Stability of the Macrocyclic Gd-DOTA Contrast Agent (DOTAREM) under Different Estuarine Environmental Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Solution Preparation
2.2. Exposure to Temperatures
2.3. Exposure to pH 2, 4, 6, 8 and 10
2.4. Exposure to Sunlight
2.5. Matrix Elimination and Pre-Concentration in seaFAST
2.6. Gd Quantification
2.7. Limit of Detection and Limit of Quantification
3. Results
3.1. Effects of Temperature
3.2. Effect of pH
3.2.1. Exposure to pH 4, 6, 8, 10
3.2.2. Exposure to pH 2
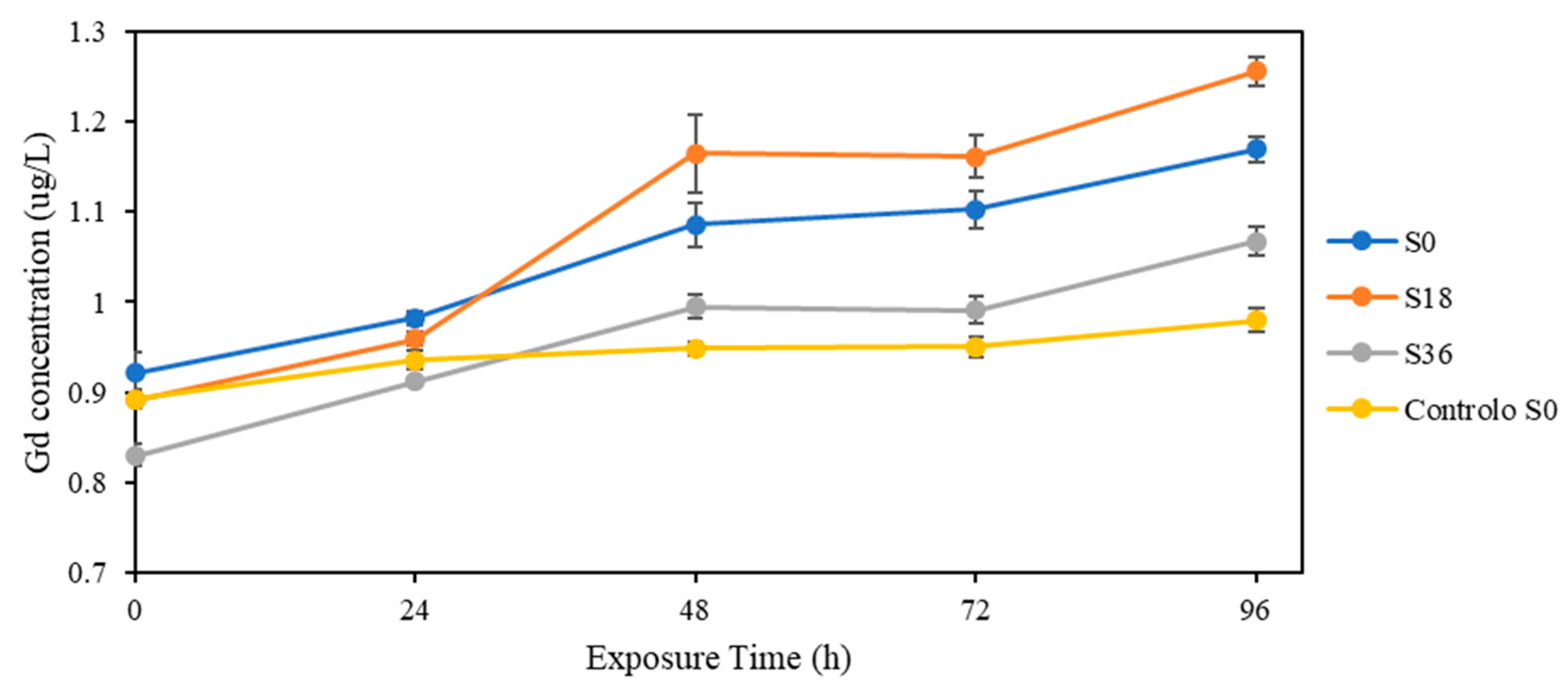
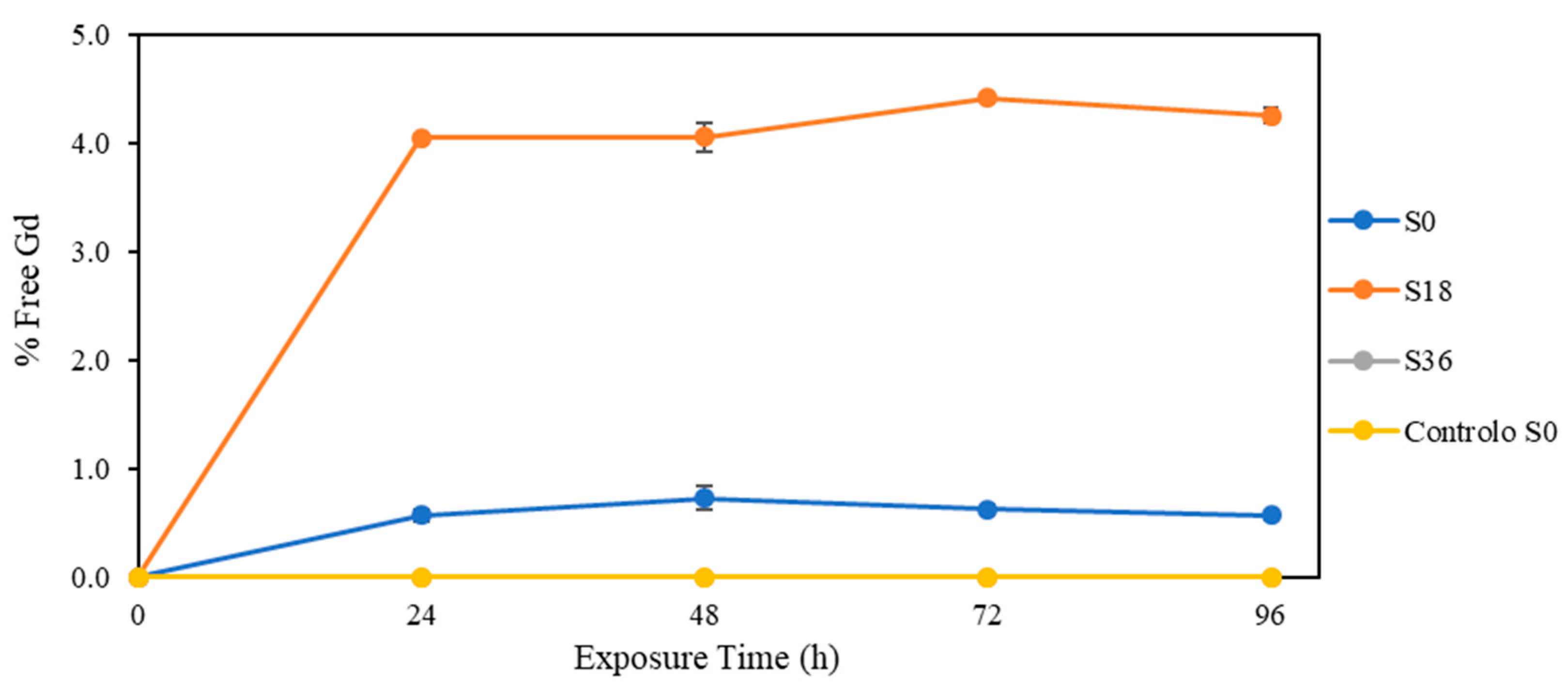
3.3. Effect of Solar Radiation
4. Discussion
4.1. Temperature
4.2. pH
4.3. Salinity
4.4. Solar Radiation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bellin, M.F. MR contrast agents, the old and the new. Eur. J. Radiol. 2006, 60, 314–323. [Google Scholar] [CrossRef]
- Shellock, F.G.; Kanal, E. Safety of magnetic resonance imaging contrast agents. J. Magn. Reson. Imag. 1999, 10, 477–484. [Google Scholar] [CrossRef]
- Tweedle, M.F.; Wedeking, P.; Kumar, K. Biodistribution of Radiolabeled, Formulated Gadopentetate, Gadoteridol, Gadoterate, and Gadodiamide in Mice and Rats. Investig. Radiol. 1995, 30, 372–380. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Robic, C.; Medina, C.; Sabatou, M.; Corot, C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam. Clin. Pharmacol. 2006, 20, 563–576. [Google Scholar] [CrossRef]
- Kanal, E. Gadolinium based contrast agents (GBCA): Safety overview after 3 decades of clinical experience. Magn. Reson. Imaging 2016, 34, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Brücher, E. Kinetic Stabilities of Gadolinium(III) Chelates Used as MRI Contrast Agents. In Top Current Chemestry; Werner, K., Ed.; Springer: Berlin, Germany, 2002; pp. 103–122. [Google Scholar]
- Port, M.; Idée, J.-M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. BioMetals 2008, 21, 469–490. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EMAs-Final-Opinion-Confirms-Restrictions-Use-Linear-Gadolinium-Agents; European Medicines Agency: Amsterdam, The Netherlands, 2017.
- Bau, M.; Dulski, E.P.S.L. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Thomsen, H.S. Are the increasing amounts of gadolinium in surface and tap water dangerous? Acta Radiol. 2017, 58, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Brünjes, R.; Hofmann, T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020, 182, 115966. [Google Scholar] [CrossRef] [PubMed]
- Elbaz-Poulichet, C.; Seidel, J.-L.; Othoniel, C. Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res. 2002, 36, 1102–1105. [Google Scholar] [CrossRef]
- Rabiet, M.; Brissaud, F.; Seidel, J.L.; Pistre, S.; Elbaz-Poulichet, F. Positive gadolinium anomalies in wastewater treatment plant effluents and aquatic environment in the Hérault watershed (South France). Chemosphere 2009, 75, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Klaver, G.; Verheul, M.; Bakker, I.; Petelet-Giraud, E. Négrel Anthropogenic Rare Earth Element in rivers: Gadolinium and lanthanum. Partitioning between the dissolved and particulate phases in the Rhine River and spatial propagation through the Rhine-Meuse Delta (the Netherlands). Appl. Geochem. 2014, 47, 186–197. [Google Scholar] [CrossRef]
- Bau, M.; Knappe, A. Dulski Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States. Chem. Der Erde. 2006, 66, 143–152. [Google Scholar] [CrossRef]
- Knappe, A.; Möller, P.; Dulski, P.; Pekdeger, A. Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chem. Der Erde. 2005, 65, 167–189. [Google Scholar] [CrossRef]
- Da Costa, A.R.B.; Rousseau, T.C.C.; Maia, P.D.; Amorim, A.M.; Sodré, F.F.; Teixeira, C.E. Anthropogenic gadolinium in estuaries and tropical Atlantic coastal waters from Fortaleza, Northeast Brazil. Appl. Geochem. 2021, 127, 104908. [Google Scholar] [CrossRef]
- Nozaki, Y.; Lerche, D.; Alibo, D.S.; Tsutsumi, M. Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: Evidence for anthropogenic Gd and In. Geochim. Cosmochim. Acta 2000, 64, 3975–3982. [Google Scholar] [CrossRef]
- Pereto, C.; Lerat-Hardy, A.; Baudrimont, M.; Coynel, A. European fluxes of medical gadolinium to the ocean: A model based on healthcare databases. Environ. Int. Internet 2023, 173, 107868. [Google Scholar] [CrossRef]
- Braun, M.; Zavanyi, G.; Laczovics, A.; Berényi, E.; Szabó, S. Can aquatic macrophytes be biofilters for gadolinium based contrasting agents? Water Res. 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Henriques, B.; Coppola, F.; Monteiro, R.; Pinto, J.; Viana, T.; Pretti, C.; Soares, A.; Freitas, R.; Pereira, E. Toxicological assessment of anthropogenic Gadolinium in seawater: Biochemical effects in mussels Mytilus galloprovincialis. Sci. Total Environ. 2019, 664, 626–634. [Google Scholar] [CrossRef]
- Perrat, E.; Parant, M.; Py, J.-S.; Rosin, C.; Cossu-Leguille, C. Bioaccumulation of gadolinium in freshwater bivalves. Environ. Sci. Pollut. Res. 2017, 24, 12405–12415. [Google Scholar] [CrossRef] [PubMed]
- Pereto, C.; Coynel, A.; Lerat-Hardy, A.; Gourves, P.-Y.; Schäfer, J.; Baudrimont, M. Corbicula fluminea: A sentinel species for urban Rare Earth Element origin. Sci. Total Environ. 2020, 732, 138552. [Google Scholar] [CrossRef] [PubMed]
- Haneder, S.; Kucharczyk, W.; Schoenberg, S.O.; Michaely, H.J. Safety of Magnetic Resonance Contrast Media: A Review With Special Focus on Nephrogenic Systemic Fibrosis. Top. Magn. Reson. Imaging 2015, 24, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Calabi, L.; Corana, F.; Fontana, S.; Losi, P.; Maiocchi, A.; Paleari, M.; Valtancoli, B. Thermodynamic and structural properties of Gd(III) complexes with polyamino-polycarboxylic ligands: Basic compounds for the development of MRI contrast agents [Internet]. Coord. Chem. Rev. 2000, 204, 292–309. [Google Scholar] [CrossRef]
- Brookman, B.; Mann, I. Eurachem Guide: Selection, Use and Interpretation of Proficiency Testing (PT) Schemes, 3rd ed.; Laboratory of the Government Chemis: Middlesex, UK, 2021. [Google Scholar]
- Ribeiro, A.R.; Pedrosa, M.; Moreira, N.F.F.; Pereira, M.F.R.; Silva, A.M.T. Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU. J. Chromatogr. A 2015, 1418, 140–149. [Google Scholar] [CrossRef]
- Wysocka, I.A.; Rogowska, A.M.; Kostrz-Sikora, A. Investigation of anthropogenic gadolinium in tap water of polish cities: Gdańsk, Kraków, Warszawa, and Wrocław. Environ. Pollut. 2023, 323, 121289. [Google Scholar] [CrossRef]
- Bichler, A.; Muellegger, C.; Brünjes, R.; Hofmann, T. Quantification of river water infiltration in shallow aquifers using acesulfame and anthropogenic gadolinium. Hydrol. Process. 2016, 30, 1742–1756. [Google Scholar] [CrossRef]
- Birka, M.; Wehe, C.A.; Hachmöller, O.; Sperling, M.; Karst, U. Tracing gadolinium-based contrast agents from surface water to drinking water by means of speciation analysis. J. Chromatogr. A 2016, 1440, 105–111. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Matthews, J.B.R.; Berger, S.; et al. (Eds.) Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; Routledge: Oxford, UK, 2005. [Google Scholar]
- Candeias, C.; Ávila, P.F.; Da Silva, E.F.; Ferreira, A.; Salgueiro, A.R.; Teixeira, J. Acid mine drainage from the Panasqueira mine and its influence on Zêzere river (Central Portugal). J. Afr. Earth Sci. 2014, 99, 705–712. [Google Scholar] [CrossRef]
- Olías, M.; Cánovas, C.R.; Basallote, M.D.; Lozano, A. Geochemical behaviour of rare earth elements (REE) along a river reach receiving inputs of acid mine drainage. Chem. Geol. 2018, 493, 468–477. [Google Scholar] [CrossRef]
- Wang, X.; Jin, T.; Comblin, V.; Lopez-Mut, A.; Merciny, E.; Desreux, J.F. A kinetic investigation of the lanthanide DOTA chelates. Stability and rates of formation and of dissociation of a macrocyclic gadolinium (III) polyaza polycarboxylic MRI contrast agent. Inorg. Chem. Internet 1992, 31, 1095–1099. [Google Scholar] [CrossRef]
- Birka, M.; Roscher, J.; Holtkamp, M.; Sperling, M.; Karst, U. Investigating the stability of gadolinium-based contrast agents towards UV radiation. Water Res. 2016, 91, 244–250. [Google Scholar] [CrossRef] [PubMed]
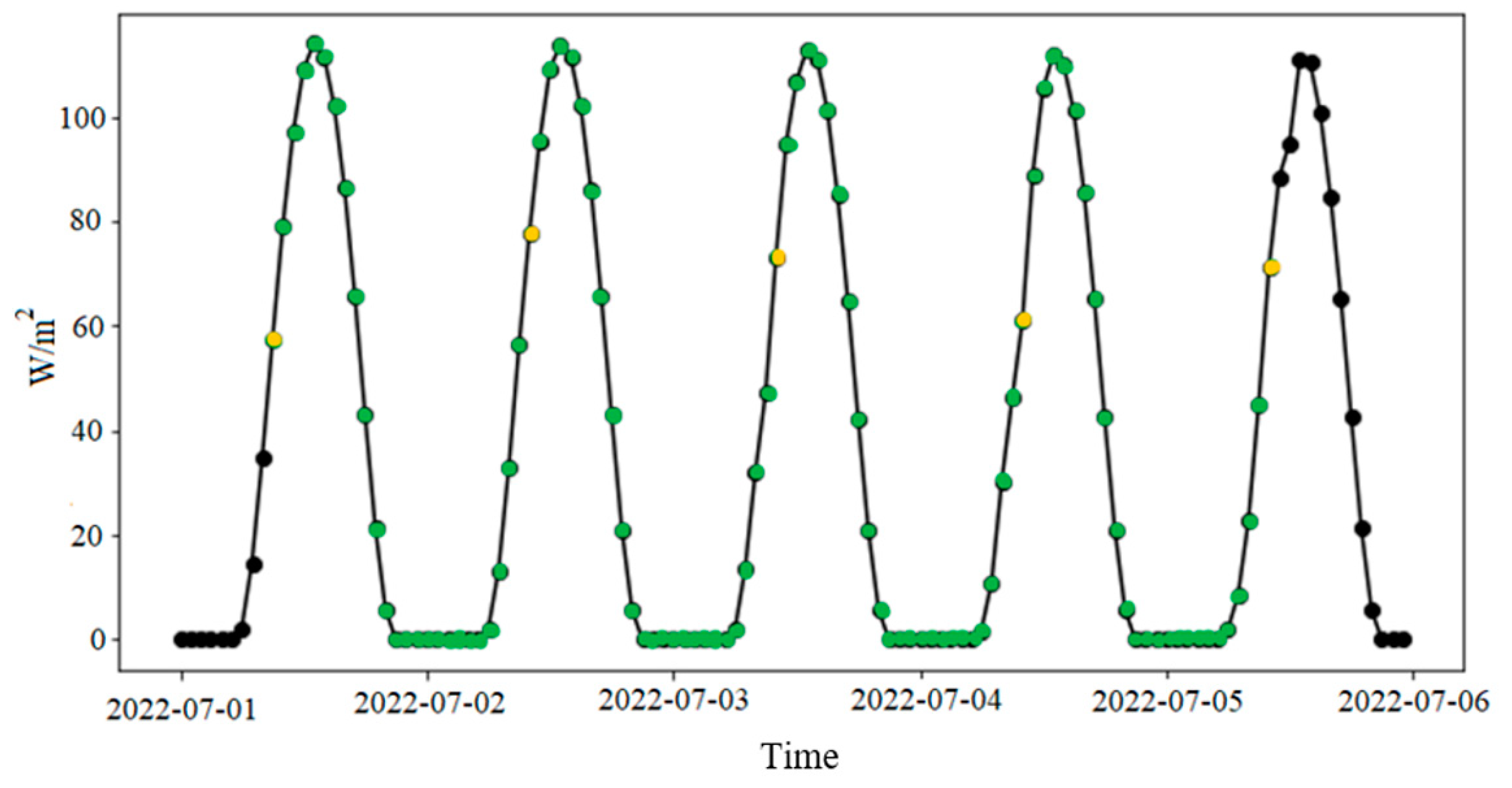

| Exposure Days | UV Daily Mean Irradiance (W/m2) |
|---|---|
| 1st day | 39.2 |
| 2nd day | 38.5 |
| 3rd day | 37.8 |
| 4th day | 36.5 |
| Temperature 15 °C | Temperature 40 °C | ||||||
|---|---|---|---|---|---|---|---|
| Salinity (PSU) | Exposure Time (Days) | [Gd]free (μg/L) | [Gd]total (μg/L) | Salinity (PSU) | Exposure Time (Days) | [Gd]free (μg/L) | [Gd]total (μg/L) |
| 0 | 0 | <LODm | 1.14 ± 0.02 | 0 | 0 | <LODm | 1.19 ± 0.02 |
| 1 | 1 | ||||||
| 2 | 2 | ||||||
| 3 | 3 | ||||||
| 4 | 4 | ||||||
| 18 | 0 | <LODm | 1.02 ± 0.02 | 18 | 0 | <LODm | 1.04 ± 0.03 |
| 1 | 1 | ||||||
| 2 | 2 | ||||||
| 3 | 3 | ||||||
| 4 | 4 | ||||||
| 36 | 0 | <LODm | 0.92 ± 0.07 | 36 | 0 | <LODm | 1.05 ± 0.05 |
| 1 | 1 | ||||||
| 2 | 2 | ||||||
| 3 | 3 | ||||||
| 4 | 4 | ||||||
| Salinity (PSU) | pH | Exposure Time (Days) | [Gd]free (μg/L) | [Gd]total (μg/L) |
|---|---|---|---|---|
| 0 | 4 | 0 | <LODm <LOQm | 1.12 ± 0.02 |
| 36 | ||||
| 6 | 0 | <LODm | ||
| 36 | ||||
| 8 | 0 | |||
| 36 | ||||
| 18 | 4 | 0 | <LODm <LOQm | 1.04 ± 0.03 |
| 36 | ||||
| 6 | 0 | <LODm | ||
| 36 | ||||
| 8 | 0 | |||
| 36 | ||||
| 36 | 4 | 0 | <LODm <LOQm | 1.00 ± 0.04 |
| 36 | ||||
| 6 | 0 | <LODm | ||
| 36 | ||||
| 8 | 0 | |||
| 36 |
| Salinity (PSU) | Exposure Times (Days) | [Gd]free (μg/L) | [Gd]total (μg/L) |
|---|---|---|---|
| 0 | 0 | <LODm | 1.09 ± 0.02 |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 36 | |||
| 18 | 0 | <LODm | 1.00 ± 0.05 |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 36 | |||
| 36 | 0 | <LODm | 0.95 ± 0.03 |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 36 |
| Salinity (PSU) | Exposure Time (Days) | [Gd]average (μg/L) | [Gd]total (μg/L) |
|---|---|---|---|
| 0 | 0 | <LOQm | 1.12 ± 0.02 |
| 36 | 0.31 ± 0.01 | ||
| 1 | <LOQm | 1.15 ± 0.02 | |
| 2 | <LOQm | ||
| 3 | <LOQm | ||
| 4 | 0.007 ± 3 × 10−4 | ||
| 18 | 0.037 ± 0.001 | ||
| 18 | 0 | <LOQm | 1.04 ± 0.03 |
| 36 | 0.074 ± 0.001 | ||
| 1 | <LOQm | 1.04 ± 0.02 | |
| 2 | <LOQm | ||
| 3 | <LOQm | ||
| 4 | 0.006 ± 1 × 10−4 | ||
| 18 | 0.030 ± 0.001 | ||
| 36 | 0 | <LOQm | 1.00 ± 0.04 |
| 36 | 0.039 ± 4 × 10−4 | ||
| 1 | <LOQm | 1.01 ± 0.05 | |
| 2 | 0.006 ± 3 × 10−5 | ||
| 3 | 0.008 ± 0.003 | ||
| 4 | 0.010 ± 8 × 10−5 | ||
| 18 | 0.031 ± 0.001 |
| Salinity | Exposure Time (Days) | [Gd]free (μg/L) | [Gd]total (μg/L) |
|---|---|---|---|
| 0 | 0 | <LODm | 0.94 ± 0.02 |
| 24 | 0.006 ± 0.001 | 1.00 ± 0.01 | |
| 48 | 0.008 ± 0.001 | 1.11 ± 0.03 | |
| 72 | 0.007 ± 0.001 | 1.13 ± 0.02 | |
| 96 | 0.007 ± 0.004 | 1.19 ± 0.01 | |
| 18 | 0 | <LODm | 0.91 ± 0.01 |
| 24 | 0.040 ± 0.0003 | 0.98 ± 0.01 | |
| 48 | 0.048 ± 0.002 | 1.19 ± 0.04 | |
| 72 | 0.052 ± 0.001 | 1.19 ± 0.02 | |
| 96 | 0.054 ± 0.001 | 1.28 ± 0.02 | |
| 36 | 0 | <LODm <LODm | 0.85 ± 0.01 |
| 24 | 0.93 ± 0.00 | ||
| 48 | <LOQm | 1.02 ± 0.01 | |
| 72 | 1.01 ± 0.02 | ||
| 96 | 1.09 ± 0.02 | ||
| Control (S0) | 0 | <LODm | 0.91 ± 0.01 |
| 24 | 0.96 ± 0.01 | ||
| 48 | 0.97 ± 0.01 | ||
| 72 | 0.97 ± 0.01 | ||
| 96 | 1.00 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, A.; Brito, P. Stability of the Macrocyclic Gd-DOTA Contrast Agent (DOTAREM) under Different Estuarine Environmental Conditions. Oceans 2023, 4, 381-393. https://doi.org/10.3390/oceans4040026
Guerreiro A, Brito P. Stability of the Macrocyclic Gd-DOTA Contrast Agent (DOTAREM) under Different Estuarine Environmental Conditions. Oceans. 2023; 4(4):381-393. https://doi.org/10.3390/oceans4040026
Chicago/Turabian StyleGuerreiro, Ana, and Pedro Brito. 2023. "Stability of the Macrocyclic Gd-DOTA Contrast Agent (DOTAREM) under Different Estuarine Environmental Conditions" Oceans 4, no. 4: 381-393. https://doi.org/10.3390/oceans4040026






