Abstract
Tetrabromobisphenol A (TBBPA) is a fire-retardant containing bromine, produced in large quantities worldwide and extensively used in several industrial products. This compound was identified as a potential contaminant of the environment, causing toxicity to organisms. However, its toxicity remains poorly understood in marine bivalves. The first objective of this work was to evaluate the impact of TBBPA on mussels (Mytilus galloprovincialis) exposed for 28 days to various concentrations of TBBPA (0, 1, 10, and 100 µg·L−1), by assessing stress biomarkers’ responses (Glutathione S-transferase, superoxide dismutase, catalase, lipid peroxidation, total antioxidant capacity, total ubiquitin, caspase-3 and acetylcholinesterase). The results showed that lower concentrations (1 and 10 µg·L−1) were efficiently detoxified, as suggested by GST activities, which were supported by the responses of the other biomarkers. The most pronounced effects were observed in animals exposed to the highest concentration of TBBPA (100 µg·L−1), suggesting oxidative stress. Additionally, significant strong correlations were found between total antioxidant capacity and some biomarkers (superoxide dismutase and lipid peroxidation), showing that processes involved in oxidative stress fighting are working to avoid cell injury. In brief, mussels’ defense mechanisms were capable of dealing with exposure to the lower concentrations tested. Despite this, the risk of consuming shellfish or other fishery products contaminated with TBBPA should be a cause for concern.
1. Introduction
Discharging various chemical substances into the environment has increased the number of emerging contaminants (e.g., endocrine disruptors, pharmaceuticals, brominated and perfluorinated compounds, and personal care products), which has raised significant concern worldwide [1,2]. Flame retardants (FRs) constitute a group of synthetic compounds commonly incorporated into a wide range of industrial and household items, including plastics, polymers, textiles, and electronic equipment, to reinforce their resistance to combustion. They can be categorized into organic (halogenated), inorganic, organophosphorus, and nitrogen-based compounds [3]. Brominated FRs (BFRs) are the most commercialized due to their low cost and high efficiency, comprising mainly hexabromocyclododecane (HBCDs), polybrominated diphenyl ethers (PBDEs), brominated phenols (BrPhs) and tetrabromobisphenol A (TBBPA) [3,4]. TBBPA emerges as one of the most widely used FRs globally, exhibiting substantial production volumes and widespread use [5,6]. Thus, water samples have shown the levels of TBBPA, from various regions of the world, ranging from non-detected (n.d.) to 4.87 μg·L−1 of magnitude [7,8] and in sediments between n.d. and 500 of µg·kg−1 [8,9].
Despite the absence of current limitations on the manufacture and use of TBBPA, its inclusion by the Convention for the Protection of the Marine Environment of the Northeast Atlantic (OSPAR) on the substances requiring priority action [10] implies urgent assessment and intervention. Thus, releasing these compounds into aquatic ecosystems represents an increased risk to aquatic biota due to their persistent nature, promoting an accumulation in sediments and marine organisms [11,12]. Furthermore, TBBPA’s high lipophilicity and heat stability lead to bioconcentration and biomagnification across trophic levels [4,13]. Moreover, the consumption of contaminated seafood could endanger human health. However, studies to detect their presence in food are still insufficient [14], and frequent monitoring should be carried out.
Additionally, identifying TBBPA as a potential endocrine disruptor [15], and its associated neurotoxic, immunotoxic, nephrotoxic, and hepatotoxic effects, highlights the urgency of understanding its toxicity [16,17,18]. Furthermore, it is recognized by the EU as carcinogenic (https://echa.europa.eu/, accessed on 6 February 2024). Although the debate surrounding the toxicity of TBBPA persists [19], evidence indicates its harmful impact on several aquatic organisms, including algae, bivalves, crustaceans, and fish [20], implicating fisheries as potential sources of human exposure. Consuming food contaminated with TBBPA can lead to diverse health problems, including its capacity to disrupt the endocrine system [1,21].
Regarding LC50 values found for TBBPA, Jiang et al. [22] found 7.4 mg·L−1 (96h-LC50) in juvenile clams (Ruditapes philippinarum). However, we found no LC50 information for mussels. In a review by Yang et al. [8], it was reported that the 96 h LC50 for freshwater fishes (Pimephales promelas and Danio rerio) ranged from 0.006 to 3.0 mg·L−1. But, we should be cautious about some of these results, since in some cases the experiment conditions were not the same.
Mussels, such as M. galloprovincialis, are widely distributed in Europe from the Mediterranean to the U.K. and Norway coasts [23]. Thus, mussels are often utilized in toxicity studies as biological models due to their filtration capabilities, wide distribution, and capability to bioaccumulate contaminants [24], enabling the understanding of ecological disturbances induced by exposure to contaminants and functioning as indicators of aquatic pollution in environmental monitoring programs [25].
Biomarkers of response, including indicators of oxidative stress and markers of cellular damage, are essential in strategies for monitoring and evaluating the effects of contaminants on living organisms. Biomarkers can, therefore, be utilized as early warning indicators to assess the condition of the environment [26,27]. Multi-biomarker methodologies have been recommended to assess the impacts of toxic substances and understand the impact on ecosystems [28]. Exposure to various types of pollutants may result in an excess of reactive oxygen species (ROS) that can induce oxidative stress and cell damage in cells, as the organism is unable to remove the excess of ROS produced [29,30,31,32]. To evaluate the consequences of exposure to contaminants, antioxidant enzymes can be helpful [31] in addition to other biomarkers that suggest damage at a cellular level (e.g., lipid peroxidation, caspase, and ubiquitin) [33,34]. However, there are few studies on antioxidant enzymes and other biomarkers of response (e.g., caspase, ubiquitin) in mussels exposed to TBBPA. Thus, multi-biomarker studies are essential for comprehending the effects of exposure to these compounds in marine biota.
The purpose of this work was to study the effects of TBBPA on the mussel (Mytilus galloprovincialis), a common marine model, by exposing the animal for 28 days to three different concentrations of TBBPA (1, 10, and 100 µg·L−1) and then analyzing oxidative stress biomarkers, such as GST (glutathione S-transferase), SOD (superoxide dismutase), CAT (catalase), LPO (lipid peroxidation), and TAC (total antioxidant capacity). Protein degradation signaling was examined by assessing total ubiquitin, cellular apoptosis was evaluated by measuring caspase (CASP-3) levels, and neurotoxicity was assessed by analyzing acetylcholinesterase (AChE) activity. In addition, an integrated biomarker analysis was also performed to provide further information on the effects on mussels, allowing a better understanding of the risk of exposure to the marine ecosystem.
2. Materials and Methods
2.1. TBBPA Stock Solution
To prepare the TBBPA (CAS No. 79-94-7; ≈99.3%; ref. 330396, Sigma-Aldrich, Shanghai, China), a stock solution was prepared, where 0.1 g of the compound was dissolved in 100 mL of methanol (≈99.8% (v/v); Honeywell, Seelze, Germany). An appropriate volume was then added to each aquarium to attain the concentrations to be tested (1, 10 and 100 µg·L−1). An additional aquarium with filtered seawater was used as a control. Considering the dilution factor, the methanol concentration in each aquarium was estimated to be ≤0.01%.
2.2. Experimental Trials
M. galloprovincialis (Lamarck, 1819) were manually gathered at Guincho coast (Cascais, Lisbon, Portugal) in January/February 2023. Only mussels of comparable lengths were collected to prevent variations in bioaccumulation and biomarker responses due to size or age. The animals (n = 40; 1.270 g ± 0.365) were transported in a thermal box to the laboratory facilities at NOVA School of Science and Technology. They were acclimatized in an aquarium with 50 L of seawater (from the same collection site—Guincho), with filtration, water recirculation, and aeration (>6 mg·L−1 dissolved O2). They were maintained at a pH of 8.1 ± 0.2; a temperature of 20.0 ± 1.0 °C; a salinity of 33 ± 1 g·L−1; a photoperiod of 12 h of light and 12 h of darkness; and continuously aerated. Water quality parameters were checked daily in each aquarium (temperature, pH, and salinity). The animals were randomly distributed by four aquariums containing 8 animals each. Then, for 28 days, mussels were tested at different concentrations of TBBPA (0, 1, 10, and 100 µg·L−1) diluted in seawater from the collection site. TBBPA concentrations selected for the exposure tests were based on values determined in seawater elsewhere [7,8]. However, higher concentrations were also assayed to better comprehend the effects on animals. In addition, a control aquarium was also used with ≤0.01% methanol in seawater. The animals were fed three times per week with Chlorella sp. (Shine superfood, Setúbal, Portugal).
Ethics
This work has been approved by the competent national authorities and has complied with all applicable laws regarding animal welfare. The researchers hold a level C certification from the European Federation for Laboratory Animal Science (FELASA) for carrying out experiments on animals. The published results are consistent with the ARRIVE recommendations, covering the 3 Rs of animal welfare.
2.3. Sample Treatment
Following the exposure period, the organisms were gathered, weighted, and the whole organism was removed. Then, a tissue homogenizer (Tissue Master 125, Kennesaw, GA, USA) was used to homogenize the samples in a solution of 3.0 mL phosphate-buffered saline solution (PBS; comprising NaCl (140 mM; Panreac, Barcelona, Spain), Na2HPO4 (10 mM; Sigma-Aldrich, St. Louis, MO, USA), KCl (3 mM; Merck, Darmstatd, Germany), and KH2PO4 (2 mM; Sigma-Aldrich, Steinheim, Germany)) at pH 7.3 ± 0.2. Subsequently, the samples were centrifuged for 10 min at 4 °C at 15,000× g using a centrifuge (VWR, model CT 15RE, Tokyo, Japan). The supernatant was transferred to microtubes (1.5 mL) and kept at −45 °C until biomarkers were analyzed.
2.4. Total Protein
Bradford method was used to determine the total cytosolic protein content [35]. Standards were prepared with Bovine Serum Albumin (BSA; Nzytech, Lisboa, Portugal) to construct a calibration curve, ranging from 0 to 4 mg·mL−1. Then, in a 96-well microplate (Greiner Bio-One, GmbH, Kremsmünster, Austria), 20 µL of each standard or sample and 180 µL of Bradford Reagent were pipetted into each well. A microplate reader (Synergy HTX, Multi-Mode Reader, BioTek, Winooski, VT, USA) was used to read the absorbance in each well at 595 nm. Total protein concentration in samples were obtained from the calibration curve, and the results were expressed as mg·mL−1. The cytosolic protein concentration (PROT) was determined for normalization purposes.
2.5. Biomarkers Analyses
2.5.1. Glutathione S-Transferase (GST) Activity
A protocol that was first published by Habig et al. [36] but adapted and optimized for 96-well microplates was used. To determine the specific activity of GST, a molar extinction coefficient of 5.3 mM−1·cm−1 for CDNB was utilized. Thus, 20 µL of the sample and 180 µL of the substrate solution (19.6 mL of PBS buffer, 200 µL of reduced L-Glutathione (200 mM; GSH; Sigma-Aldrich, St. Louis, MO, USA), and 200 µL at 100 mM of 1-chloro-2,4-dinitrobenzene (CDNB; Sigma-Aldrich, St. Louis, MO, USA)) were pipetted into each well of the microplate. A microplate reader (Synergy HTX, BioTek) was used to read the absorbance at 340 nm, every minute, for six minutes. GST activity results were expressed relative to cytosolic protein content.
2.5.2. Superoxide Dismutase (SOD) Activity
The NBT method described by Sun et al. [37] was performed to measure SOD activity, with adaptations for a 96-well microplate. Quickly, 200 μL of potassium phosphate buffer (50 mM; pH 8.0) was pipetted into each well, followed by 10 μL of EDTA (3 mM; Riedel-Haën, Seelze, Germany), 10 μL of xanthine (3 mM; Sigma-Aldrich, China), 10 μL of sodium chloride nitroblue tetrazolium (0.75 mM; NBT; Sigma-Aldrich, Germany), and 10 μL of sample. Next, 10 µL of xanthine oxidase (XOD, Sigma-Aldrich, Germany) was added to begin the reaction. The absorbance was monitored at 560 nm, every two minutes, for 20 min in a microplate reader (Synergy HTX, BioTek). Results were expressed in relation to cytosolic protein content.
2.5.3. Catalase (CAT) Activity
The method described by Johansson and Borg [38] was used to determine CAT activity, after being adapted for use in 96-well microplates. Thus, 20 µL of sample, 30 µL of methanol (Honeywell), and 100 µL of potassium phosphate buffer (100 mM; pH 7.0; Sigma) were pipetted to each microplate well. Next, 20 µL of hydrogen peroxide (0.035 M; Sigma-Aldrich, Germany) was pipetted to each well to initiate the reaction. The microplate was then continuously shaken in an orbital shaker (Optic Ivymen System, JP Selecta, Barcelona Spain) for 20 min while incubating in the dark at room temperature. After adding 30 µL of KOH (10 M; ChemLab, Zedelgem, Belgium) and 30 µL of 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (32.4 mM in HCl 0.5 M; purpald; Aldrich, Germany), the microplate was incubated again under the same conditions as mentioned previously but this time for ten minutes. Finally, 10 µL of potassium periodate (65.2 mM in KOH 0.5 M; Sigma-Aldrich) was pipetted into each well and incubated for 5 min in the dark at room temperature. Then, the microplate was measured with a Synergy HTX microplate reader (BioTek) at 540 nm. A calibration curve was built using formaldehyde (AppliChem, Darmstadt, Germany) as standards, ranging from 0 to 150 µM. The CAT results were then presented in relation to the cytosolic protein content.
2.5.4. Acetylcholinesterase (AChE) Activity
AChE activity was quantified by following the Ellman et al. [39] method, adapted to 96-well microplates. Hence, 50 μL of sample and 250 μL of a mixture containing sodium phosphate buffer (50 mM; pH 8.0; Sigma-Aldrich), 5,5′-dithio-bis-2-nitrobenzoic acid (10 mM; DTNB; Sigma-Aldrich, St. Louis, MO, USA), and acetylcholine (75 mM; ACTI; Sigma, UK) were pipetted into the microplate wells. Next, the microplate wells were measured at 415 nm, each minute for ten minutes, with a microplate reader (Synergy HTX, BioTek) and the results were plotted as a function of the amount of cytosolic protein in each sample.
2.5.5. Lipid Peroxidation (LPO)
LPO assessment followed the thiobarbituric acid assay, as described by Madeira et al. [40]. A calibration curve was plotted for concentrations between 0 and 0.1 µM of Malondialdehyde (MDA, Merck, Germany) in Mili-Q ultrapure water. A volume of 5 µL of sample or standard, 45 µL of PBS buffer, 12.5 μL Sodium Dodecyl Sulfate (8.1% (w/v); SDS; Sigma-Aldrich, Germany), 93.5 μL Trichloroacetic Acid (20% (w/v); TCA; Panreac, Barcelona, Spain), 93.5 μL thiobarbituric acid (1% (w/v); TBA; Sigma-Aldrich, Germany), and 50.5 μL of ultrapure water were pipetted into 1.5 mL microtubes. This mixture was subjected to a brief centrifugation (3000× g rpm) for 30 s, followed by placing the microtubes, with punctured caps, in a dry bath (Thermobloc Digital, Labnet, Dusseldorf, Germany) for ten minutes at 100 °C. Next, samples were chilled on ice, 62.5 μL of ultrapure water was added to each microtube and subsequently centrifugated (3000× g rpm) for 30 s. Then, 150 μL of each sample was added to the wells of the microplate, and the absorbance was measured at 530 nm in a Synergy HTX microplate reader (BioTek). The results were then presented as a function of total cytosolic protein content.
2.5.6. Total Antioxidant Capacity (TAC)
Total antioxidant capacity assay was performed as described by Kambayashi et al. [41]. Standards of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox; Aldrich, Russian Federation) were diluted in potassium phosphate buffer (potassium phosphate monobasic (5mM; pH 7.4; Sigma), glucose (5.55 mM) and sodium chloride (154 mM; NaCl; Panreac)), with concentrations between 0 and 0.330 mM to construct a calibration curve. Afterwards, 10 µL of sample or standard, 10 µL of myoglobin (90 µM; Sigma, USA), and 150 µL of 2,2-azino-bis 3- ethylbenzothiazoline-6-sulphonic acid (600 µM; ABTS; Alfa Aesar, Karlsruhe, Germany) were pipetted into the microplate wells (Greiner Bio-one, GmbH, Kremsmünster, Austria). To start the reaction, 40 µL of hydrogen peroxide (500 µM; Sigma-Aldrich) was pipetted into each well. The microplate was incubated for 5 min at room temperature and the absorbance was read on a microplate reader Synergy HTX (BioTek). Results were normalized to the total cytosolic protein content.
2.5.7. Caspase-3 (CASP-3)
Caspase-3 was determined by Enzyme-Linked Immunosorbent Assay (ELISA) [42]. Caspase-3 standards were prepared in PBS buffer (human caspase 3, recombinant human active cc119; Merck, Rahway, NJ, USA) with concentrations between 0 and 5 µg·mL−1 to obtain a calibration curve. A total of 50 µL of sample or standard was pipetted into each microplate well (Greiner Bio-one, Microlon 600 High Binding, Frickenhausen, Germany Germany), and the microplate was incubated at 4 °C for 24 h. Afterwards, a solution containing PBS-Tween solution (0.05% v/v; Panreac; Spain) was used to wash the microplate wells. Next, the microplate wells were blocked by pipetting 100 µL of BSA blocking solution (1% (w/v) in PBS; Nzytech, Lisboa, Portugal) and incubated for 90 min at ambient temperature. The microplate was rewashed three times with the same washing solution. Right away, a primary antibody (anti-caspase 3 antibody ab13847, Abcam, Amsterdam, the Netherlands) was prepared in 1% BSA (w/v) to obtain a final concentration of 1.5 µg·mL−1 and 50 µL was pipetted into the microplate wells. Then, the microplate was incubated at 4 °C for 24 h. Next, the microplate was rewashed three times and 50 µL of secondary antibody (anti-mouse IgG Fc specific-alkaline phosphatase), diluted in PBS (1% BSA w/v) to a concentration of 1.0 µg·mL−1, was pipetted into the microplate wells. Then, the microplate was incubated for 90 min at 37 °C. The microplate was washed again and 50 µL of substrate solution (157 mg of trizma hydrochloride (Tris-HCl; Sigma, USA), 58 mg NaCl (Panreac), 50 μL MgCl2 (5 mM; Fluka, BioChemika, Buchs, Switzerland), and 10 mg of 4-nitrophenyl phosphate disodium salt hexahydrate (pNPP; Sigma-Aldrich, Gillingham, UK) prepared in 10 mL of distillate water, pH 9) was added to microplate wells. After the new incubation period (15 min) at ambient temperature, 50 µL of STOP solution (3 M NaOH (Panreac, Spain)) was pipetted into the microplate wells and the absorbance was read in a microplate reader Synergy HTX (BioTek) at 405 nm. The results were expressed as a function of the total cytosolic protein content.
2.5.8. Total Ubiquitin (UBI)
To determine total ubiquitin, an ELISA assay was performed, following the same procedure as described before [42]. The ubiquitin standards (UBPBio; Dallas, TX, USA) were prepared in a range of concentrations between 0 and 0.8 µg·mL−1 to build a calibration curve. A primary ubiquitin antibody (P4D1; Sc-8017; Santa Cruz Biotechnology, Dallas, TX, USA) was diluted to 1.5 µg·mL−1 in 1% BSA (w/v) and pipetted into each microplate well. Next, the same steps were followed as described above for the caspase-3 assay. Results were expressed as a function of the total cytosolic protein content.
2.6. Statistical Analysis
Statistics were carried out with the software Prism 9 (GraphPad software; version 9.5.1). Depending on whether parametric assumptions were satisfied, statistical comparisons were carried out using either the Kruskal–Wallis or the one-way ANOVA test, which were both found using Dunnett’s multiple comparison test. Whenever the parametric assumptions were fulfilled, then the one-way ANOVA test was carried out, whereas if these assumptions were not met, then the Kruskal–Wallis test was performed.
The non-parametric Spearman correlation was employed to measure the strength and direction of linear relationships between pairs of variables.
For IBR, to normalize the different biomarkers in the various concentrations, the difference between the mean of each group and the sample was calculated and divided for the standard deviation of each treatment as described in Madeira et al. [40]. Then, an integrated biomarker response (IBR) index was computed, and corresponding radar plots were created to compare the different concentrations. IBR was computed using Excel and the procedure was outlined by Beliaeff and Burgeot [43].
3. Results
3.1. Mortality Rate
No deaths were recorded during the exposure assays.
3.2. Biomarkers of Oxidative Stress
3.2.1. Glutathione-S-Transferase (GST)
GST activity results (mean ± standard deviation) are depicted in Figure 1A. Statistical analysis showed a significant increase in enzyme activity when comparing the control group with animals exposed to 100 µg·L−1 of TBBPA (p < 0.05) and between animals exposed to 1 µg·L−1 and animals exposed to 100 µg·L−1 of TBBPA (p < 0.01).
3.2.2. Superoxide Dismutase (SOD)
SOD activity, mean ± standard deviation, for each group is shown in Figure 1B. Statistics revealed a significant increase between mussels tested for 1 µg·L−1 and animals tested for 100 µg·L−1 of TBBPA (p < 0.05).
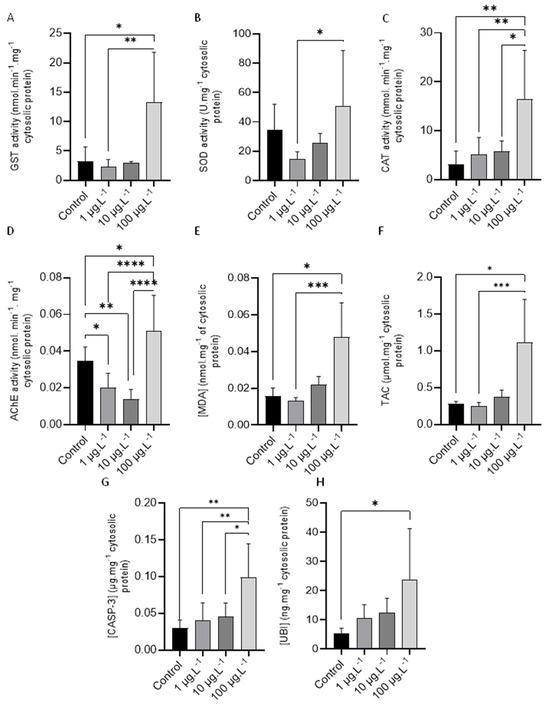
Figure 1.
(A) GST activity, (B) SOD activity, (C) CAT activity, (D) AChE activity, (E) MDA concentration, (F) TAC concentration, (G) CASP-3, and (H) UBI concentrations in mussels exposed to various concentrations (0, 1, 10, and 100 µg·L−1) of TBBPA. One-way ANOVA was performed in CAT, SOD, AChE, and CASP-3. Kruskal–Wallis test was performed in GST, TAC, and UBI. All data are presented as mean ± s.d. *—p < 0.05, **—p < 0.01, ***—p < 0.001 and ****—p < 0.0001.
3.2.3. Catalase (CAT)
Figure 1C shows CAT activity results (mean ± standard deviation) in animals tested to different tested TBBPA concentrations. A significant increase (p < 0.05) in this enzyme activity can be seen between the animals tested to the highest concentration of TBBPA (100 μg·L−1) and the other concentrations tested, as well as the control group.
3.2.4. Acetylcholinesterase (AChE)
AChE activity results (mean ± standard deviation) are shown in Figure 1D. Statistical analysis revealed a noteworthy increase in enzyme activity, when comparing each concentration (0, 1, and 10 µg·L−1) with the highest concentration (p < 0.05, p < 0.0001 and p < 0.0001, respectively). On the contrary, a significant decrease was observed comparing the animals tested to 1 µg·L−1 and control animals (p < 0.05) and between animals tested to 10 µg·L−1 (p < 0.01) and control animals.
3.2.5. Lipid Peroxidation (LPO)
LPO results (mean ± standard deviation), expressed in terms of MDA concentrations, are presented in Figure 1E. A notable increase in MDA concentration can be seen between control animals (p < 0.05) and mussels exposed to 100 µg·L−1 and between animals tested to 1 µg·L−1 and those tested to 100 µg·L−1 (p < 0.001) of TBBPA.
3.2.6. Total Antioxidant Capacity (TAC)
TAC values (mean ± standard deviation) determined in animals tested to the different TBBPA concentrations are presented in Figure 1F. Statistical analysis detected a significant increase in total antioxidant capacity between control animals and those tested to 100 µg·L−1 of TBBPA (p < 0.05) and between the animals tested to 1 µg·L−1 and those tested to 100 µg·L−1 (p < 0.001) of TBBPA.
3.2.7. Caspase-3 (CASP-3)
The levels of CASP-3 (mean ± standard deviation) determined in the animals exposed to TBBPA are shown in Figure 1G. A significant increase (p < 0.05) is evident when comparing the highest concentration (100 µg·L−1) with the other tested concentrations (0 µg·L−1, 1 µg·L−1, and 10 µg·L−1 of TBBPA).
3.2.8. Total Ubiquitin (UBI)
The results of the total UBI concentration (mean ± standard deviation) are presented in Figure 1H, where a significant increase (p < 0.05) can be observed between animals tested to 100 µg·L−1 of TBBPA and control animals.
3.3. IBR Index
The radar plot of IBR index is presented in Figure 2. Differences are only observed when comparing animals tested to the highest concentration (100 µg·L−1) with all other tested animals. Thus, a decrease in MDA concentrations and in AChE activities can be observed, along with a slight decrease in UBI concentrations. GST showed no differences among all tested animals. For SOD, CAT, CASP-3, and TAC, there was a slight increase, as shown by the IBR radar plot.
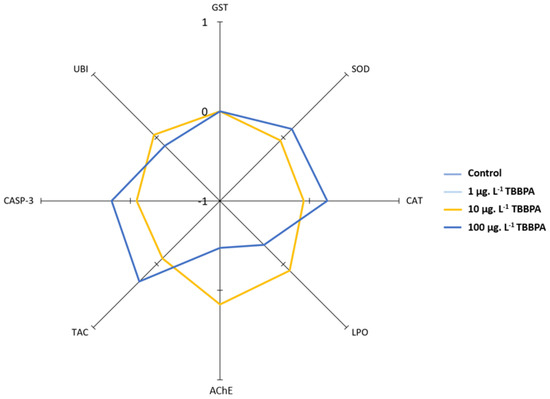
Figure 2.
Radar plot of IBR index in mussels exposed to different TBBPA concentrations. Control, 1 µg·L−1, and 10 µg·L−1 concentrations are overlapped (yellow line) and are, therefore, not distinguishable.
3.4. Correlation Analyses
The results of correlation analysis (Spearman) are presented in Table 1. GST shows a statistically significant moderate positive correlation with LPO (p < 0.001), while SOD exhibits a significant moderate positive correlation with TAC (p < 0.0001). Moreover, results indicate a strong significant correlation between LPO and TAC (p < 0.0001) and a significant moderate positive correlation with LPO and UBI (p < 0.001).

Table 1.
Spearman correlation matrix, with relevant correlations highlighted in bold. Significant differences are marked with asterisks. *—p < 0.05, **—p < 0.01, ***—p < 0.001 and ****—p < 0.0001.
4. Discussion
Numerous research studies have focused on the detection of TBBPA and its effects on humans and other animals, including aquatic biota [19,44,45,46,47]. However, to our knowledge, no studies are available on TBBPA effects in marine organisms combining the response of antioxidant enzymes, acetylcholinesterase, caspase-3, and ubiquitin, which are key markers of neurotoxicity [48], apoptosis [49], and the degradation of damaged proteins via the proteasome [50], respectively.
The evaluation of biomarker responses, such as enzymes involved in the biotransformation process (e.g., CYP1A, GST), antioxidant enzymes, and other specific biomarkers, is essential for assessing xenobiotic toxicity in biota and evaluating ecosystem health [51,52]. Therefore, utilizing biomarkers to study the impact of xenobiotics in living organisms is a useful tool, as they provide an early indication on the presence of stressors [53].
The TBBPA concentrations selected for this study include previously reported environmentally relevant concentrations found in water (up to 4.87 µg·L−1) [54]. Still, higher concentrations were also tested to help us assess toxicity in mussels.
GST, an enzyme mainly linked to the detoxification of xenobiotics, increased only in mussels tested to the most elevated concentration (100 μg·L−1 of TBBPA). This suggests that animals can detoxify TBBA at the lower concentrations tested. In a study by Hu et al. [55], who exposed scallops (Chlamys farreri) for ten days followed by another ten days of depuration, it was demonstrated that TBBPA bioaccumulated in mussels’ gills and digestive glands. Furthermore, GST increased in both organs analyzed, suggesting a detoxification response. However, the scallops were exposed to much higher levels than in the current work. Another study by the same author who exposed microalgae to 400 µg·L−1 of TBBPA, which was then used to feed scallops [56], also showed increased GST and SOD and suggested oxidative stress.
Yang et al. [57] conducted exposure tests on freshwater fish (Carassius auratus), finding a significant increase in GST activity following an eight-day exposure assay to 0.5 mg·L−1 TBBPA. An alteration in the regulation of GST gene expression was reported by Gong et al. [58], who observed a rise in GST expression in the gills of C. farreri exposed to TBBPA. Furthermore, Hu et al. [59] exposed C. farreri to TBBPA (varying from 0.2 to 0.8 mg·L−1) and found that GST activity and gene expression levels, but also UDP-glucuronosyltransferase (UGT), increased accordingly with the time and concentration tested, demonstrating its role on TBBPA detoxification. Thus, various studies performed in different aquatic species seem to indicate a consistent increase in GST activity in response to exposure to TBBPA, suggesting activation of xenobiotic detoxification mechanisms. This is also corroborated by the rapid metabolization of TBBPA into hydrophilic conjugates of sulfate and glucuronide, which are easily eliminated by the organisms [60].
However, it should be emphasized that most studies in aquatic animals tested concentrations of TBBPA beyond the most elevated concentration tested in the current work.
The activities of SOD and CAT also support the GST results, as only the highest concentration tested (100 µg·L−1) seems to increase the responses of both antioxidant enzymes determined in exposed mussels, suggesting a response to oxidative stress by eliminating the overproduction of reactive oxygen species (ROS) and neutralizing the excess of H2O2, as these enzymes act together to defend cells against oxidative stress. Indeed, oxidative stress occurs when ROS production exceeds the ability of cells to remove them [61,62]. Noteworthily, TAC results followed the same pattern of antioxidant enzymes, suggesting that GST and antioxidant mechanisms are sufficient to deal with the exposure to lower TBBPA concentrations.
Antioxidant enzymes (e.g., SOD and CAT) act together to protect cells by converting reactive radicals into non-reactive molecules [39] and, thus, avoid the establishment of stress oxidative damage.
Studies by Hu et al. 2015 [55] also reported a substantial elevation in SOD activity in C. farreri tested to higher levels (0.2 to 0.8 mg·L−1) of TBBPA, suggesting that the response of SOD activity to TBBPA exposure may be concentration-dependent. The differences observed in SOD activity in these studies, and the present study, may be attributed to the different concentrations of TBBPA tested.
Studies by Wu et al. [45] on zebrafish (Danio rerio) embryos and larvae tested to 0.1 to 1.0 mg·L−1 of TBBPA revealed no significant alterations in oxidative stress enzymes (SOD, CAT, and Glutathione Peroxidase—GPx), while animals tested at concentrations ranging from 0.4 to 1.0 mg·L−1 showed decreased enzyme activities. Feng et al. [48] reported developmental neurotoxicity in juvenile zebrafish exposed to different concentrations, varying from 0.86 to 193.5 µg·L−1 of TBBPA bis(2-hydroxyethyl) ether (TBBPA-DHEE). Interestingly, they found that females were more susceptible than males. Likewise, a proteomic and metabolomic study carried out in the gills of M. galloprovincialis exposed to TBBPA (10 μg·L−1), for 30 days, showed different responses between males and females, with females being more susceptible. They found changes in nine metabolites, mostly related to energy metabolism and osmotic regulation in females, while in males, alterations were related only to osmotic regulation. Proteomic analyses revealed higher levels of proteins linked to energy metabolism and defense mechanisms in males [63].
Lipid peroxidation (LPO) results from the action of free radicals on cell biological membranes [64]. Therefore, it can be used as a biomarker of cell damage upon exposure to xenobiotics [65]. Oxidative damage following exposure to contaminants is often reported. For instance, Emamnouil et al. [66] showed oxidative damage in Mytilus edulis collected in a U.K. contaminated site. However, the mussels were able to recover after depuration. In this work, LPO exhibited a trend to rise according to tested concentrations. However, this was only statistically significant for mussels tested to 100 µg·L−1 of TBBPA, which is in accordance with antioxidant enzyme levels and supports the hypothesis that cells’ antioxidant mechanisms are enough to fight oxidative stress at the lower TBBPA concentrations tested but can lead to oxidative stress in mussels tested to the most elevated concentration. Our findings are consistent with previous research conducted on zebrafish embryos, where an increase in LPO was observed, suggesting oxidative stress [67].
AChE has a central role in the proper functioning of neurotransmission and muscle contraction, and is used as a marker of neurotoxicity [68]. In the current work, mussels tested at lower levels of TBBPA (1 and 10 μg·L−1) displayed a reduction in AChE activity. However, after the animals were tested to 100 μg·L−1 of TBBPA, there was a significant increase in AChE activity. This behavior is not clear, but may suggest that exposure to different levels of TBBPA can have different effects on enzyme activity. The elevation in AChE noticed in animals tested to the highest TBBPA concentration is consistent with findings by Zhu et al. [69] in zebrafish and with Liu et al. [70], who reported increased AChE in rat pheochromocytoma cells exposed to TBBPA. The rise in AChE activities was associated with ROS and apoptosis [71], which is consistent with our results in mussels exposed to the highest TBBPA concentration. Interestingly, some studies reported increased AChE activity and lipid peroxidation in fish containing microplastics, which authors attributed to the disruption of vesicles containing acetylcholine [72,73].
Caspase-3 holds a critical role in the regulation of apoptosis or programmed cell death, being activated during the apoptotic process, which is essential for removing unwanted or damaged cells from the body [74], while ubiquitin plays an important role in marking proteins for degradation [75]. In addition, the imbalance of the ubiquitin-proteasome system has been associated with various diseases (e.g., tumors, neurodegenerative disorders, and inflammation) [76].
Research conducted in zebrafish larvae and embryos exposed to TBBPA showed developmental toxicity due to oxidative stress and apoptosis [45]. Moreover, Han et al. [77] reported that TBBPA also caused apoptosis, mitochondrial dysfunction, and increased ROS in carp hepatocytes after exposure. Interestingly, it was also shown that degraded TBBPA (e.g., monoBr-BPA, diBr-BPA, and triBr-BPA) presented stronger toxicity to HeLa cells than parent TBBPA, leading to apoptosis [78]. Our results suggest that exposing mussels to 100 µg·L−1 of TBBPA raised the production of both caspase-3 and ubiquitin, suggesting that this concentration can lead to apoptosis and increased damaged proteins. No-significant changes were observed for caspase-3 and ubiquitin in mussels exposed to 1 and 10 µg·L−1, which may suggest that these concentrations are not enough to cause significant apoptosis and protein damaging.
Overall, the results suggest that when considering environmentally relevant concentrations, significant oxidative stress it is not expected to occur in mussels. Nonetheless, we must consider TBBPA bioaccumulation in the marine biota. While some authors reported moderate TBBPA bioaccumulation [79], others stated that this compound is rapidly absorbed and accumulates in several aquatic species [80]. The potential for trophic transfer and bioamplification should also be emphasised as a risk to the aquatic ecosystem [13]. Finally, there should be greater concern about the consumption of fishery products, as several studies have unequivocally shown levels of TBBPA that could impact human health [3,81].
5. Conclusions
The strong positive correlation between TAC and some biomarkers (SOD and LPO) indicates that the organism’s global antioxidant capacity is linked to the response of these biomarkers, suggesting that when faced with oxidative stress, antioxidant mechanisms are activated to neutralize oxidative stress and its adverse effects. The biomarker responses of animals tested at 1 and 10 µg·L−1 are low or moderate and no noteworthy effects were detected at these concentrations. However, animals tested to the most elevated concentration of TBBPA (100 µg·L−1) showed more pronounced responses that can lead to oxidative stress and concomitant cellular damage. Although the higher concentrations tested are not considered environmentally relevant, they help to understand the effect on biota exposed to TBBPA and the risk if higher quantities of this compound are discharged into the environment.
Author Contributions
Conceptualization, M.D. and S.C.; methodology, M.D., S.G., I.J.F. and S.C.; writing—original draft preparation, S.G., S.C., M.S. and M.D.; writing—review and editing, supervision, S.C., M.S., M.D., C.M. and I.J.F.; project administration, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences, UCIBIO; the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy, i4HB; and by the Associate Laboratory for Green Chemistry, LAQV, which is financed by national funds from FCT/MCTES (UID/QUI/50006/2019).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of FCT NOVA and by national authorities ICNF (SC-044726/2023) and DGRM; PT2024OPCM00674901.
Data Availability Statement
All data is presented in the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gereckea, A.C.; Schmid, P.; Bogdal, C.; Kohler, M.; Zennegg, M.; Heeb, N.V. Brominated Flame Retardants—Endocrine-Disrupting Chemicals in the Swiss Environment. Chimia 2008, 62, 352. [Google Scholar] [CrossRef]
- Fabbri, R.; Montagna, M.; Balbi, T.; Raffo, E.; Palumbo, F.; Canesi, L. Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in Mytilus galloprovincialis. Mar. Environ. Res. 2014, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poma, G.; Malysheva, S.V.; Goscinny, S.; Malarvannan, G.; Voorspoels, S.; Covaci, A.; Van Loco, J. Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere 2018, 194, 256–265. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Li, X.; Sha, W.; Xue, Z.; Zhou, Z.; Ma, Y.; Zhu, S.; Guo, Z.; Zhao, B.; et al. Toxicological evaluation of TBBPA by common carp (Cyprinus carpio) about the in vivo/vitro disturbance of the AHR pathway. Sci. Total Environ. 2023, 904, 166622. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, H.; Yu, H.; Wang, X.; Wu, J.; Xue, Y. Bioaccumulation and physiological effects of tetrabromobisphenol A in coontail Ceratophyllum demersum L. Chemosphere 2008, 70, 1787–1795. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, Z.; Qu, G.; Shen, Z.; Liang, X.; Shi, J.; Jiang, G. Identification of transformation/degradation products of tetrabromobisphenol A and its derivatives. TrAC Trends Anal. Chem. 2019, 111, 85–99. [Google Scholar] [CrossRef]
- Blanco, E.; Casais, M.C.; Mejuto, M.C.; Cela, R. Analysis of tetrabromobisphenol A and other phenolic compounds in water samples by non-aqueous capillary electrophoresis coupled to photodiode array ultraviolet detection. J. Chromatogr. A 2005, 1071, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-W.; Yan, Z.-G.; Xu, F.-F.; Wang, S.-R.; Wu, F.-C. Development of freshwater aquatic life criteria for Tetrabromobisphenol A in China. Environ. Pollut. 2012, 169, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Saint-Louis, R.; Pelletier, E. LC-ESI-MS-MS method for the analysis of tetrabromobisphenol A in sediment and sewage sludge. Analyst 2004, 129, 724–730. [Google Scholar] [CrossRef]
- OSPAR Convention (Convention for the Protection of the Marine Environment of the North-East Atlantic). Available online: https://www.ospar.org/site/assets/files/1169/ospar_convention.pdf (accessed on 5 January 2024).
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Maulvault, A.L.; Tediosi, A.; Fernández-Tejedor, M.; Van den Heuvel, F.; Kotterman, M.; Marques, A.; Barceló, D. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ. Res. 2015, 143, 56–64. [Google Scholar] [CrossRef]
- Kotthoff, M.; Rüdel, H.; Jürling, H. Detection of tetrabromobisphenol A and its mono- and dimethyl derivatives in fish, sediment and suspended particulate matter from European freshwaters and estuaries. Anal. Bioanal. Chem. 2017, 409, 3685–3694. [Google Scholar] [CrossRef]
- Sun, C.-S.; Yuan, S.-W.; Hou, R.; Zhang, S.-Q.; Huang, Q.-Y.; Lin, L.; Li, H.-X.; Liu, S.; Cheng, Y.-Y.; Li, Z.-H.; et al. First insights into the bioaccumulation, biotransformation and trophic transfer of typical tetrabromobisphenol A (TBBPA) analogues along a simulated aquatic food chain. J. Hazard. Mater. 2024, 465, 133390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Jeong, Y.; Kim, D.; Kang, G.-J.; Kang, Y. Assessment of Tetrabromobisphenol and Hexabromocyclododecanes exposure and risk characterization using occurrence data in foods. Food Chem. Toxicol. 2020, 137, 111121. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, C.; Li, F.; Zhan, J.; Sun, T.; Tang, J.; Wu, H. Tetrabromobisphenol A induced reproductive endocrine-disrupting effects in mussel Mytilus galloprovincialis. J. Hazard. Mater. 2021, 416, 126228. [Google Scholar] [CrossRef]
- Mariussen, E.; Fonnum, F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Int. 2003, 43, 533–542. [Google Scholar] [CrossRef]
- Pullen, S.; Boecker, R.; Tiegs, G. The flame retardants tetrabromobisphenol A and tetrabromobisphenol A–bisallylether suppress the induction of interleukin-2 receptor α chain (CD25) in murine splenocytes. Toxicology 2003, 184, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Ito, Y.; Yamaguchi, M.; Mitumori, K.; Koizumi, M.; Hasegawa, R.; Kamata, E.; Ema, M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004, 150, 145–155. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; Sun, P.; Wang, Z.; Wang, L. Acute and chronic toxicity of tetrabromobisphenol A to three aquatic species under different pH conditions. Aquat. Toxicol. 2015, 164, 145–154. [Google Scholar] [CrossRef]
- Pittinger, C.A.; Pecquet, A.M. Review of historical aquatic toxicity and bioconcentration data for the brominated flame retardant tetrabromobisphenol A (TBBPA): Effects to fish, invertebrates, algae, and microbial communities. Environ. Sci. Pollut. R. 2018, 25, 14361–14372. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Ren, C.; Li, F.; Xu, Y.; Wu, H.; Ji, C. Time- and dose-dependent detoxification and reproductive endocrine disruption induced by tetrabromobisphenol A (TBBPA) in mussel Mytilus galloprovincialis. Mar. Environ. Res. 2023, 183, 105839. [Google Scholar] [CrossRef]
- Jiang, S.; Miao, J.; Wang, X.; Liu, P.; Pan, L. Inhibition of growth in juvenile manila clam Ruditapes philippinarum: Potential adverse outcome pathway of TBBPA. Chemosphere 2019, 224, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, K.; Mlouka, R.; Clerandeau, C.; Banni, M.; Cachot, J. Natural distribution of pure and hybrid Mytilus sp. along the south Mediterranean and North-east Atlantic coasts and sensitivity of D-larvae stages to temperature increases and metal pollution. Sci. Total Environ. 2021, 756, 143675. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Farrington, J.W.; Tripp, B.W.; Tanabe, S.; Subramanian, A.; Sericano, J.L.; Wade, T.L.; Knap, A.H.; Edward, D. Goldberg’s proposal of “the Mussel Watch”: Reflections after 40 years. Mar. Pollut. Bull. 2016, 110, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Cajaraville, M.P.; Bebianno, M.J.; Blasco, J.; Porte, C.; Sarasquete, C.; Viarengo, A. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci. Total Environ. 2000, 247, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Gagné, F.; Marin, M.G.; Ricciardi, F.; Blaise, C. Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates: A review. Environ. Int. 2008, 34, 531–545. [Google Scholar] [CrossRef] [PubMed]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Phar 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Almroth, B.C.; Sturve, J.; Berglund, Å.; Förlin, L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 2005, 73, 171–180. [Google Scholar] [CrossRef]
- Borković, S.S.; Šaponjić, J.S.; Pavlović, S.Z.; Blagojević, D.P.; Milošević, S.M.; Kovačević, T.B.; Radojičić, R.M.; Spasić, M.B.; Žikić, R.V.; Saičić, Z.S. The activity of antioxidant defence enzymes in the mussel Mytilus galloprovincialis from the Adriatic Sea. Comp. Biochem. Phys. C 2005, 141, 366–374. [Google Scholar] [CrossRef]
- Rodrigues, I.; Ferreira, I.J.; Duarte, R.M.B.O.; Diniz, M. Effects of Exposure to Urban Atmospheric Particulate Matter Suspended in Seawater on the Mussel Mytilus galloprovincialis. Environments 2024, 11, 12. [Google Scholar] [CrossRef]
- Afsa, S.; De Marco, G.; Giannetto, A.; Parrino, V.; Cappello, T.; ben Mansour, H.; Maisano, M. Histological endpoints and oxidative stress transcriptional responses in the Mediterranean mussel Mytilus galloprovincialis exposed to realistic doses of salicylic acid. Environ. Toxicol. Phar 2022, 92, 103855. [Google Scholar] [CrossRef] [PubMed]
- Chora, S.; McDonagh, B.; Sheehan, D.; Starita-Geribaldi, M.; Roméo, M.; Bebianno, M.J. Ubiquitination and carbonylation as markers of oxidative-stress in Ruditapes decussatus. Mar. Environ. Res. 2008, 66, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.H.; Håkan Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Leal, M.C.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal stress and energy metabolism in two circumtropical decapod crustaceans: Responses to acute temperature events. Mar. Environ. Res. 2018, 141, 148–158. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W.; Hibino, Y.; Hitomi, Y.; Nakamura, H.; Ogino, K. Efficient Assay for Total Antioxidant Capacity in Human Plasma Using a 96-Well Microplate. J. Clin. Biochem. Nutr. 2008, 44, 46–51. [Google Scholar] [CrossRef]
- Lopes, A.R.; Sampaio, E.; Santos, C.; Couto, A.; Pegado, M.R.; Diniz, M.; Munday, P.L.; Rummer, J.L.; Rosa, R. Absence of cellular damage in tropical newly hatched sharks (Chiloscyllium plagiosum) under ocean acidification conditions. Cell Stress Chaperon 2018, 23, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Lorusso, L.C.; Ciacci, C.; Betti, M.; Gallo, G. Effects of the brominated flame retardant tetrabromobisphenol-A (TBBPA) on cell signaling and function of Mytilus hemocytes: Involvement of MAP kinases and protein kinase C. Aquat. Toxicol. 2005, 75, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 2016, 31, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Jarosiewicz, M.; Krokosz, A.; Marczak, A.; Bukowska, B. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere 2019, 227, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hao, C.; Xiang, M.; Tian, J.; Kuang, H.; Li, Z. Potential obesogenic effects of TBBPA and its alternatives TBBPS and TCBPA revealed by metabolic perturbations in human hepatoma cells. Sci. Total Environ. 2022, 832, 154847. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xu, T.; Zuo, J.; Luo, M.; Mao, G.; Chen, Y.; Ding, Y.; Okeke, E.S.; Wu, X.; Yang, L. The potential mechanisms of TBBPA bis(2-hydroxyethyl) ether induced developmental neurotoxicity in juvenile zebrafish (Danio rerio). Comp. Biochem. Phys. C 2023, 265, 109530. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087312. [Google Scholar] [CrossRef]
- Abbas, R.; Larisch, S. Killing by Degradation: Regulation of Apoptosis by the Ubiquitin-Proteasome-System. Cells 2021, 10, 3465. [Google Scholar] [CrossRef]
- Gil, F.; Pla, A. Biomarkers as biological indicators of xenobiotic exposure. J. Appl. Toxicol. 2001, 21, 245–255. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. Biomarkers based tools to assess environmental and chemical stressors in aquatic systems. Ecol. Indic. 2021, 122, 107207. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, H.; Yan, Z. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. R. 2012, 19, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Pan, L.; Xiu, M.; Jin, Q. Exposure of Chlamys farreri to tetrabromobisphenol A: Accumulation and multibiomarker responses. Environ. Sci. Pollut. R. 2015, 22, 12224–12234. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Pan, L.; Xiu, M.; Liu, D. Dietary accumulation of tetrabromobisphenol A and its effects on the scallop Chlamys farreri. Comp. Biochem. Phys. C 2015, 167, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, F.; Zheng, B.; Wu, F.; Wang, S. Multibiomarker responses upon exposure to tetrabromobisphenol A in the freshwater fish Carassius auratus. Aquat. Toxicol. 2013, 142–143, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Pan, L.; Miao, J.; Liu, N. Application of SSH and quantitative real time PCR to construction of gene expression profiles from scallop Chlamys farreri in response to exposure to tetrabromobisphenol A. Environ. Toxicol. Phar 2012, 34, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Pan, L.; Xiu, M.; Jin, Q.; Wang, G.; Wang, C. Bioaccumulation and detoxification responses in the scallop Chlamys farreri exposed to tetrabromobisphenol A (TBBPA). Environ. Toxicol. Phar 2015, 39, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Fini, J.-B.; Riu, A.; Debrauwer, L.; Hillenweck, A.; Le Mével, S.; Chevolleau, S.; Boulahtouf, A.; Palmier, K.; Balaguer, P.; Cravedi, J.-P.; et al. Parallel Biotransformation of Tetrabromobisphenol A in Xenopus laevis and Mammals: Xenopus as a Model for Endocrine Perturbation Studies. Toxicol. Sci. 2012, 125, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.A.; Bainy, A.C.D.; Dafre, A.L.; Gomes, O.F.; Medeiros, M.H.G.; Di Mascio, P. Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J. Exp. Mar. Biol. Ecol. 2005, 318, 21–30. [Google Scholar] [CrossRef]
- López-Barea, J.; Pueyo, C. Mutagen content and metabolic activation of promutagens by molluscs as biomarkers of marine pollution. Mutat. Res. 1998, 399, 3–15. [Google Scholar] [CrossRef]
- Ji, C.; Li, F.; Wang, Q.; Zhao, J.; Sun, Z.; Wu, H. An integrated proteomic and metabolomic study on the gender-specific responses of mussels Mytilus galloprovincialis to tetrabromobisphenol A (TBBPA). Chemosphere 2016, 144, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Oruç, E.Ö.; Usta, D. Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ. Toxicol. Phar 2007, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.J.; Meneses, L.; Paiva, A.; Diniz, M.; Duarte, A.R.C. Assessment of deep eutectic solvents toxicity in zebrafish (Danio rerio). Chemosphere 2022, 299, 134415. [Google Scholar] [CrossRef]
- Emmanouil, C.; Green, R.M.; Willey, F.R.; Chipman, J.K. Oxidative damage in gill of Mytilus edulis from Merseyside, UK, and reversibility after depuration. Environ. Pollut. 2008, 151, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liang, Y.; Chen, M.; Wang, X. Assessing the toxicity of TBBPA and HBCD by zebrafish embryo toxicity assay and biomarker analysis. Environ. Toxicol. 2009, 24, 334–342. [Google Scholar] [CrossRef]
- Matozzo, V.; Tomei, A.; Marin, M.G. Acetylcholinesterase as a biomarker of exposure to neurotoxic compounds in the clam Ruditapes philippinarum from the Lagoon of Venice. Mar. Pollut. Bull. 2005, 50, 1686–1693. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, G.; Yang, L.; Zhou, B. Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere 2018, 197, 353–361. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, X.; Long, Y.; Hu, L.; Qu, G.; Zhou, Q.; Jiang, G. The potential neurotoxicity of emerging tetrabromobisphenol A derivatives based on rat pheochromocytoma cells. Chemosphere 2016, 154, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.B.; Agostinho, P.; Oliveira, C.R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci. Res. 2003, 45, 117–127. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- De Marco, G.; Eliso, M.C.; Oliveri Conti, G.; Galati, M.; Billè, B.; Maisano, M.; Ferrante, M.; Cappello, T. Short-term exposure to polystyrene microplastics hampers the cellular function of gills in the Mediterranean mussel Mytilus galloprovincialis. Aquat. Toxicol. 2023, 264, 106736. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Liu, Y.Q.; Cui, Y.F. Pathways to caspase activation. Cell Biol. Int. 2005, 29, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, M. Ubiquitin and intracellular protein degradation. Curr. Opin. Cell Biol. 1992, 4, 1024–1031. [Google Scholar] [CrossRef]
- Shang, F.; Gong, X.; Taylor, A. Activity of Ubiquitin-dependent Pathway in Response to Oxidative Stress: Ubiquitin-activating enzyme is transiently up-regulated. J. Biol. Chem. 1997, 272, 23086–23093. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yang, N.; Liu, H.; Yao, Y.; Xu, S. TBBPA causes apoptosis in grass carp hepatocytes involving destroyed ER-mitochondrial function. Chemosphere 2023, 341, 139974. [Google Scholar] [CrossRef] [PubMed]
- Suyama, K.; Kesamaru, H.; Okubo, T.; Kasatani, K.; Tomohara, K.; Matsushima, A.; Nose, T. High cytotoxicity of a degraded TBBPA, dibromobisphenol A, through apoptotic and necrosis pathways. Heliyon 2023, 9, e13003. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Z.; Zhang, T.; Yu, N.; Su, G.; Giesy, J.P.; Yu, H. Pharmacokinetics and effects of tetrabromobisphenol a (TBBPA) to early life stages of zebrafish (Danio rerio). Chemosphere 2018, 190, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Yakubu, S.; Zhu, Q.; Issaka, E.; Zhang, Y.; Adams, M. A Review on Tetrabromobisphenol A: Human Biomonitoring, Toxicity, Detection and Treatment in the Environment. Molecules 2023, 28, 2505. [Google Scholar] [CrossRef]
- Cunha, S.C.; Menezes-Sousa, D.; Mello, F.V.; Miranda, J.A.T.; Fogaca, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Survey on endocrine-disrupting chemicals in seafood: Occurrence and distribution. Environ. Res. 2022, 210, 112886. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).