Influencing Factors on Oyster Recruitment and Performance Evaluation for Oyster Reef Restoration in Tianjin Coastal Zones
Abstract
1. Introduction
2. Methods
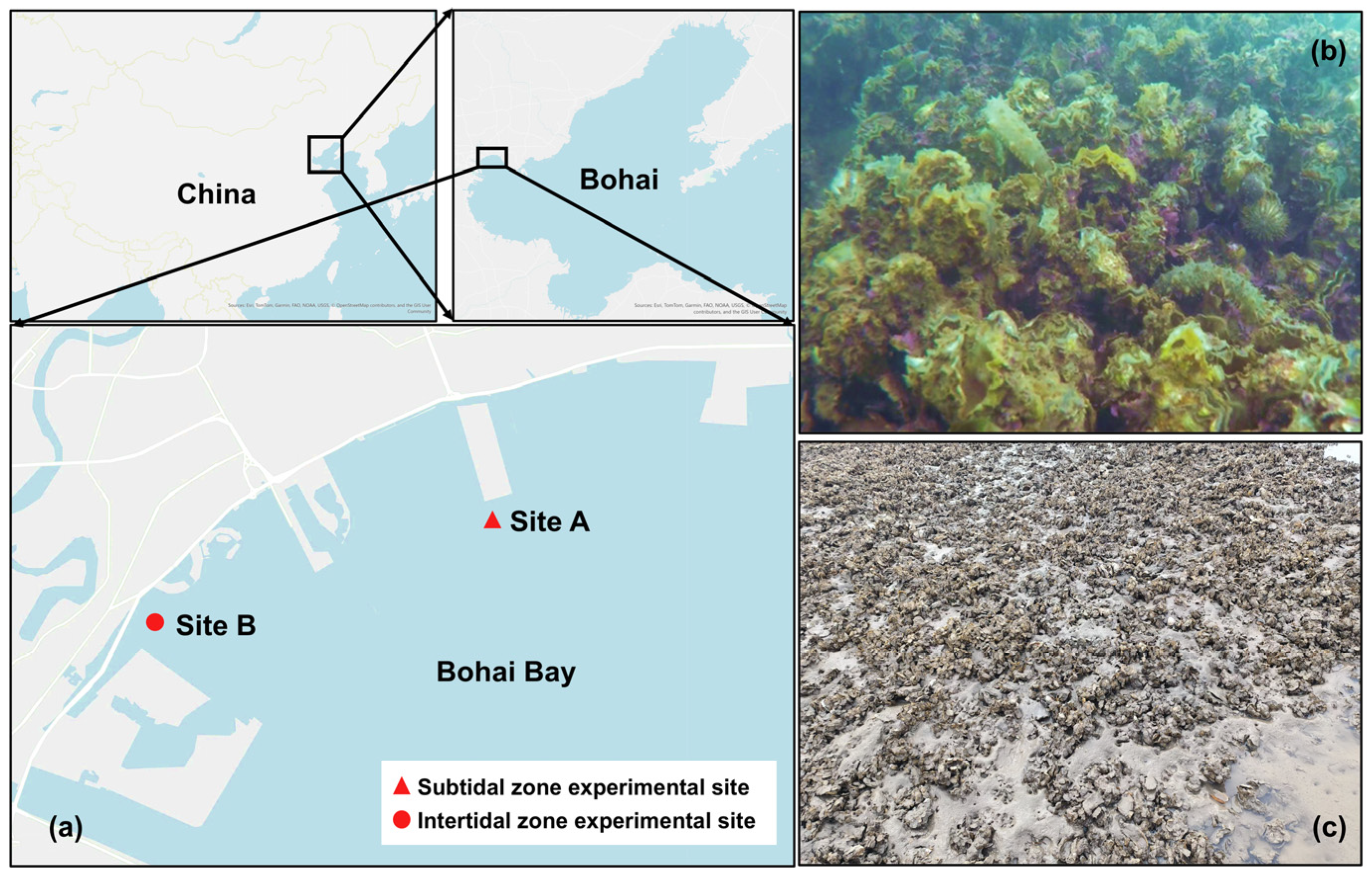
2.1. Site Description
2.2. Oyster Recruitment Experiments
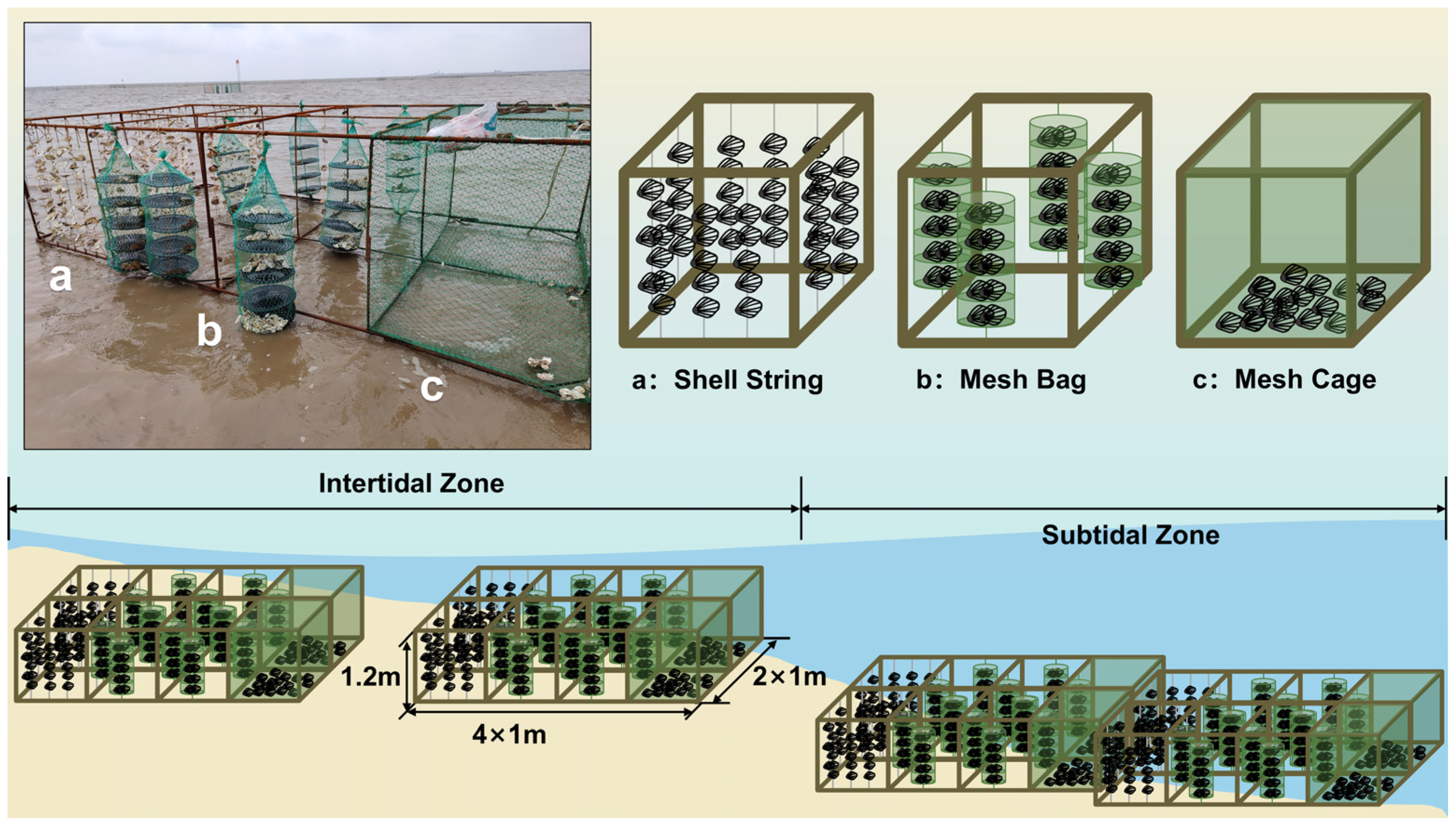
2.2.1. Effects of Three Restoration Methods on Oyster Recruitment in Intertidal and Subtidal Zones
2.2.2. Effects of Seeding with Juvenile Oysters, Shell Orientation, and Hanging Height of Shell Cultches on Oyster Recruitment
2.2.3. Effects of Environmental Variables at Different Restoration Sites on Oyster Recruitment
2.3. Statistical Methods
2.4. Comprehensive Performance Evaluation
3. Results and Discussion
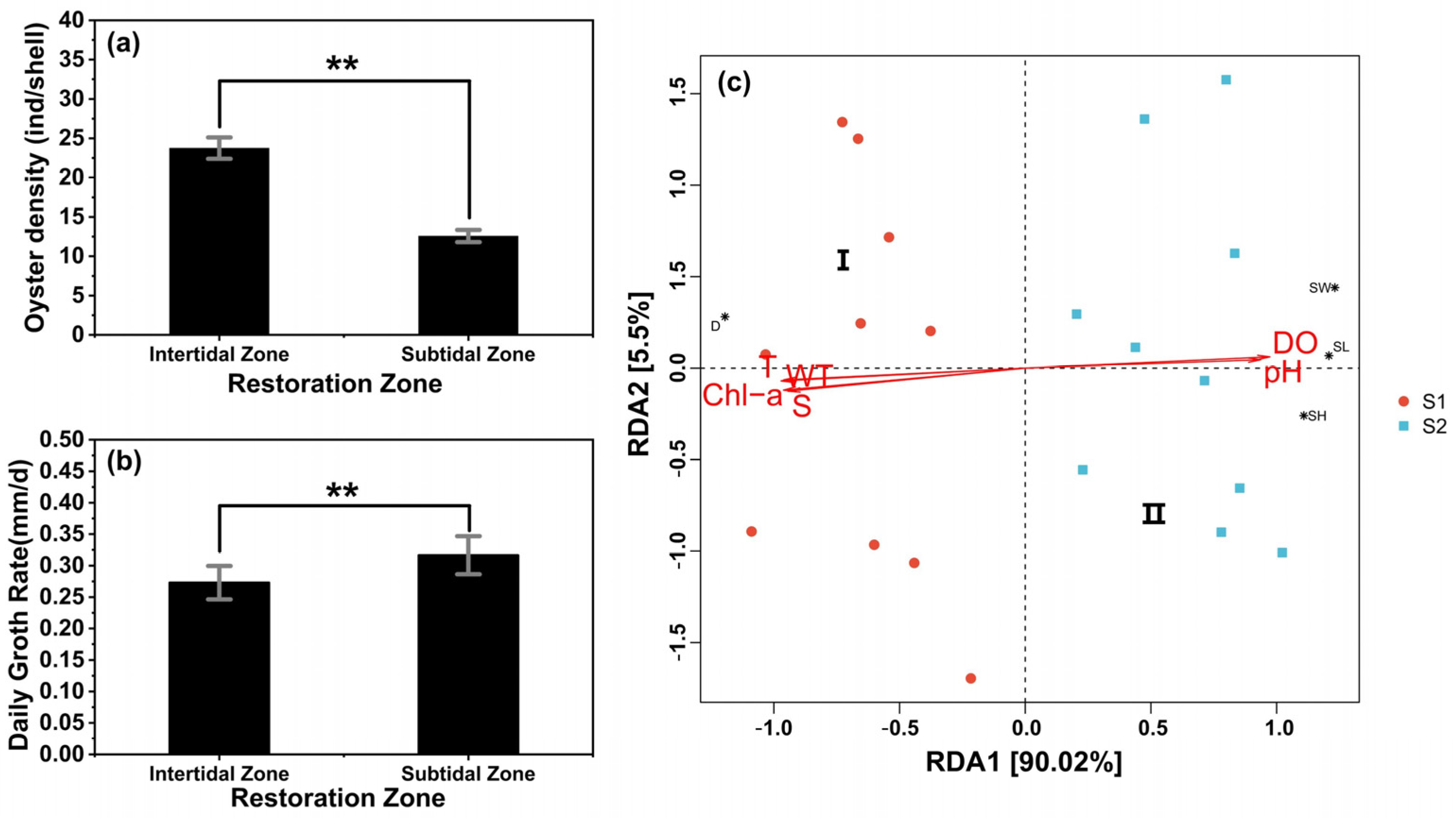
3.1. Comparison of Three Restoration Methods in the Intertidal Zone and the Subtidal Zone
3.2. Effects of Seeding with Juvenile Oyster, Shell Orientation, and Hanging Height of Cultches on Oyster Recruitment
3.3. Comparison of Intertidal Zone and Subtidal Zone and the Driving Environmental Factors
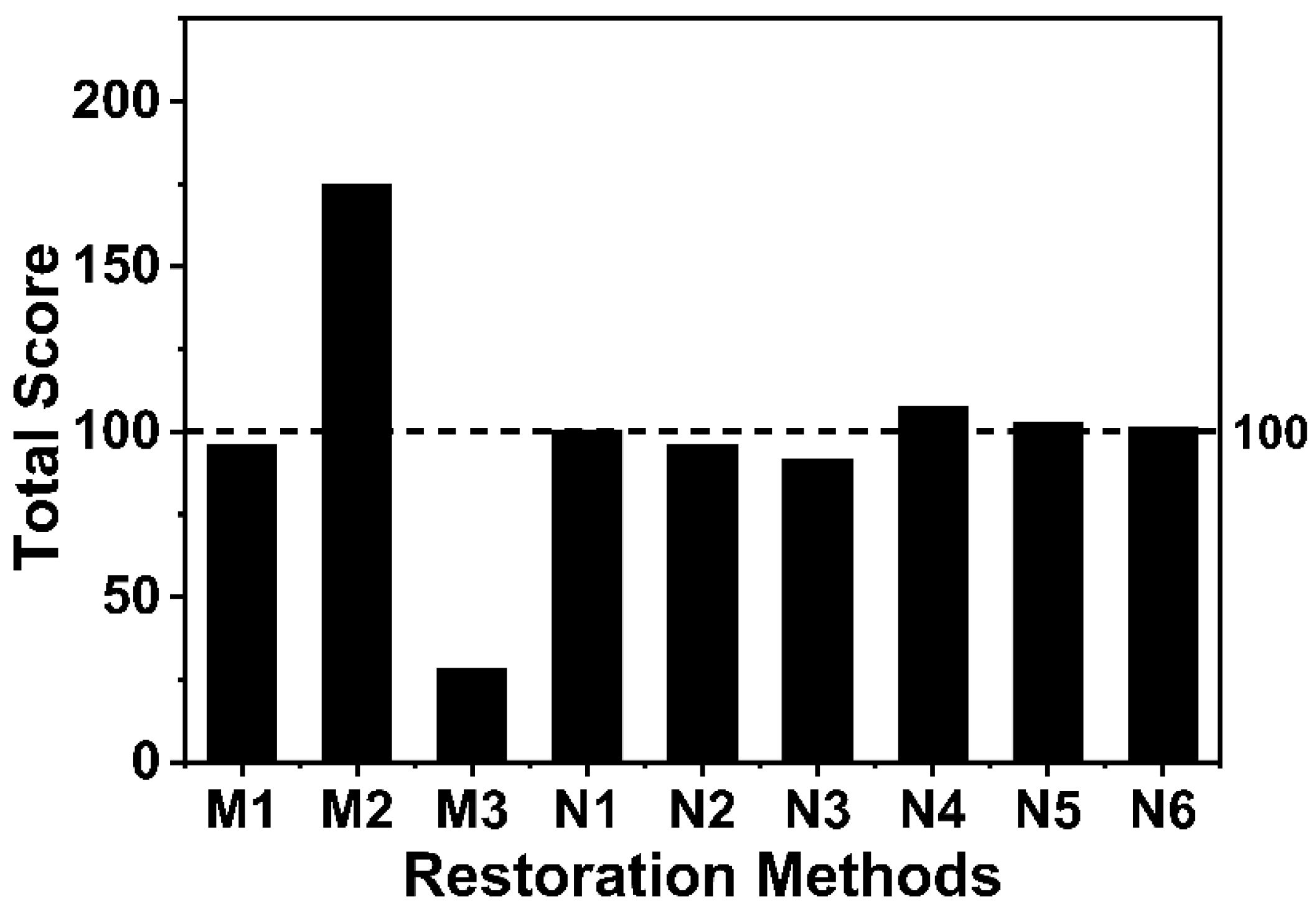
3.4. Evaluation of Different Restoration Methods in Intertidal and Subtidal Zones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cranfield, H.J.; Manighetti, B.; Michael, K.P.; Hill, A. Effects of Oyster Dredging on the Distribution of Bryozoan Biogenic Reefs and Associated Sediments in Foveaux Strait, Southern New Zealand. Cont. Shelf Res. 2003, 23, 1337–1357. [Google Scholar] [CrossRef]
- Jamil, A.; Ahmad, A.; Zhao, Y.; Zhao, Y.; Yang, C.; Li, Y.; Tu, J.; Niu, F.; Kong, W.; Liu, X. Advances in Global Oyster Reef Restoration: Innovations and Sustainable Ecological Approaches. Sustainability 2024, 16, 9795. [Google Scholar] [CrossRef]
- Quan, W.; Shen, X.; Luo, M.; Chen, Y. Ecological function and restoration measures of oyster reef in estuaries. Chin. J. Ecol. 2006, 25, 1234–1239. [Google Scholar]
- Quan, W.-M.; Zhang, J.-P.; Ping, X.-Y.; Shi, L.-Y.; Li, P.-J.; Chen, Y.-Q. Purification function and ecological services value of Crassostrea sp. in Yangtze River estuary. J. Appl. Ecol. 2007, 18, 871–876. [Google Scholar]
- Lenihan, H.S. Physical-Biological Coupling on Oyster Reefs: How Habitat Structure Influences Individual Performance. Ecol. Monogr. 1999, 69, 251–275. [Google Scholar] [CrossRef]
- Quan, W.; Feng, M.; Zhou, Z.; Wu, Z.; Tang, F.; Wang, Y.; Bao, X.; Shen, H.; Cheng, W. Ecological assessment of the oyster Crassostrea sikamea population and associated benthic communities on restored oyster reefs along Jiangsu Province coast, China. Acta Ecol. Sin. 2017, 37, 1709–1718. [Google Scholar]
- Quan, W.; Zhu, J.; Ni, Y.; Shi, L.; Chen, Y. Faunal Utilization of Constructed Intertidal Oyster (Crassostrea rivularis) Reef in the Yangtze River Estuary, China. Ecol. Eng. 2009, 35, 1466–1475. [Google Scholar] [CrossRef]
- Grabowski, J.; Peterson, C. Restoring Oyster Reefs to Recover Ecosystem Services. Theor. Ecol. Ser. 2007, 4, 281–298. [Google Scholar] [CrossRef]
- Plutchak, R.; Major, K.; Cebrian, J.; Foster, C.D.; Miller, M.-E.C.; Anton, A.; Sheehan, K.L.; Heck, K.L.; Powers, S.P. Impacts of Oyster Reef Restoration on Primary Productivity and Nutrient Dynamics in Tidal Creeks of the North Central Gulf of Mexico. Estuarise Coasts 2010, 33, 1355–1364. [Google Scholar] [CrossRef]
- Quan, W.; Humphries, A.T.; Shi, L.; Chen, Y. Determination of Trophic Transfer at a Created Intertidal Oyster (Crassostrea ariakensis) Reef in the Yangtze River Estuary Using Stable Isotope Analyses. Estuaries Coasts 2012, 35, 109–120. [Google Scholar] [CrossRef]
- Gong, P.; Li, J.; Guan, C.; Li, M.; Liu, C. Estimation and experiment of carbon sequestration by oysters attached to the enhancement artificial reefs in Laizhou Bay, Shandong, China. J. Appl. Ecol. 2014, 25, 3032–3038. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Gong, P.; Guan, C. Research Progress on Fishery Carbon Sinking Associated with Marine Ranching. Prog. Fiash. Sci. 2022, 43, 142–150. [Google Scholar] [CrossRef]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. Bioscience 2011, 61, 107–116. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Yu, H.; Du, S. Developmental Dynamics of Myogenesis in Pacific Oyster Crassostrea gigas. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2019, 227, 21–30. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef]
- Boesch, D.; Burreson, E.; Dennison, W.; Houde, E.; Kemp, M.; Kennedy, V.; Newell, R.; Paynter, K.; Orth, R.; Ulanowicz, R. Factors in the Decline of Coastal Ecosystems. Science 2001, 293, 1589–1590. [Google Scholar] [CrossRef]
- Coen, L.D.; Luckenbach, M.W. Developing Success Criteria and Goals for Evaluating Oyster Reef Restoration: Ecological Function or Resource Exploitation? Ecol. Eng. 2000, 15, 323–343. [Google Scholar] [CrossRef]
- Zu Ermgassen, P.S.E.; Grabowski, J.H.; Gair, J.R.; Powers, S.P. Quantifying Fish and Mobile Invertebrate Production from a Threatened Nursery Habitat. J. Appl. Ecol. 2016, 53, 596–606. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Borsje, B.W.; van Wesenbeeck, B.K.; Dekker, F.; Paalvast, P.; Bouma, T.J.; van Katwijk, M.M.; de Vries, M.B. How Ecological Engineering Can Serve in Coastal Protection. Ecol. Eng. 2011, 37, 113–122. [Google Scholar] [CrossRef]
- Ray, N.E.; Terlizzi, D.E.; Kangas, P.C. Nitrogen and Phosphorus Removal by the Algal Turf Scrubber at an Oyster Aquaculture Facility. Ecol. Eng. 2015, 78, 27–32. [Google Scholar] [CrossRef]
- Volety, A.K.; Haynes, L.; Goodman, P.; Gorman, P. Ecological Condition and Value of Oyster Reefs of the Southwest Florida Shelf Ecosystem. Ecol. Indic. 2014, 44, 108–119. [Google Scholar] [CrossRef]
- Goelz, T.; Vogt, B.; Hartley, T. Alternative Substrates Used for Oyster Reef Restoration: A Review. J. Shellfish Res. 2020, 39, 1–12. [Google Scholar] [CrossRef]
- Colden, A.M.; Latour, R.J.; Lipcius, R.N. Reef Height Drives Threshold Dynamics of Restored Oyster Reefs. Mar. Ecol. Prog. Ser. 2017, 582, 1–13. [Google Scholar] [CrossRef]
- McAfee, D.; McLeod, I.M.; Bostrom-Einarsson, L.; Gillies, C.L. The Value and Opportunity of Restoring Australia’s Lost Rock Oyster Reefs. Restor. Ecol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Brumbaugh, R.D.; Coen, L.D. Contemporary Approaches for Small-Scale Oyster Reef Restoration to Address Substrate Versus Recruitment Limitation: A Review and Comments Relevant for the Olympia Oyster, Ostrea Lurida Carpenter 1864. J. Shellfish Res. 2009, 28, 147–161. [Google Scholar] [CrossRef]
- Harwell, H.D.; Kingsley-Smith, P.R.; Kellogg, M.L.; Allen, S.M.; Allen, S.K.; Meritt, D.W.; Paynter, K.T.; Luckenbach, M.W. A Comparison of Crassostrea virginica and C. ariakensis in Chesapeake Bay: Does Oyster Species Affect Habitat Function? J. Shellfish Res. 2010, 29, 253–269. [Google Scholar] [CrossRef]
- Li, J.; Shang, Z.; Chen, Y.; Tian, L.; Jiang, X.; Wang, F.; Hu, Y.; Li, Y.; Yang, P.; Wen, M.; et al. Research status and protection suggestions on oyster reef in Bohai Bay. North China Geol. 2020, 43, 317–333. [Google Scholar]
- Xu, X.; Zeng, X.; Li, Y.; Gao, Y. A Preliminary Study on the Influencing Factors of Newborn Oyster in Tianjin Dashentang Marine Ranching. J. Ocean Technol. 2024, 43, 70–77. [Google Scholar]
- Yu, Q.; Zhang, X.; Han, X. Elementary Study on the Fishery Resources Conservation and the Ecological Restoration of the Live Oyster Reef in Dashentang, Tianjin. Mar. Econ. 2014, 4, 16–22. [Google Scholar] [CrossRef]
- Yin, X.; Chen, H.; Qiao, Y.; Chen, L. Present Condition and Management Countermeasures of Dashentang Oyster Reef Marine Special Reserve in Tianjin. Trans. Ocean. Limnol. 2015, 1, 162–166. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Wang, Y.; Gu, Z.; Yang, W.; Jia, L. Investigation and study on the subsidence status of artificial fish reefs in Tianjin Dashengtang Marine Ranching. J. Mar. Inf. Technol. Appl. 2024, 39, 72–78. [Google Scholar]
- Fang, E.; Wang, H.; Zeng, X.; Wang, Y.; Xu, X.; Zhang, X. Preliminary Acoustic Investigation of Artificial Reef Area in Dashentang Marine Ranch, Tianjin. Tianjin Agric. Sci. 2023, 29, 42–47+52. [Google Scholar]
- Sha, W.; Xu, Y.; Kan, W.; Jiang, H.; Zhang, J.; Lu, X.; Li, H.; Zhu, L.; Feng, J. Characteristics analysis of symbiotic community of Spartina alterniflora-Crassostrea gigas in intertidal zone of Bohai Bay. Acta Ecol. Sin. 2024, 44, 7738–7747. [Google Scholar] [CrossRef]
- Scyphers, S.B.; Powers, S.P.; Heck, K.L.; Byron, D. Oyster Reefs as Natural Breakwaters Mitigate Shoreline Loss and Facilitate Fisheries. PLoS ONE 2011, 6, e22396. [Google Scholar] [CrossRef]
- Guo, B.; Chen, W.; Gao, Y.; Zhang, B.; Wang, S.; Wang, Y.; Zhang, X.; Zeng, X. Community Characteristics of Attaching Organisms on the Artificial Reefs Built in Different Years in Dashentang, Tianjin. Prog. Fiash. Sci. 2020, 41, 12–18. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, E.; Guo, B.; Gao, Y.; Hou, C.; Wang, Q. Preliminary evaluation of the effect of habitat restoration of Tianjin Da Shentang Special Marine Reserve. Hebei Fish. 2014, 11, 23–29+42. [Google Scholar] [CrossRef]
- The Specification for Marine Monitoring—Part 4: Seawater Analysis (GB 17378.4-2007). Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=9FB14D0EE23D77A96D54A9BDAAF6EA07 (accessed on 23 July 2022).
- Technical Guideline on Coastal Ecological Rehabilitation for Hazard Mitigation—Part 6: Oyster Reef (T/CAOE 21.6-2020). Available online: http://www.scsio.cas.cn/gczx/xzzx_197139/xgxyjszlk/st/202309/P020230921652878576312.pdf (accessed on 23 July 2022).
- Technical Directives for Investigation and Assessment of Coastal Ecosystem Status—Part 7: Oyster Reefs (T/CAOE 20.7). Available online: https://gi.mnr.gov.cn/202401/P020240104653088805622.pdf (accessed on 23 July 2022).
- Breitburg, D.; Palmer, M.; Loher, T. Larval Distributions and the Spatial Patterns of Settlement of an Oyster Reef Fish: Responses to Flow and Structure. Mar. Ecol. Prog. Ser. 1995, 125, 45–60. [Google Scholar] [CrossRef]
- Virnstein, R.W. The Importance of Predation by Crabs and Fishes on Benthic Infauna in Chesapeake Bay. Ecology 1977, 58, 1199–1217. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Fang, J.; Ni, X.; Chen, W.; Xie, S.; Yu, R.; Zheng, X. Evaluation of the Effect of Raft Culture of “Haida Series” Crassostrea gigas Varieties in Nanji Sea Area. Period. Ocean Univ. China 2023, 53, 24–32. [Google Scholar] [CrossRef]
- Lipcius, R.N.; Burke, R.P.; McCulloch, D.N.; Schreiber, S.J.; Schulte, D.M.; Seitz, R.D.; Shen, J. Overcoming Restoration Paradigms: Value of the Historical Record and Metapopulation Dynamics in Native Oyster Restoration. Front. Mar. Sci. 2015, 2, 65. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.; James, M.; Donnan, D.W.; Henry, T.B.; Møller, L.F.; Sanderson, W.G. Conservation and Restoration of a Keystone Species: Understanding the Settlement Preferences of the European Oyster (Ostrea edulis). Mar. Pollut. Bull. 2019, 138, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.B.; Luckenbach, M.W.; Kingsley-Smith, P.R. Oyster Reef Community Interactions: The Effect of Resident Fauna on Oyster (Crassostrea Spp.) Larval Recruitment. J. Exp. Mar. Biol. Ecol. 2010, 391, 169–177. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Zimmer-Faust, R.K.; Tamplin, M.L. Natural Sources and Properties of Chemical Inducers Mediating Settlement of Oyster Larvae: A Re-Examination. Biol. Bul. 1992, 183, 327–338. [Google Scholar] [CrossRef]
- Bayne, B.L. Biology of Oysters; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-803472-9. [Google Scholar]
- Ridge, J.T.; Rodriguez, A.B.; Fodrie, F.J.; Lindquist, N.L.; Brodeur, M.C.; Coleman, S.E.; Grabowski, J.H.; Theuerkauf, E.J. Maximizing Oyster-Reef Growth Supports Green Infrastructure with Accelerating Sea-Level Rise. Sci. Rep. 2015, 5, 14785. [Google Scholar] [CrossRef]
- North, E.W.; Schlag, Z.; Hood, R.R.; Li, M.; Zhong, L.; Gross, T.; Kennedy, V.S. Vertical Swimming Behavior Influences the Dispersal of Simulated Oyster Larvae in a Coupled Particle-Tracking and Hydrodynamic Model of Chesapeake Bay. Mar. Ecol. Prog. Ser. 2008, 359, 99–115. [Google Scholar] [CrossRef]
- Johnson, M.W.; Powers, S.P.; Senne, J.; Park, K. Assessing in Situ Tolerances of Eastern Oysters (Crassostrea virginica) Under Moderate Hypoxic Regimes: Implications for Restoration. J. Shellfish Res. 2009, 28, 185–192. [Google Scholar] [CrossRef]
- Lemasson, A.J.; Fletcher, S.; Hall-Spencer, J.M.; Knights, A.M. Linking the Biological Impacts of Ocean Acidification on Oysters to Changes in Ecosystem Services: A Review. J. Exp. Mar. Biol. Ecol. 2017, 492, 49–62. [Google Scholar] [CrossRef]
- Dunphy, B.J.; Wells, R.M.G.; Jeffs, A.G. Oxygen Consumption and Enzyme Activity of the Subtidal Flat Oyster (Ostrea chilensis) and Intertidal Pacific Oyster (Crassostrea gigas): Responses to Temperature and Starvation. N. Z. J. Mar. Freshw. Res. 2006, 40, 149–158. [Google Scholar] [CrossRef]
- Meng, J.; Wang, T.; Li, L.; Zhang, G. Inducible Variation in Anaerobic Energy Metabolism Reflects Hypoxia Tolerance across the Intertidal and Subtidal Distribution of the Pacific Oyster (Crassostrea gigas). Mar. Environ. Res. 2018, 138, 135–143. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, T.; Fan, R.; Ren, G.; Tang, B.; Quan, W. Artificial breeding of Suminoe oyster Crassostrea ariakensis. Fish. Inf. Strategy 2019, 34, 121–127. [Google Scholar] [CrossRef]
- Kennedy, V.S.; Newell, R.I.E.; Eble, A.F.; Leffler, M.; Harpe, S.R. The Eastern Oyster: Crassostrea Virginica. Nat. Environ. Factors 1996, 13, 467–513. [Google Scholar]
- Lowe, M.R.; Sehlinger, T.; Soniat, T.M.; Peyre, M.K.L. Interactive Effects of Water Temperature and Salinity on Growth and Mortality of Eastern Oysters, Crassostrea virginica: A Meta-Analysis Using 40 Years of Monitoring Data. J. Shellfish Res. 2017, 36, 683–697. [Google Scholar] [CrossRef]


| Group No. | Zone Type | Seeding with Juvenile Oyster | Restoration Method | ||||
|---|---|---|---|---|---|---|---|
| Intertidal Zone | Subtidal Zone | With Seeding | Without Seeding | Mesh Bag | Shell String | Mesh Cage | |
| M1 | ◯ | ◯ | ◯ | ||||
| M2 | ◯ | ◯ | ◯ | ||||
| M3 | ◯ | ◯ | ◯ | ||||
| N1 | ◯ | ◯ | ◯ | ||||
| N2 | ◯ | ◯ | ◯ | ||||
| N3 | ◯ | ◯ | ◯ | ||||
| N4 | ◯ | ◯ | ◯ | ||||
| N5 | ◯ | ◯ | ◯ | ||||
| N6 | ◯ | ◯ | ◯ | ||||
| Restoration Method | Score A (Cost) | Score B (Settlement Abundance) | Score C (Average Daily Growth) | Score D (Marine Water Quality) | Score E (Reef Sedimentation) | Total Score |
|---|---|---|---|---|---|---|
| M1 | 37.21 | 10.92 | 37.74 | 5.08 | 5 | 95.95 |
| M2 | 26.49 | 98.98 | 39.34 | 5.03 | 5 | 174.84 |
| M3 | 18.80 | 0.00 | 0.00 | 4.39 | 5 | 28.19 |
| N1 | 30.45 | 31.47 | 28.58 | 5 | 5 | 100.50 |
| N2 | 31.79 | 26.97 | 27.27 | 5 | 5 | 96.03 |
| N3 | 31.36 | 22.80 | 27.73 | 5 | 5 | 91.89 |
| N4 | 27.69 | 37.32 | 32.82 | 5 | 5 | 107.83 |
| N5 | 29.73 | 31.70 | 31.52 | 5 | 5 | 102.95 |
| N6 | 29.35 | 29.74 | 32.08 | 5 | 5 | 101.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, C.; Zhang, B.; Li, Y.; Tu, J.; Niu, F.; Kong, W.; Wang, Z.; Liu, X. Influencing Factors on Oyster Recruitment and Performance Evaluation for Oyster Reef Restoration in Tianjin Coastal Zones. Oceans 2025, 6, 20. https://doi.org/10.3390/oceans6020020
Zhao Y, Yang C, Zhang B, Li Y, Tu J, Niu F, Kong W, Wang Z, Liu X. Influencing Factors on Oyster Recruitment and Performance Evaluation for Oyster Reef Restoration in Tianjin Coastal Zones. Oceans. 2025; 6(2):20. https://doi.org/10.3390/oceans6020020
Chicago/Turabian StyleZhao, Yuxuan, Chen Yang, Bo Zhang, Yanping Li, Jianbo Tu, Fuxin Niu, Wenliang Kong, Zhiyun Wang, and Xianhua Liu. 2025. "Influencing Factors on Oyster Recruitment and Performance Evaluation for Oyster Reef Restoration in Tianjin Coastal Zones" Oceans 6, no. 2: 20. https://doi.org/10.3390/oceans6020020
APA StyleZhao, Y., Yang, C., Zhang, B., Li, Y., Tu, J., Niu, F., Kong, W., Wang, Z., & Liu, X. (2025). Influencing Factors on Oyster Recruitment and Performance Evaluation for Oyster Reef Restoration in Tianjin Coastal Zones. Oceans, 6(2), 20. https://doi.org/10.3390/oceans6020020






