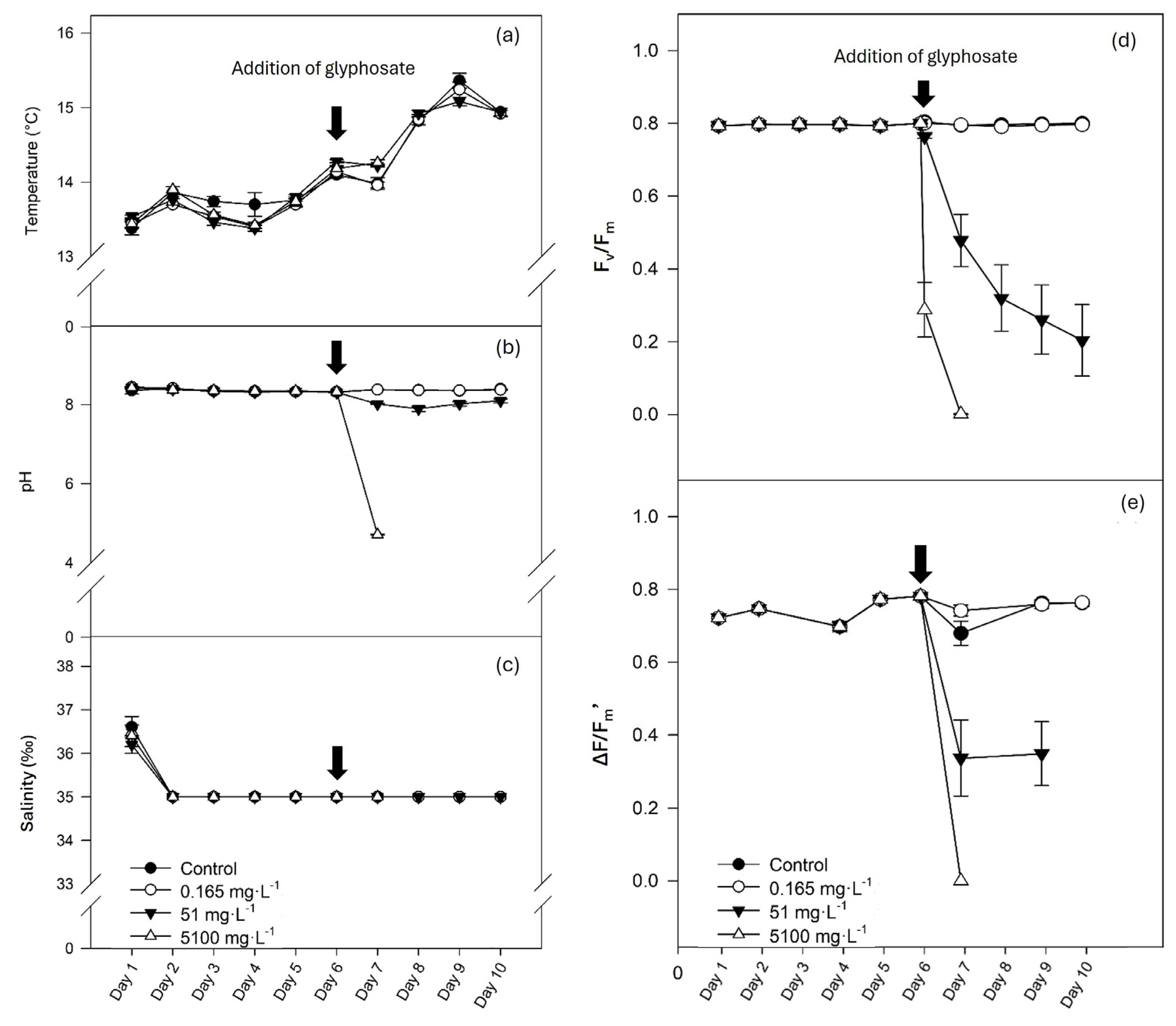
3.1. Water Physical–Chemical Parameters
3.2. Photosynthetic Performance
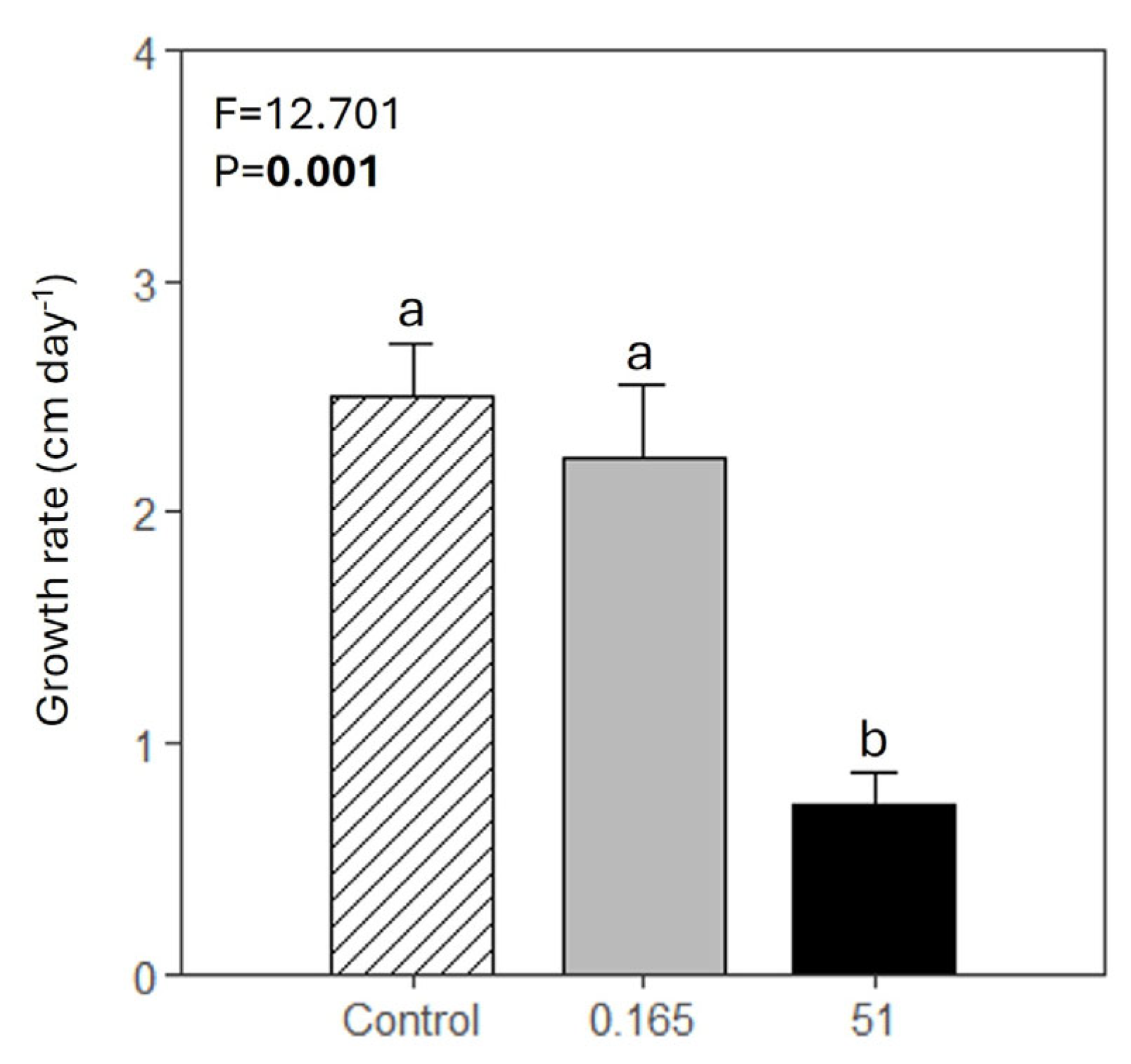
3.3. Growth Rate
3.4. Photosynthetic Activity
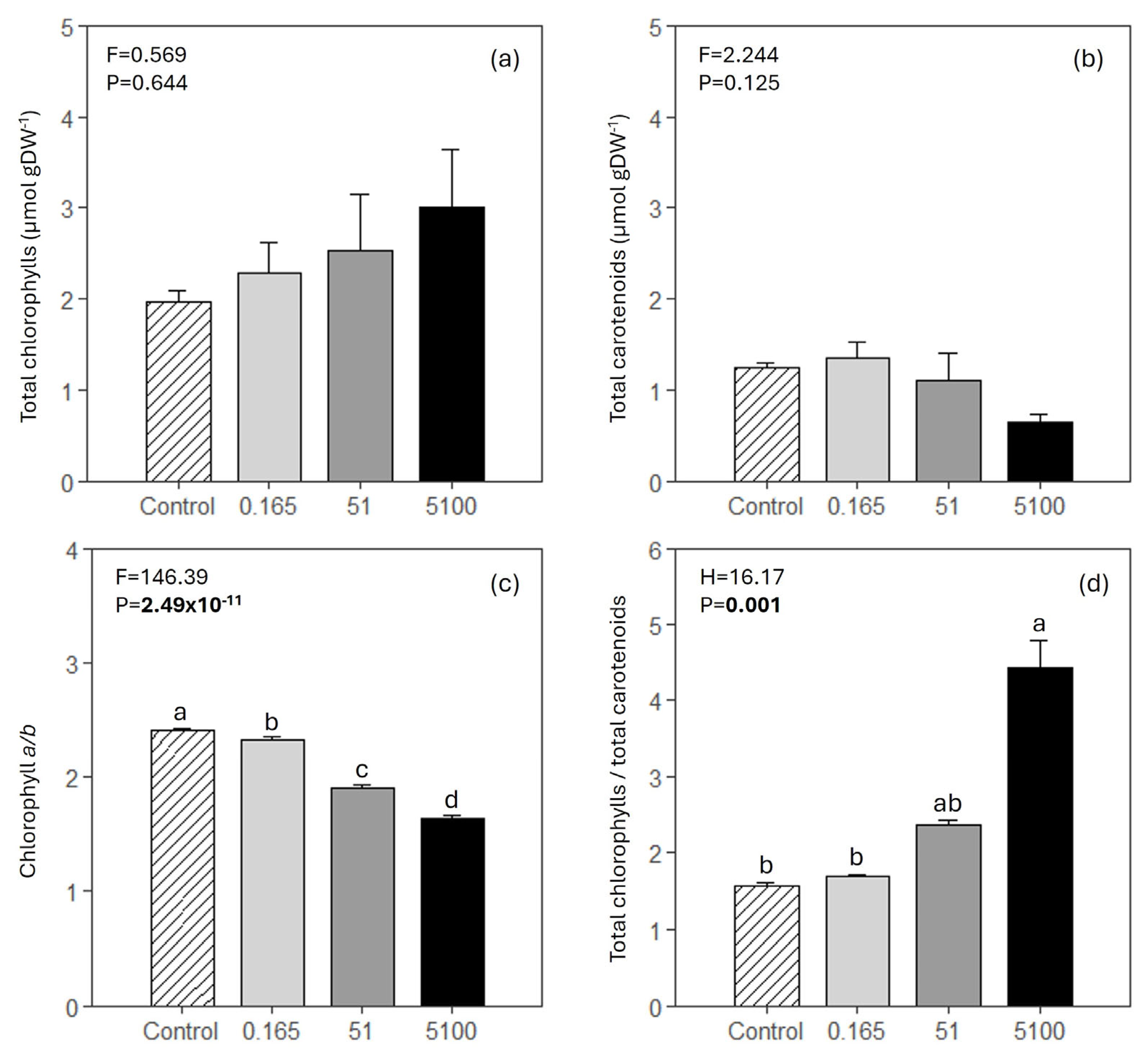
3.5. Photosynthetic Pigments
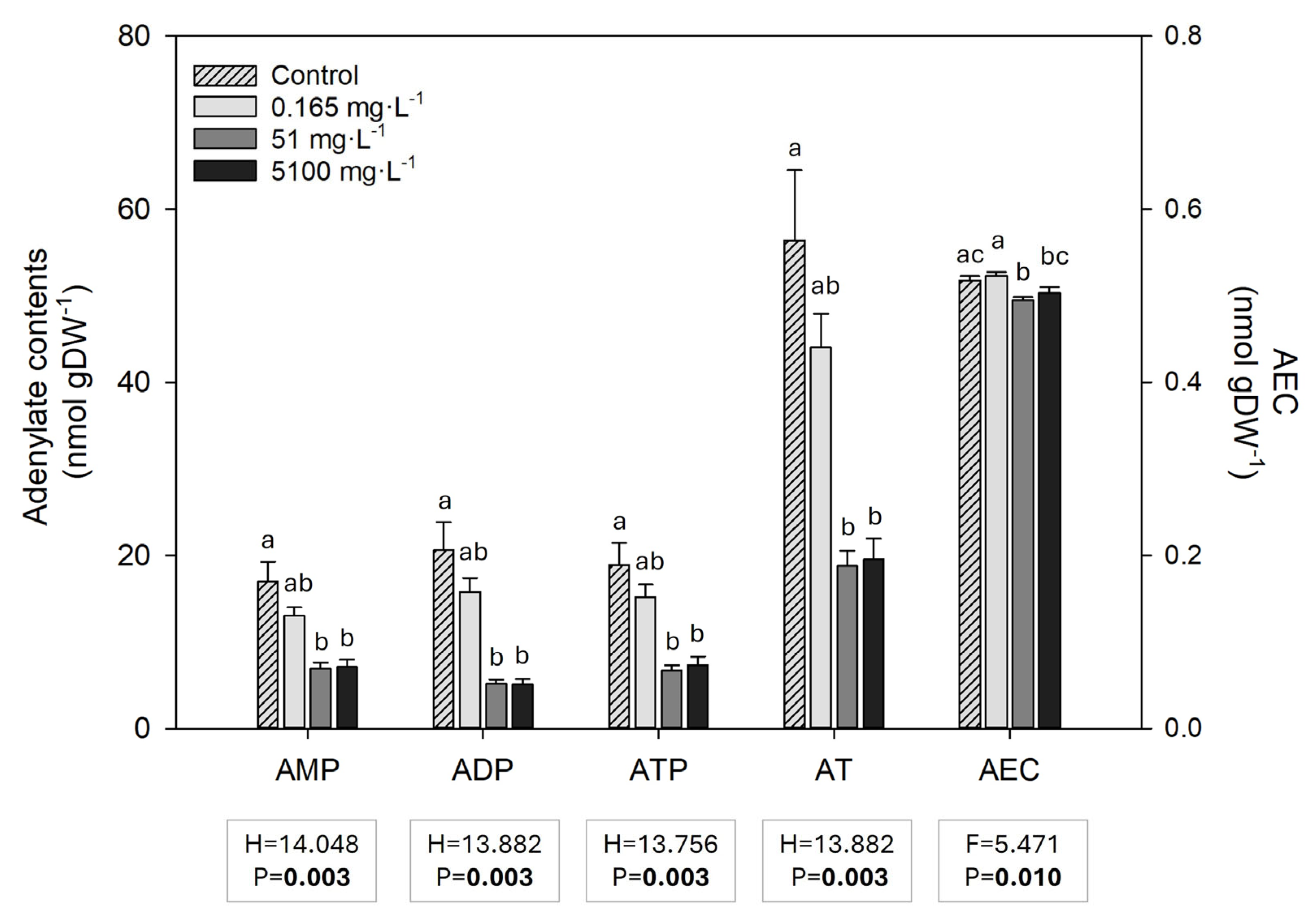
3.6. Adenylate Compounds (ATP, ADP and AMP) and Energy Charge (AEC)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 4302. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Organophosphate Insecticides and Herbicides; IARC: Sydney, Australia, 2017; ISBN 978-92-832-0178-6. [Google Scholar]
- Madani, N.A.; Carpenter, D.O. Effects of glyphosate and glyphosate-based herbicides like Roundup™ on the mammalian nervous system: A review. Environ. Res. 2022, 214, 113933. [Google Scholar] [CrossRef] [PubMed]
- Torretta, V.; Katsoyiannis, I.A.; Viotti, P.; Rada, E.C. Critical review of the effects of glyphosate exposure to the environment and humans through the food supply chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef]
- Walsh, L.; Hill, C.; Ross, R.P. Impact of glyphosate (RoundupTM) on the composition and functionality of the gut microbiome. Gut Microbes 2023, 15, 2263935. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2023/2660 of 28 November 2023. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/2660/oj (accessed on 1 August 2025).
- Decreto-Lei n.º 35/2017, de 24 de Março. Diário da República n.º 60/2017, Série I de 2017-03-24. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/35-2017-106654351 (accessed on 1 August 2025).
- Antier, C.; Kudsk, P.; Reboud, X.; Ulber, L.; Baret, P.V.; Messéan, A. Glyphosate use in the European agricultural sector and a framework for its further monitoring. Sustainability 2020, 12, 5682. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Solomon, K.; Thompson, D. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Environ. Health Part B 2003, 6, 289–324. [Google Scholar] [CrossRef]
- Vereecken, H. Mobility and leaching of glyphosate: A review. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Carretta, L.; Masin, R.; Zanin, G. Review of studies analysing glyphosate and aminomethylphosphonic acid (AMPA) occurrence in groundwater. Environ. Rev. 2022, 99, 88–109. [Google Scholar] [CrossRef]
- Maqueda, C.; Undabeytia, T.; Villaverde, J.; Morillo, E. Behaviour of glyphosate in a reservoir and the surrounding agricultural soils. Sci. Total Environ. 2017, 593, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Rendón-von Osten, J.R.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Toledo, J.; Castro, R.; Rivero-Pérez, N.; Bello-Mendoza, R.; Sánchez, D. Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico. Bull. Environ. Contam. Toxicol. 2014, 93, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, J.; Kantiani, L.; Llorca, M.; Rubio, F.; Ginebreda, A.; Fraile, J.; Garrido, T.; Farré, M. Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2335–2345. [Google Scholar] [CrossRef]
- Skeff, W.; Neumann, C.; Schulz-Bull, D.E. Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study. Mar. Pollut. Bull. 2015, 100, 577–585. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Daouk, S.; Copin, P.J.; Rossi, L.; Chèvre, N.; Pfeifer, H.R. Dynamics and environmental risk assessment of the herbicide glyphosate and its metabolite AMPA in a small vineyard river of the Lake Geneva catchment. Environ. Toxicol. Chem. 2013, 32, 2035–2044. [Google Scholar] [CrossRef]
- Huntscha, S.; Stravs, M.A.; Bühlmann, A.; Ahrens, C.H.; Frey, J.E.; Pomati, F.; Hollender, J.; Buerge, I.J.; Balmer, M.E.; Poiger, T. Seasonal dynamics of glyphosate and AMPA in Lake Greifensee: Rapid microbial degradation in the epilimnion during summer. Environ. Sci. Technol. 2018, 52, 4641–4649. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Fajon, C.; Bardot, C.; Bonnemoy, F.; Portelli, C.; Bohatier, J. Longitudinal changes in microbial planktonic communities of a French river in relation to pesticide and nutrient inputs. Aquat. Toxicol. 2008, 86, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Pupke, D.; Daniel, L.; Proefrock, D. Optimization of an enrichment and LC-MS/MS method for the analysis of glyphosate and aminomethylphosphonic acid (AMPA) in saline natural water samples without derivatization. J. Chromatogr. Sep. Tech. 2016, 7, 338. [Google Scholar] [CrossRef]
- Villeneuve, A.; Larroudé, S.; Humbert, J.F. Herbicide contamination of freshwater ecosystems: Impact on microbial communities. In Pesticides-Formulations, Effects, Fate; InTech (Open Access Publisher): Rijeka, Croatia, 2011; pp. 285–312. ISBN 9533075325. [Google Scholar]
- Burgeot, T.; Gagnaire, B.; Renault, T.; Haure, J.; Moraga, D.; David, E.; Boutet, I.; Sauriau, P.G.; Malet, N.; Bouchet, V.; et al. Oyster summer mortality risks associated with environmental stress. In Summer Mortality of Pacific Oyster Crassostrea Gigas: The Morest Project; Samain, J.F., Mc Combie, H., Eds.; Editions Quæ: Versailles, France, 2008; pp. 107–151. ISBN 2759215334. [Google Scholar]
- Feltracco, M.; Barbaro, E.; Morabito, E.; Zangrando, R.; Piazza, R.; Barbante, C.; Gambaro, A. Assessing glyphosate in water, marine particulate matter, and sediments in the Lagoon of Venice. Environ. Sci. Pollut. Res. 2022, 29, 16383–16391. [Google Scholar] [CrossRef]
- Wirth, M.A.; Schulz-Bull, D.E.; Kanwischer, M. The challenge of detecting the herbicide glyphosate and its metabolite AMPA in seawater–method development and application in the Baltic Sea. Chemosphere 2021, 262, 128327. [Google Scholar] [CrossRef]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef]
- Newton, A.; Mudge, S.M. Temperature and salinity regimes in a shallow, mesotidal lagoon, the Ria Formosa, Portugal. Estuar. Coast. Shelf Sci. 2003, 57, 73–85. [Google Scholar] [CrossRef]
- Ito, P.; Martins, M.; Gotha, S.V.S.C.; Santos, R.; de los Santos, C.B. Seagrasses in coastal wetlands of the Algarve region (southern Portugal): Past and present distribution and extent. J. Sea Res. 2025, 205, 102580. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Scott, A.; Arias-Ortiz, A.; Jones, B.; Kennedy, H.; Mazarrasa, I.; McKenzie, L.; Nordlund, L.M.; De La Torre-Castro, M.D.; Unsworth, R.K.; et al. Seagrass ecosystem services: Assessment and scale of benefits. In Out of the Blue: The Value of Seagrasses to the Environment and to People; GRID-Arendal, Ed.; United Nations Environment Programme: Nairobi, Kenya, 2020; pp. 19–21. Available online: https://wedocs.unep.org/20.500.11822/32636 (accessed on 5 December 2024).
- Andrade, J.P. Aspectos Geomorfológicos, Ecológicos e Socioeconómicos da Ria Formosa; Universidade do Algarve: Faro, Portugal, 1985; 91p. [Google Scholar]
- Aníbal, J.; Gomes, A.; Mendes, I.; Moura, D. (Eds.) Ria Formosa: Challenges of a Coastal Lagoon in a Changing Environment, 1st ed.; University of Algarve: Faro, Portugal, 2019; ISBN 978-989-8859-72-3. [Google Scholar]
- Erzini, K.; Parreira, F.; Sadat, Z.; Castro, M.; Bentes, L.; Coelho, R.; Gonçalves, J.M.; Lino, P.G.; Martinez-Crego, B.; Monteiro, P.; et al. Influence of seagrass meadows on nursery and fish provisioning ecosystem services delivered by Ria Formosa, a coastal lagoon in Portugal. Ecosyst. Serv. 2022, 58, 101490. [Google Scholar] [CrossRef]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Dunic, J.C.; Brown, C.J.; Connolly, R.M.; Turschwell, M.P.; Côté, I.M. Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Glob. Change Biol. 2021, 27, 4096–4109. [Google Scholar] [CrossRef] [PubMed]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Carapeto, A.; Francisco, A.; Pereira, P.; Porto, M. Lista Vermelha da Flora Vascular de Portugal Continental; Sociedade Portuguesa de Botânica, Associação Portuguesa de Ciência da Vegetação—PHYTOS e Instituto da Conservação da Natureza e das Florestas (coord.); Coleção «Botânica em Português»; Imprensa Nacional: Lisbon, Portugal, 2020; Volume 7, p. 374. ISBN 978-972-27-2876-8. [Google Scholar]
- Cunha, A.H.; Assis, J.F.; Serrão, E.A. Seagrasses in Portugal: A most endangered marine habitat. Aquat. Bot. 2013, 104, 193–203. [Google Scholar] [CrossRef]
- Casal-Porras, I.; de Los Santos, C.B.; Martins, M.; Santos, R.; Pérez-Lloréns, J.L.; Brun, F.G. Sedimentary organic carbon and nitrogen stocks of intertidal seagrass meadows in a dynamic and impacted wetland: Effects of coastal infrastructure constructions and meadow establishment time. J. Environ. Manag. 2022, 322, 115841. [Google Scholar] [CrossRef]
- Guimarães, M.H.M.; Cunha, A.H.; Nzinga, R.L.; Marques, J.F. The distribution of seagrass (Zostera noltii) in the Ria Formosa lagoon system and the implications of clam farming on its conservation. J. Nat. Conserv. 2012, 20, 30–40. [Google Scholar] [CrossRef]
- Newton, A.; Icely, J.D.; Falcão, M.; Nobre, A.; Nunes, J.P.; Ferreira, J.G.; Vale, C. Evaluation of eutrophication in the Ria Formosa coastal lagoon, Portugal. Cont. Shelf Res. 2003, 23, 1945–1961. [Google Scholar] [CrossRef]
- Cravo, A.; Barbosa, A.B.; Correia, C.; Matos, A.; Caetano, S.; Lima, M.J.; Jacob, J. Unravelling the effects of treated wastewater discharges on the water quality in a coastal lagoon system (Ria Formosa, South Portugal): Relevance of hydrodynamic conditions. Mar. Pollut. Bull. 2022, 174, 113296. [Google Scholar] [CrossRef]
- Cravo, A.; Fernandes, D.; Damião, T.; Pereira, C.; Reis, M.P. Determining the footprint of sewage discharges in a coastal lagoon in South-Western Europe. Mar. Pollut. Bull. 2015, 96, 197–209. [Google Scholar] [CrossRef]
- Newton, A.; Icely, J.; Cristina, S.; Perillo, G.M.; Turner, R.E.; Ashan, D.; Cragg, S.; Luo, Y.; Tu, C.; Li, Y.; et al. Anthropogenic, direct pressures on coastal wetlands. Front. Ecol. Evol. 2020, 8, 144. [Google Scholar] [CrossRef]
- Tett, P.; Gilpin, L.; Svendsen, H.; Erlandsson, C.P.; Larsson, U.; Kratzer, S.; Fouilland, E.; Janzen, C.; Lee, J.Y.; Grenz, C.; et al. Eutrophication and some European waters of restricted exchange. Cont. Shelf Res. 2003, 23, 1635–1671. [Google Scholar] [CrossRef]
- Choi, C.J.; Berges, J.A.; Young, E.B. Rapid effects of diverse toxic water pollutants on chlorophyll a fluorescence: Variable responses among freshwater microalgae. Water Res. 2012, 46, 2615–2626. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Feijão, E.; Matos, A.R.; Cabrita, M.T.; Utkin, A.B.; Novais, S.C.; Lemos, M.F.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Effects of glyphosate-based herbicide on primary production and physiological fitness of the macroalgae Ulva lactuca. Toxics 2022, 10, 430. [Google Scholar] [CrossRef]
- de Campos Oliveira, R.; Boas, L.K.V.; Branco, C.C.Z. Effect of herbicides based on glyphosate on the photosynthesis of green macroalgae in tropical lotic environments. Fundam. Appl. Limnol. 2021, 195, 85–93. [Google Scholar] [CrossRef]
- Falace, A.; Tamburello, L.; Guarnieri, G.; Kaleb, S.; Papa, L.; Fraschetti, S. Effects of a glyphosate-based herbicide on Fucus virsoides (Fucales, Ochrophyta) photosynthetic efficiency. Environ. Pollut. 2018, 243, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Felline, S.; Del Coco, L.; Kaleb, S.; Guarnieri, G.; Fraschetti, S.; Terlizzi, A.; Fanizzi, F.P.; Falace, A. The response of the algae Fucus virsoides (Fucales, Ochrophyta) to Roundup® solution exposure: A metabolomics approach. Environ. Pollut. 2019, 254, 112977. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Visintin, A.; Kaleb, S.; Spazzali, F.; Pallavicini, A.; Falace, A. Gene expression response of the alga Fucus virsoides (Fucales, Ochrophyta) to glyphosate solution exposure. Environ. Pollut. 2020, 267, 115483. [Google Scholar] [CrossRef] [PubMed]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; dos Santos Afonso, M.; de Molina, M.D.C.R.; Juárez, Á.B. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef]
- Kittle, R.P.; McDermid, K.J. Glyphosate herbicide toxicity to native Hawaiian macroalgal and seagrass species. J. Appl. Phycol. 2016, 28, 2597–2604. [Google Scholar] [CrossRef]
- Pang, T.; Liu, J.; Liu, Q.; Zhang, L.; Lin, W. Impacts of glyphosate on photosynthetic behaviors in Kappaphycus alvarezii and Neosiphonia savatieri detected by JIP-test. J. Appl. Phycol. 2012, 24, 467–473. [Google Scholar] [CrossRef]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of glyphosate and its metabolite aminomethylphosphonic acid on aquatic plants in different ecological niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef]
- Romero, D.M.; de Molina, M.C.R.; Juárez, Á.B. Oxidative stress induced by a commercial glyphosate formulation in a tolerant strain of Chlorella kessleri. Ecotoxicol. Environ. Saf. 2011, 74, 741–747. [Google Scholar] [CrossRef]
- Castro, A.D.J.V.; Colares, I.G.; dos Santos Franco, T.C.R.; Cutrim, M.V.J.; Luvizotto-Santos, R. Using a toxicity test with Ruppia maritima (Linnaeus) to assess the effects of Roundup. Mar. Pollut. Bull. 2015, 91, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Leonard, H.; Springer, E.; Provoncha, T. Glyphosate Herbicide Impacts on the Seagrasses Halodule wrightii and Ruppia maritima from a Subtropical Florida Estuary. J. Mar. Sci. Eng. 2024, 12, 1941. [Google Scholar] [CrossRef]
- Ralph, P.J. Herbicide toxicity of Halophila ovalis assessed by chlorophyll a fluorescence. Aquat. Bot. 2000, 66, 141–152. [Google Scholar] [CrossRef]
- Silvera, O.; Harris, R.J.; Arrington, D.A. Measuring herbicide (73.3% glyphosate) exposure response in Halophila ovalis (previously johnsonii) and Halodule wrightii seagrass. Mar. Pollut. Bull. 2024, 198, 115885. [Google Scholar] [CrossRef]
- van Wyk, J.W.; Adams, J.B.; von der Heyden, S. Conservation implications of herbicides on seagrasses: Sublethal glyphosate exposure decreases fitness in the endangered Zostera capensis. PeerJ 2022, 10, e14295. [Google Scholar] [CrossRef]
- Worm, B.; Reusch, T.B. Do nutrient availability and plant density limit seagrass colonization in the Baltic Sea? Mar. Ecol. Prog. Ser. 2000, 200, 159–166. [Google Scholar] [CrossRef]
- Silva, J.; Barrote, I.; Costa, M.M.; Albano, S.; Santos, R. Physiological responses of Zostera marina and Cymodocea nodosa to light-limitation stress. PLoS ONE 2013, 8, e81058. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Abadía, J.; Abadía, A. Iron and Plant Pigments. In Iron Chelation in Plants and Soil Microorganisms; Barton, L.L., Hemming, B., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 327–343. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- de las Rivas, J.; Abadía, A.; Abadía, J. A new reversed phase-HPLC method resolving all major higher plant photosynthetic pigments. Plant Physiol. 1989, 91, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Abadía, A.; Morales, F.; Abadía, J. Fe resupply to Fe-deficient sugar beet plants leads to rapid changes in the violaxanthin cycle and other photosynthetic characteristics without significant de novo chlorophyll synthesis. Photosynth. Res. 2004, 79, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Coolen, E.J.; Arts, I.C.; Swennen, E.L.; Bast, A.; Stuart, M.A.C.; Dagnelie, P.C. Simultaneous determination of adenosine triphosphate and its metabolites in human whole blood by RP-HPLC and UV-detection. J. Chromatogr. B 2008, 864, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, Y.; Luo, Y.; Jiang, W. A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high performance liquid chromatography. Food Technol. Biotechnol. 2006, 1, 44. [Google Scholar]
- Atkinson, D.E.; Walton, G.M. Adenosine triphosphate conservation in metabolic regulation: Rat liver citrate cleavage enzyme. J. Biol. Chem. 1967, 242, 3239–3241. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 15 October 2024).
- Martin, B.C.; Alarcon, M.S.; Gleeson, D.; Middleton, J.A.; Fraser, M.W.; Ryan, M.H.; Holmer, M.; Kendrick, G.A.; Kilminster, K. Root microbiomes as indicators of seagrass health. FEMS Microbiol. Ecol. 2020, 96, fiz201. [Google Scholar] [CrossRef]
- Bassil, E.; Blumwald, E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr. Opin. Plant Biol. 2014, 22, 1–6. [Google Scholar] [CrossRef]
- Banister, R.B.; Schwarz, M.T.; Fine, M.; Ritchie, K.B.; Muller, E.M. Instability and stasis among the microbiome of seagrass leaves, roots and rhizomes, and nearby sediments within a natural pH gradient. Microb. Ecol. 2022, 84, 703–716. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Kühl, M.; Nielsen, D.A.; Pedersen, O.; Larkum, A.W. Rhizome, root/sediment interactions, aerenchyma and internal pressure changes in seagrasses. In Seagrasses of Australia: Structure, Ecology and Conservation; Springer Nature: Berlin, Germany, 2018; pp. 393–418. [Google Scholar] [CrossRef]
- Atkinson, D.E. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-dependent inhibition of photosynthesis in willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Smedbol, É.; Lucotte, M.; Labrecque, M.; Lepage, L.; Juneau, P. Phytoplankton growth and PSII efficiency sensitivity to a glyphosate-based herbicide (Factor 540®). Aquat. Toxicol. 2017, 192, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Juneau, P. Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: Is the mitochondrial electron transport chain a target of this herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Yamamoto, Y. Damage to photosystem II by lipid peroxidation products. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem. Phys. Lipids 1987, 44, 327–340. [Google Scholar] [CrossRef]
- Ljones, T. Nitrogen fixation and bioenergetics: The role of ATP in nitrogenase catalysis. FEBS Lett. 1979, 98, 1–8. [Google Scholar] [CrossRef]
- Nielsen, L.W.; Dahllöf, I. Direct and indirect effects of the herbicides Glyphosate, Bentazone and MCPA on eelgrass (Zostera marina). Aquat. Toxicol. 2007, 82, 47–54. [Google Scholar] [CrossRef]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid (AMPA), an overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Orcaray, L.; Zulet, A.; Zabalza, A.; Royuela, M. Impairment of carbon metabolism induced by the herbicide glyphosate. J. Plant Physiol. 2012, 169, 27–33. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Podestá, F.E. The functional organization and control of plant respiration. Crit. Rev. Plant Sci. 2006, 25, 159–198. [Google Scholar] [CrossRef]
- Cedergreen, N.; Olesen, C.F. Can glyphosate stimulate photosynthesis? Pestic. Biochem. Physiol. 2010, 96, 140–148. [Google Scholar] [CrossRef]
- Swanson, N.L.; Hoy, J.; Seneff, S. Evidence that glyphosate is a causative agent in chronic sub-clinical metabolic acidosis and mitochondrial dysfunction. Int. J. Hum. Nutr. Funct. Med. 2016, 4, 32–52. [Google Scholar]
- Fuchs, B.; Saikkonen, K.; Helander, M. Glyphosate-modulated biosynthesis driving plant defense and species interactions. Trends Plant Sci. 2021, 26, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Strilbyska, O.M.; Tsiumpala, S.A.; Kozachyshyn, I.I.; Strutynska, T.; Burdyliuk, N.; Lushchak, V.I.; Lushchak, O. The effects of low-toxic herbicide Roundup and glyphosate on mitochondria. EXCLI J. 2022, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Helman, R.J.; Miranda, L.A.; dos Santos Afonso, M.; Salibián, A. Subcellular energy balance of Odontesthes bonariensis exposed to a glyphosate-based herbicide. Ecotoxicol. Environ. Saf. 2015, 114, 157–163. [Google Scholar] [CrossRef]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef]
- Sikorski, Ł.; Baciak, M.; Bęś, A.; Adomas, B. The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species. Aquat. Toxicol. 2019, 209, 70–80. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Kremer, R.J.; de Oliveira, R.S., Jr.; Constantin, J. Glyphosate effects on photosynthesis, nutrient accumulation, and nodulation in glyphosate-resistant soybean. J. Plant Nutr. Soil Sci. 2012, 175, 319–333. [Google Scholar] [CrossRef]
- Costa, M.M.; Silva, J.; Barrote, I.; Santos, R. Heatwave effects on the photosynthesis and antioxidant activity of the seagrass Cymodocea nodosa under contrasting light regimes. Oceans 2021, 2, 448–460. [Google Scholar] [CrossRef]
- Biswal, A.K.; Pattanayak, G.K.; Pandey, S.S.; Leelavathi, S.; Reddy, V.S.; Govindjee; Tripathy, B.C. Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Physiol. 2012, 159, 433–449. [Google Scholar] [CrossRef]
- Chen, L.H.; Xu, M.; Cheng, Z.; Yang, L.T. Effects of nitrogen deficiency on the photosynthesis, chlorophyll a fluorescence, antioxidant system, and sulfur compounds in Oryza sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef]
- Cruz, J.L.; Mosquim, P.R.; Pelacani, C.R.; Araújo, W.L.; DaMatta, F.M. Photosynthesis impairment in cassava leaves in response to nitrogen deficiency. Plant Soil 2003, 257, 417–423. [Google Scholar] [CrossRef]
- Kasajima, I. Difference in oxidative stress tolerance between rice cultivars estimated with chlorophyll fluorescence analysis. BMC Res. Notes 2017, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Sandmann, G.; Römer, S.; Fraser, P.D. Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab. Eng. 2006, 8, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Caffarri, S.; Tibiletti, T.; Jennings, R.C.; Santabarbara, S. A comparison between plant photosystem I and photosystem II architecture and functioning. Curr. Protein Pept. Sci. 2014, 15, 296–331. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Scarlett, A.; Donkin, P.; Fileman, T.W.; Evans, S.V.; Donkin, M.E. Risk posed by the antifouling agent Irgarol 1051 to the seagrass, Zostera marina. Aquat. Toxicol. 1999, 45, 159–170. [Google Scholar] [CrossRef]
- Bouvier, F.; D’Harlingues, A.; Hugueney, P.; Marin, E.; Marion-Poll, A.; Camara, B. Xanthophyll biosynthesis: Cloning, expression, functional reconstitution, and regulation of beta-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 1996, 271, 28861–28867. [Google Scholar] [CrossRef]
- Gilmore, A.M. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol. Plant. 1997, 99, 197–209. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Maccario, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic. Biochem. Physiol. 2016, 130, 65–70. [Google Scholar] [CrossRef]
- de Campos Oliveira, R.; Boas, L.K.V.; Branco, C.C.Z. Assessment of the potential toxicity of glyphosate-based herbicides on the photosynthesis of Nitella microcarpa var. wrightii (Charophyceae). Phycologia 2016, 55, 577–584. [Google Scholar] [CrossRef]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Lipok, J.; Studnik, H.; Gruyaert, S. The toxicity of Roundup® 360 SL formulation and its main constituents: Glyphosate and isopropylamine towards non-target water photoautotrophs. Ecotoxicol. Environ. Saf. 2010, 73, 1681–1688. [Google Scholar] [CrossRef]
- Mann, R.M.; Bidwell, J.R. The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch. Environ. Contam. Toxicol. 1999, 36, 193–199. [Google Scholar] [CrossRef]
- Mendes, E.J.; Malage, L.; Rocha, D.C.; Kitamura, R.S.A.; Gomes, S.M.A.; Navarro-Silva, M.A.; Gomes, M.P. Isolated and combined effects of glyphosate and its by-product aminomethylphosphonic acid on the physiology and water remediation capacity of Salvinia molesta. J. Hazard. Mater. 2021, 417, 125694. [Google Scholar] [CrossRef]
- Pérez, G.L.; Vera, M.S.; Miranda, L.A. Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. Herbic. Environ. 2011, 16, 343–368. [Google Scholar]
- Relyea, R.A. The lethal impact of Roundup on aquatic and terrestrial amphibians. Ecol. Appl. 2005, 15, 1118–1124. [Google Scholar] [CrossRef]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Newman, M.M.; Hoilett, N.; Lorenz, N.; Dick, R.P.; Liles, M.R.; Ramsier, C.; Kloepper, J.W. Glyphosate effects on soil rhizosphere-associated bacterial communities. Sci. Total Environ. 2016, 543, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.; Finckh, M.R.; He, M.; Ritsema, C.J.; Harkes, P.; Knuth, D.; Geissen, V. Indirect effects of the herbicide glyphosate on plant, animal and human health through its effects on microbial communities. Front. Environ. Sci. 2021, 9, 763917. [Google Scholar] [CrossRef]
- Denny, M. Wave-Energy Dissipation: Seaweeds and Marine Plants Are Ecosystem Engineers. Fluids 2021, 6, 151. [Google Scholar] [CrossRef]
- Bester, K. Effects of pesticides on seagrass beds. Helgol. Mar. Res. 2000, 54, 95–98. [Google Scholar] [CrossRef]
- Diepens, N.J.; Buffan-Dubau, E.; Budzinski, H.; Kallerhoff, J.; Merlina, G.; Silvestre, J.; Auby, I.; Tapie, N.; Elger, A. Toxicity effects of an environmental realistic herbicide mixture on the seagrass Zostera noltei. Environ. Pollut. 2017, 222, 393–403. [Google Scholar] [CrossRef]

| Glyphosate Equivalent (mg L−1) | Dark Respiration (μmol O2 gDW−1 h−1) | Pmax (μmol O2 gDW−1 h−1) | α | Ik (μmol m−2 s−1) |
|---|---|---|---|---|
| 0 (Control) | −3.448 ± 0.807 | 832.704 ± 32.927 a | 4.409 ± 0.450 b | 188.869 ± 20.661 a |
| 0.165 | −17.336 ± 6.752 | 945.414 ± 89.636 a | 5.149 ± 1.168 b | 183.615 ± 45.154 a |
| 51 | −22.893 ± 4.750 | 108.436 ± 3.512 b | 17.361 ± 4.237 a | 6.246 ± 1.538 b |
| F | 3.493 | 53.421 | 10.111 | 51.621 |
| P | 0.067 | <0.001 | 0.003 | <0.001 |
| Pigments (μmol gDW−1) | Glyphosate Equivalent (mg L−1) | F | P | |||
|---|---|---|---|---|---|---|
| 0 (Control) | 0.165 | 51 | 5100 | |||
| Chl a | 1.396 ± 0.081 | 1.604 ± 0.230 | 1.651 ± 0.396 | 1.850 ± 0.386 | 0.257 | 0.855 |
| Chl b | 0.580 ± 0.036 | 0.690 ± 0.095 | 0.879 ± 0.216 | 1.152 ± 0.256 | 1.434 | 0.272 |
| β-Carotene | 0.392 ± 0.015 a | 0.426 ± 0.053 a | 0.288 ± 0.077 a | 0.105 ± 0.014 b | 6.684 | 0.004 |
| Lutein | 0.362 ± 0.013 | 0.393 ± 0.057 | 0.467 ± 0.119 | 0.419 ± 0.082 | 0.226 | 0.877 |
| Lutein epoxide | 0.008 ± 0.001 | 0.006 ± 0.001 | 0.008 ± 0.002 | 0.005 ± 0.001 | 0.630 | 0.607 |
| Neoxanthin | 0.148 ± 0.005 | 0.167 ± 0.026 | 0.111 ± 0.034 | 0.087 ± 0.025 | 1.427 | 0.277 |
| Violaxanthin (V) | 0.332 ± 0.012 a | 0.346 ± 0.039 a | 0.227 ± 0.059 a | 0.053 ± 0.09 b | 8.256 | 0.002 |
| Antheraxanthin (A) | 0.006 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.949 | 0.442 |
| Zeaxanthin (Z) | 0.010 ± 0.001 | 0.007 ± 0.001 | 0.010 ± 0.002 | 0.007 ± 0.002 | 0.998 | 0.421 |
| V + A + Z | 0.346 ± 0.012 a | 0.357 ± 0.041 a | 0.240 ± 0.062 a | 0.064 ± 0.011 b | 7.550 | 0.003 |
| DES = (A + Z)/(V + A + Z) | 0.040 ± 0.008 bc | 0.030 ± 0.004 c | 0.056 ± 0.003 b | 0.160 ± 0.009 a | 73.875 | 7.998 × 10−9 |
| (V + A + Z)/Chl a + b | 0.176 ± 0.006 a | 0.160 ± 0.008 a | 0.092 ± 0.003 b | 0.027 ± 0.002 c | 113.44 | 4.643 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguette, A.; Pes, K.; Vasconcellos, B.; Costa, M.; Silva, J.; Barrote, I. Glyphosate: A Terrestrial Threat to Marine Plants? A Study on the Seagrass Zostera marina. Oceans 2025, 6, 51. https://doi.org/10.3390/oceans6030051
Deguette A, Pes K, Vasconcellos B, Costa M, Silva J, Barrote I. Glyphosate: A Terrestrial Threat to Marine Plants? A Study on the Seagrass Zostera marina. Oceans. 2025; 6(3):51. https://doi.org/10.3390/oceans6030051
Chicago/Turabian StyleDeguette, Alizé, Katia Pes, Bernard Vasconcellos, Monya Costa, João Silva, and Isabel Barrote. 2025. "Glyphosate: A Terrestrial Threat to Marine Plants? A Study on the Seagrass Zostera marina" Oceans 6, no. 3: 51. https://doi.org/10.3390/oceans6030051
APA StyleDeguette, A., Pes, K., Vasconcellos, B., Costa, M., Silva, J., & Barrote, I. (2025). Glyphosate: A Terrestrial Threat to Marine Plants? A Study on the Seagrass Zostera marina. Oceans, 6(3), 51. https://doi.org/10.3390/oceans6030051








