Fin Whale Acoustic Presence Increases by 3 d/y in the Migratory Corridor off Cape Leeuwin, Western Australia—An Indicator of Population Growth?
Abstract
1. Introduction
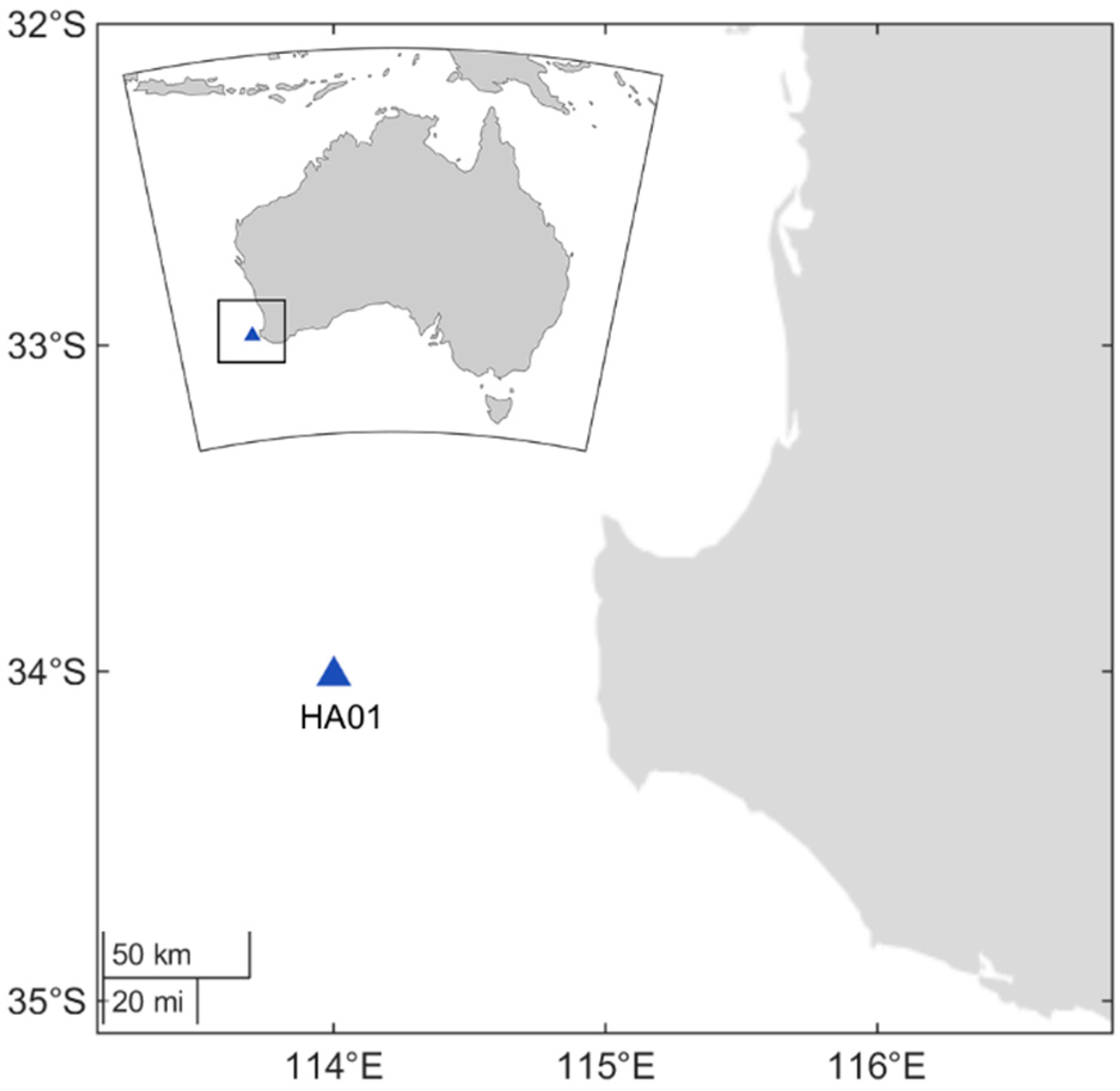
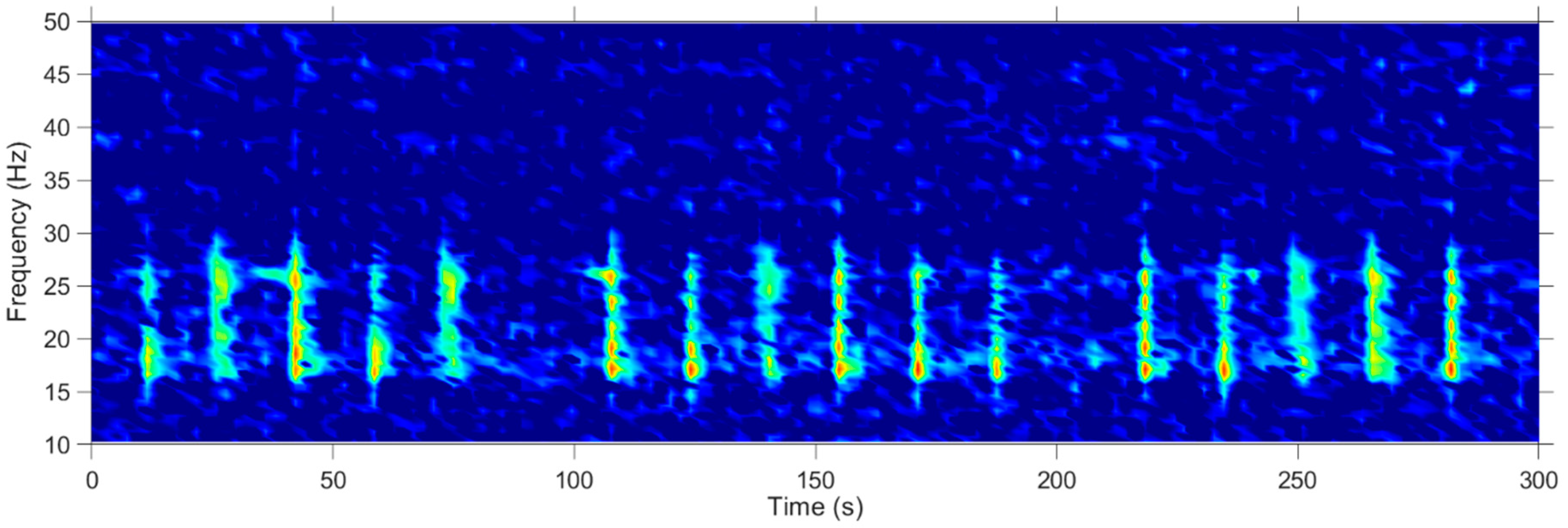
2. Materials and Methods
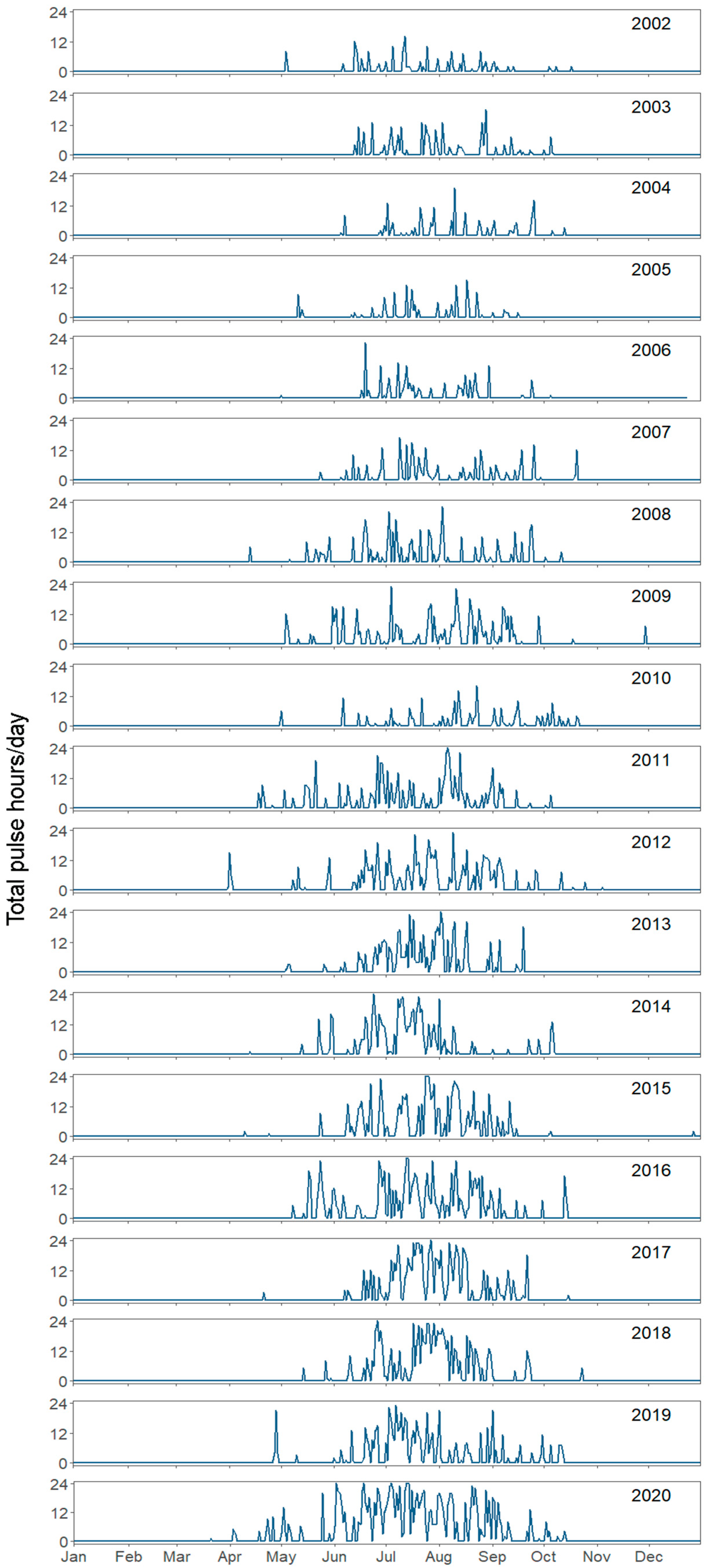
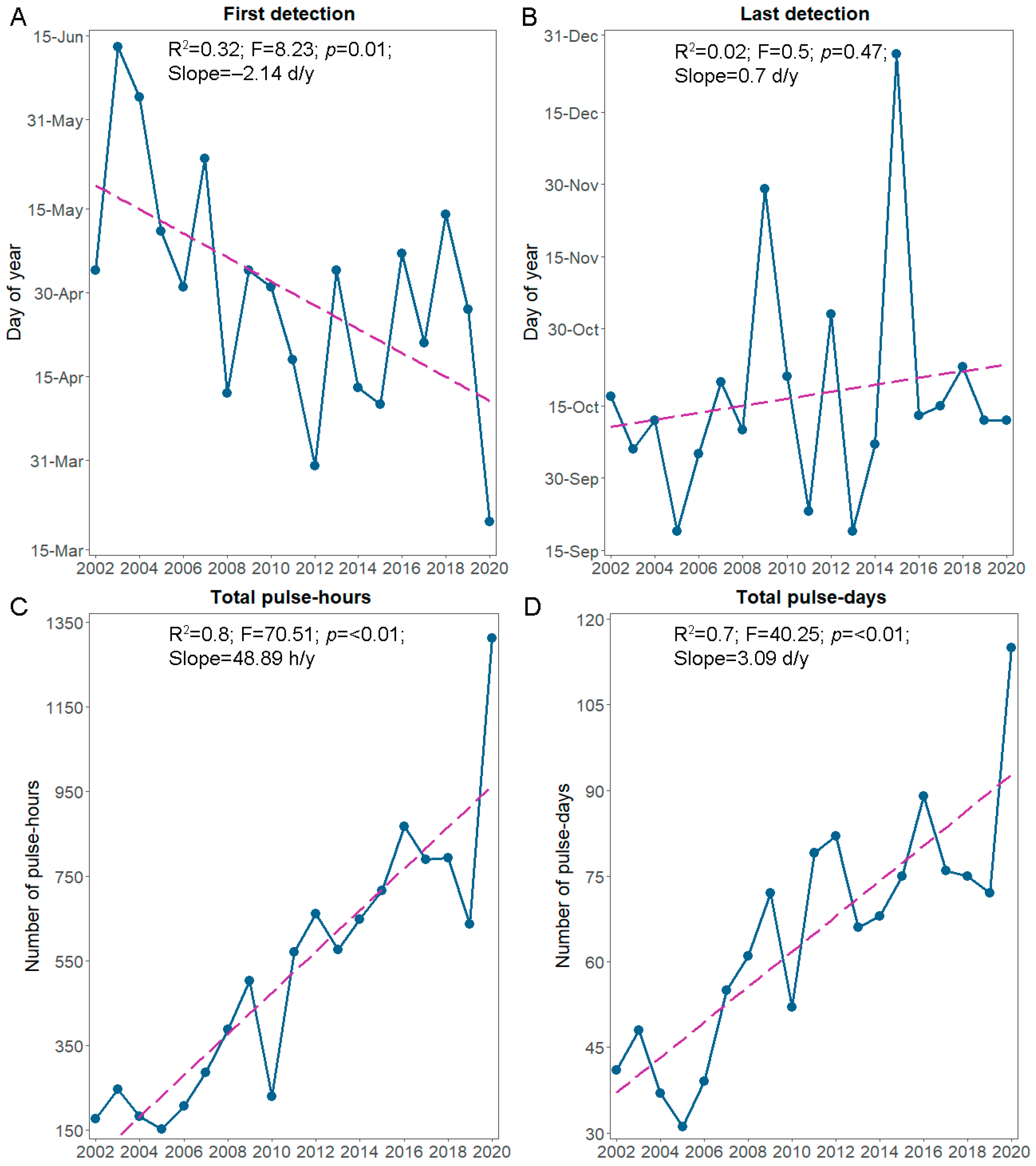
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PAM | Passive acoustic monitoring |
References
- Lockyer, C.; Brown, S. The migration of whales. In Animal Migration; Cambridge University Press Binghamton: New York, NY, USA, 1981; Volume 13, pp. 105–137. [Google Scholar]
- Corkeron, P.J.; Connor, R.C. Why Do Baleen Whales Migrate? Mar. Mammal Sci. 1999, 15, 1228–1245. [Google Scholar] [CrossRef]
- Mizroch, S.A.; Rice, D.W.; Breiwick, J.M. The fin whale, Balaenoptera physalus. Mar. Fish. Rev. 1984, 46, 20–24. [Google Scholar]
- Simon, M.; Stafford, K.M.; Beedholm, K.; Lee, C.M.; Madsen, P.T. Singing behavior of fin whales in the Davis Strait with implications for mating, migration and foraging. J. Acoust. Soc. Am. 2010, 128, 3200–3210. [Google Scholar] [CrossRef]
- Tsujii, K.; Otsuki, M.; Akamatsu, T.; Matsuo, I.; Amakasu, K.; Kitamura, M.; Kikuchi, T.; Miyashita, K.; Mitani, Y. The migration of fin whales into the southern Chukchi Sea as monitored with passive acoustics. ICES J. Mar. Sci. 2016, 73, 2085–2092. [Google Scholar] [CrossRef]
- Širović, A.; Williams, L.N.; Kerosky, S.M.; Wiggins, S.M.; Hildebrand, J.A. Temporal separation of two fin whale call types across the eastern North Pacific. Mar. Biol. 2013, 160, 47–57. [Google Scholar] [CrossRef]
- Aulich, M.G.; McCauley, R.D.; Miller, B.S.; Samaran, F.; Giorli, G.; Saunders, B.J.; Erbe, C. Seasonal distribution of the fin whale (Balaenoptera physalus) in Antarctic and Australian waters based on passive acoustics. Front. Mar. Sci. 2022, 9, 864153. [Google Scholar] [CrossRef]
- Davis, G.E.; Baumgartner, M.F.; Corkeron, P.J.; Bell, J.; Berchok, C.; Bonnell, J.M.; Bort Thornton, J.; Brault, S.; Buchanan, G.A.; Cholewiak, D.M.; et al. Exploring movement patterns and changing distributions of baleen whales in the western North Atlantic using a decade of passive acoustic data. Glob. Change Biol. 2020, 26, 4812–4840. [Google Scholar] [CrossRef]
- Watkins, W.A.; Tyack, P.; Moore, K.E.; Bird, J.E. The 20-Hz signals of finback whales (Balaenoptera physalus). J. Acoust. Soc. Am. 1987, 82, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.O.; Findley, L.T.; Vidal, O. 20-Hz pulses and other vocalizations of fin whales, Balaenoptera physalus, in the Gulf of California, Mexico. J. Acoust. Soc. Am. 1992, 92, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.A.; Zhu, C.; Schinault, M.E.; Kaplan, A.I.; Handegard, N.O.; Godø, O.R.; Ahonen, H.; Makris, N.C.; Wang, D.; Huang, W.; et al. Temporal–spatial, spectral, and source level distributions of fin whale vocalizations in the Norwegian Sea observed with a coherent hydrophone array. ICES J. Mar. Sci. 2019, 76, 268–283. [Google Scholar] [CrossRef]
- Miksis-Olds, J.L.; Harris, D.V.; Heaney, K.D. Comparison of estimated 20-Hz pulse fin whale source levels from the tropical Pacific and Eastern North Atlantic Oceans to other recorded populations. J. Acoust. Soc. Am. 2019, 146, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Širović, A.; Hildebrand, J.A.; Wiggins, S.M. Blue and fin whale call source levels and propagation range in the Southern Ocean. J. Acoust. Soc. Am. 2007, 122, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Weirathmueller, M.J.; Wilcock, W.S.D.; Soule, D.C. Source levels of fin whale 20 Hz pulses measured in the Northeast Pacific Ocean. J. Acoust. Soc. Am. 2013, 133, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Croll, D.A.; Clark, C.W.; Acevedo, A.; Tershy, B.; Flores, S.; Gedamke, J.; Urban, J. Only male fin whales sing loud songs. Nature 2002, 417, 809. [Google Scholar] [CrossRef]
- McDonald, M.A.; Hildebrand, J.A.; Webb, S.C. Blue and fin whales observed on a seafloor array in the Northeast Pacific. J. Acoust. Soc. Am. 1995, 98, 712–721. [Google Scholar] [CrossRef]
- Aulich, M.G.; McCauley, R.D.; Saunders, B.J.; Parsons, M.J.G. Fin whale (Balaenoptera physalus) migration in Australian waters using passive acoustic monitoring. Sci. Rep. 2019, 9, 8840. [Google Scholar] [CrossRef]
- Gosby, C.; Erbe, C.; Harvey, E.S.; Figueroa Landero, M.M.; McCauley, R.D. Vocalizing humpback whales (Megaptera novaeangliae) migrating from Antarctic feeding grounds arrive earlier and earlier in the Perth Canyon, Western Australia. Front. Mar. Sci. 2022, 9, 1086763. [Google Scholar] [CrossRef]
- Szesciorka, A.R.; Ballance, L.T.; Širović, A.; Rice, A.; Ohman, M.D.; Hildebrand, J.A.; Franks, P.J.S. Timing is everything: Drivers of interannual variability in blue whale migration. Sci. Rep. 2020, 10, 7710. [Google Scholar] [CrossRef]
- Avila, I.C.; Dormann, C.F.; García, C.; Payán, L.F.; Zorrilla, M.X. Humpback whales extend their stay in a breeding ground in the Tropical Eastern Pacific. ICES J. Mar. Sci. 2019, 77, 109–118. [Google Scholar] [CrossRef]
- Laidre, K.L.; Heide-Jørgensen, M.P. Spring partitioning of Disko Bay, West Greenland, by Arctic and Subarctic baleen whales. ICES J. Mar. Sci. 2012, 69, 1226–1233. [Google Scholar] [CrossRef]
- Hauser, D.D.W.; Laidre, K.L.; Stafford, K.M.; Stern, H.L.; Suydam, R.S.; Richard, P.R. Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation. Glob. Change Biol. 2017, 23, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Ramp, C.; Delarue, J.; Palsbøll, P.J.; Sears, R.; Hammond, P.S. Adapting to a Warmer Ocean—Seasonal Shift of Baleen Whale Movements over Three Decades. PLoS ONE 2015, 10, e0121374. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.G. Balaenoptera physalus. The IUCN Red List of Threatened Species 2018: e.T2478A50349982. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T2478A50349982.en (accessed on 13 July 2020).
- Rocha, R.C.; Clapham, P.J.; Ivashchenko, Y.V. Emptying the oceans: A summary of industrial whaling catches in the 20th century. Mar. Fish. Rev. 2014, 76, 37–48. [Google Scholar] [CrossRef]
- Edwards, E.F.; Hall, C.; Moore, T.J.; Sheredy, C.; Redfern, J.V. Global distribution of fin whales Balaenoptera physalus in the post-whaling era (1980–2012). Mammal Rev. 2015, 45, 197–214. [Google Scholar] [CrossRef]
- Branch, T.; Butterworth, D. Estimates of abundance south of 60° S for cetacean species sighted frequently on the 1978/79 to 1997/98 IWC/IDCR-SOWER sighting surveys. J. Cetacean Res. Manag. 2001, 3, 251–270. [Google Scholar] [CrossRef]
- Buchan, S.J.; Gutierrez, L.; Balcazar-Cabrera, N.; Stafford, K.M. Seasonal occurrence of fin whale song off Juan Fernandez, Chile. Endanger. Species Res. 2019, 39, 135–145. [Google Scholar] [CrossRef]
- Burkhardt, E.; Van Opzeeland, I.; Cisewski, B.; Mattmüller, R.; Meister, M.; Schall, E.; Spiesecke, S.; Thomisch, K.; Zwicker, S.; Boebel, O. Seasonal and diel cycles of fin whale acoustic occurrence near Elephant Island, Antarctica. R. Soc. Open Sci. 2021, 8, 201142. [Google Scholar] [CrossRef]
- Herr, H.; Hickmott, L.; Viquerat, S.; Panigada, S. First evidence for fin whale migration into the Pacific from Antarctic feeding grounds at Elephant Island. R. Soc. Open Sci. 2022, 9, 220721. [Google Scholar] [CrossRef]
- Toro, F.; Vilina, Y.A.; Capella, J.J.; Gibbons, J. Novel coastal feeding area for eastern South Pacific fin whales (Balaenoptera physalus) in mid-latitude humboldt current waters off Chile. Aquat. Mamm. 2016, 42, 47–55. [Google Scholar] [CrossRef]
- Viquerat, S.; Waluda, C.M.; Kennedy, A.S.; Jackson, J.A.; Hevia, M.; Carroll, E.L.; Buss, D.L.; Burkhardt, E.; Thain, S.; Smith, P.; et al. Identifying seasonal distribution patterns of fin whales across the Scotia Sea and the Antarctic Peninsula region using a novel approach combining habitat suitability models and ensemble learning methods. Front. Mar. Sci. 2022, 9, 1040512. [Google Scholar] [CrossRef]
- Wood, M.; Širović, A. Characterization of fin whale song off the Western Antarctic Peninsula. PLoS ONE 2022, 17, e0264214. [Google Scholar] [CrossRef] [PubMed]
- Dréo, R.; Bouffaut, L.; Leroy, E.; Barruol, G.; Samaran, F. Baleen whale distribution and seasonal occurrence revealed by an ocean bottom seismometer network in the Western Indian Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 161, 132–144. [Google Scholar] [CrossRef]
- Miller, B.S.; Masere, C.; Milnes, M.; Cleeland, J.; Lamb, T.; Maschette, D.; Welsford, D. Heard off Heard: Acoustic detections of sperm whales (Physeter macrocephalus) and other cetaceans off Heard Island. JASA Express Lett. 2024, 4, 061201. [Google Scholar] [CrossRef]
- Sprogis, K.R.; Sutton, A.L.; Jenner, M.-N.; McCauley, R.D.; Jenner, K.C.S. Occurrence of cetaceans and seabirds along the Indian Ocean 110°E meridian from temperate to tropical waters. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2022, 205, 105184. [Google Scholar] [CrossRef]
- Todd, V.L.G.; Williamson, L.D. Cetacean distribution in relation to oceanographic features at the Kerguelen Plateau. Polar Biol. 2022, 45, 113–126. [Google Scholar] [CrossRef]
- Lawrence, M.W. Acoustic Monitoring of the Global Ocean for the CTBT. In Proceedings of the Acoustics 2004, Gold Coast, Australia, 3–5 November 2004. [Google Scholar]
- Park, J.; Haralabus, G.; Zampolli, M.; Metz, D. Low frequency ambient noise dynamics and trends in the Indian Ocean, Cape Leeuwin, Australia. J. Acoust. Soc. Am. 2023, 153, 2312. [Google Scholar] [CrossRef]
- Gavrilov, A.; McCauley, R.; Salgado-Kent, C.; Tripovich, J.; Burton, C. Vocal characteristics of pygmy blue whales and their change over time. J. Acoust. Soc. Am. 2011, 130, 3651–3660. [Google Scholar] [CrossRef]
- Leroy, E.C.; Royer, J.-Y.; Alling, A.; Maslen, B.; Rogers, T.L. Multiple pygmy blue whale acoustic populations in the Indian Ocean: Whale song identifies a possible new population. Sci. Rep. 2021, 11, 8762. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 1 November 2022).
- Morano, J.L.; Salisbury, D.P.; Rice, A.N.; Conklin, K.L.; Falk, K.L.; Clark, C.W. Seasonal and geographical patterns of fin whale song in the western North Atlantic Ocean. J. Acoust. Soc. Am. 2012, 132, 1207–1212. [Google Scholar] [CrossRef]
- Erbe, C.; Verma, A.; McCauley, R.; Gavrilov, A.; Parnum, I. The marine soundscape of the Perth Canyon. Prog. Oceanogr. 2015, 137, 38–51. [Google Scholar] [CrossRef]
- Herr, H.; Viquerat, S.; Devas, F.; Lees, A.; Wells, L.; Gregory, B.; Giffords, T.; Beecham, D.; Meyer, B. Return of large fin whale feeding aggregations to historical whaling grounds in the Southern Ocean. Sci. Rep. 2022, 12, 9458. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, A.N.; Adams, G.; Best, J.; Clapham, P.J.; Jackson, J.A.; Punt, A.E. Assessing the recovery of an Antarctic predator from historical exploitation. R. Soc. Open Sci. 2019, 6, 190368. [Google Scholar] [CrossRef]
- Calderan, S.V.; Black, A.; Branch, T.A.; Collins, M.A.; Kelly, N.; Leaper, R.; Lurcock, S.; Miller, B.S.; Moore, M.; Olson, P.A. South Georgia blue whales five decades after the end of whaling. Endanger. Species Res. 2020, 43, 359–373. [Google Scholar] [CrossRef]
- Tardy, C.; Ody, D.; Gimenez, O.; Planes, S. Abundance of fin whales (Balaenoptera physalus) in the north-western Mediterranean Sea, using photo-identification and microsatellite genotyping. Mar. Ecol. 2023, 44, e12737. [Google Scholar] [CrossRef]
- Degollada, E.; Amigó, N.; O’Callaghan, S.A.; Varola, M.; Ruggero, K.; Tort, B. A novel technique for photo-identification of the fin whale, Balaenoptera physalus, as determined by drone aerial images. Drones 2023, 7, 220. [Google Scholar] [CrossRef]
- Viquerat, S.; Herr, H. Mid-summer abundance estimates of fin whales Balaenoptera physalus around the South Orkney Islands and Elephant Island. Endanger. Species Res. 2017, 32, 515–524. [Google Scholar] [CrossRef]
- Víkingsson, G.A.; Pike, D.G.; Desportes, G.; Øien, N.; Gunnlaugsson, T.; Bloch, D. Distribution and abundance of fin whales (Balaenoptera physalus) in the Northeast and Central Atlantic as inferred from the North Atlantic sightings surveys 1987–2001. NAMMCO Sci. Publ. 2013, 7, 49–72. [Google Scholar] [CrossRef]
- Watkins, W.A. Activities and underwater sounds of fin whales. Sci. Rep. Whales Res. Inst. 1981, 33, 83–117. [Google Scholar]
- Papale, E.; Pelagatti, M.; Pedrazzi, G.; Buscaino, G. Occurrence and patterns of fin whale songs reveal alternative migration strategies in Svalbard Islands, Norway. Sci. Rep. 2023, 13, 4436. [Google Scholar] [CrossRef]
- Harris, P.; Sotirakopoulos, K.; Robinson, S.; Wang, L.; Livina, V. A statistical method for the evaluation of long term trends in underwater noise measurements. J. Acoust. Soc. Am. 2019, 145, 228–242. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Gavrilov, A. Icebergs and storms in the Southern Ocean as sources of low frequency noise along Australian southern coasts. In Proceedings of the ACOUSTICS 2019, Cape Schanck, VIC, Australia, 10–13 November 2019. [Google Scholar]
- Brewer, P.G.; Hester, K. Ocean acidification and the increasing transparency of the ocean to low-frequency sound. Oceanography 2009, 22, 86–93. [Google Scholar] [CrossRef]
- McCauley, R. Offshore Irish Noise Logger Program (March to September 2014): Analysis of Cetacean Presence, and Ambient and Anthropogenic Noise Sources; Centre for Marine Science and Technology: Perth, Australia, 2015. [Google Scholar]
- Weirathmueller, M.J.; Stafford, K.M.; Wilcock, W.S.D.; Hilmo, R.S.; Dziak, R.P.; Trehu, A.M. Spatial and temporal trends in fin whale vocalizations recorded in the NE Pacific Ocean between 2003–2013. PLoS ONE 2017, 12, e0186127. [Google Scholar] [CrossRef] [PubMed]
- Shabangu, F.W.; Findlay, K.P.; Yemane, D.; Stafford, K.M.; Van den Berg, M.; Blows, B.; Andrew, R.K. Seasonal occurrence and diel calling behaviour of Antarctic blue whales and fin whales in relation to environmental conditions off the west coast of South Africa. J. Mar. Syst. 2019, 190, 25–39. [Google Scholar] [CrossRef]
- Escajeda, E.; Stafford, K.M.; Woodgate, R.A.; Laidre, K.L. Variability in fin whale (Balaenoptera physalus) occurrence in the Bering Strait and southern Chukchi Sea in relation to environmental factors. Deep Sea Res. (Part II Top. Stud. Oceanogr.) 2020, 177, 104782. [Google Scholar] [CrossRef]
- Tulloch, V.J.D.; Plagányi, É.E.; Brown, C.; Richardson, A.J.; Matear, R. Future recovery of baleen whales is imperiled by climate change. Glob. Change Biol. 2019, 25, 1263–1281. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Atkinson, A.; Bahlburg, D.; Bernard, K.S.; Cavan, E.L.; Cox, M.J.; Hill, S.L.; Meyer, B.; Veytia, D. Climate change impacts on Antarctic krill behaviour and population dynamics. Nat. Rev. Earth Environ. 2024, 5, 43–58. [Google Scholar] [CrossRef]
- Huveneers, C.; Jaine, F.R.A.; Barnett, A.; Butcher, P.A.; Clarke, T.M.; Currey-Randall, L.M.; Dwyer, R.G.; Ferreira, L.C.; Gleiss, A.C.; Hoenner, X.; et al. The power of national acoustic tracking networks to assess the impacts of human activity on marine organisms during the COVID-19 pandemic. Biol. Conserv. 2021, 256, 108995. [Google Scholar] [CrossRef]
- Coll, M. Environmental effects of the COVID-19 pandemic from a (marine) ecological perspective. Ethics Sci. Environ. Politics 2020, 20, 41–55. [Google Scholar] [CrossRef]
- Castellote, M.; Clark, C.; Lammers, M. Acoustic and behavioural changes by fin whales (Balaenoptera physalus) in response to shipping and airgun noise. Biol. Conserv. 2012, 147, 115–122. [Google Scholar] [CrossRef]
- Panigada, S.; Pesante, G.; Zanardelli, M.; Capoulade, F.; Gannier, A.; Weinrich, M.T. Mediterranean fin whales at risk from fatal ship strikes. Mar. Pollut. Bull. 2006, 52, 1287–1298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulich, M.G.; McCauley, R.D.; Miller, B.S.; Erbe, C. Fin Whale Acoustic Presence Increases by 3 d/y in the Migratory Corridor off Cape Leeuwin, Western Australia—An Indicator of Population Growth? Oceans 2025, 6, 44. https://doi.org/10.3390/oceans6030044
Aulich MG, McCauley RD, Miller BS, Erbe C. Fin Whale Acoustic Presence Increases by 3 d/y in the Migratory Corridor off Cape Leeuwin, Western Australia—An Indicator of Population Growth? Oceans. 2025; 6(3):44. https://doi.org/10.3390/oceans6030044
Chicago/Turabian StyleAulich, Meghan G., Robert D. McCauley, Brian S. Miller, and Christine Erbe. 2025. "Fin Whale Acoustic Presence Increases by 3 d/y in the Migratory Corridor off Cape Leeuwin, Western Australia—An Indicator of Population Growth?" Oceans 6, no. 3: 44. https://doi.org/10.3390/oceans6030044
APA StyleAulich, M. G., McCauley, R. D., Miller, B. S., & Erbe, C. (2025). Fin Whale Acoustic Presence Increases by 3 d/y in the Migratory Corridor off Cape Leeuwin, Western Australia—An Indicator of Population Growth? Oceans, 6(3), 44. https://doi.org/10.3390/oceans6030044







