Abstract
Analyses of the spatial and temporal patterns of 26 years of stranding events (1995–2011 and 2012–2021, n = 568) in Indonesia were conducted to improve the country’s stranding response. The Emerging Hot Spot Analysis was used to obtain the spatial and temporal hotspot patterns. A total of 92.4% events were single stranding, while the remaining were of mass stranding events. More stranding events were recorded between 2012 and 2021 in more dispersed locations compared to the previous period. Within the constraints of our sampling limitations, East Kalimantan and Bali were single stranding hotspots and consecutive hotspots. East Java and Sabu-Raijua in East Nusa Tenggara were mass stranding hotspots. Temporally, Raja Ampat (West Papua) experienced a significant increase in case numbers. The presence of active NGOs, individuals or government agencies in some locations might have inflated the numbers of reported cases compared to areas with less active institutions and/or individuals. However, our results still give a good understanding of the progression of Indonesia’s stranding responses and good guidance of resource allocation for the stranding network. Several locations in Indonesia that need more efforts (e.g., more training workshops on rescue and necropsies) have been identified in this paper. Suggestions to improve data collection (including georeferencing tips) have also been included.
1. Introduction
Despite there being at least 34 cetacean (whales and dolphins) species in Indonesia [1,2], our understanding of this taxon in the Archipelago is still very limited. With the advent of social media, the public interest in this taxon is steadily increasing, particularly as the public are more frequently exposed to news of cetacean stranding events. Stranding events have helped scientists compiling lists of species utilising certain waters [3,4]. Nevertheless, two questions are often asked by members of the public upon responding to stranding news: why are these animals stranded and what can we do to help them?
While many causes of stranding events are still unknown, a few studies have identified some causes of marine mammal stranding events. By the late 1980s, debris (particularly plastics) started to be detected in marine mammal stomach [5]. Bycatch could be another explanation, where bycaught marine mammals could be discarded alive or dead and eventually stranded [6]. Boat strikes can also injure these animals, which would also lead to stranding events [7]. Pollution may induce tumours or cancer in marine mammals, reducing their immunity and making them susceptible to strandings [8]. There is mounting evidence that the use of Mid Frequency Active Sonar (MFAS), such as those used by submarines, can disturb cetaceans by way of embolism, which could also lead to lethal stranding events [9,10,11,12]. In addition to these anthropogenic explanations, some natural causes were also linked to stranding events, such as solar storms and their prevalence to sperm whale stranding events [13,14,15].
Due to the close associations between the anthropogenic explanations of marine mammal stranding events and global ocean health [16,17,18], stranding events are good proxies to understand the health of the ocean. However, despite the above global explanations of stranding events, only ~34 necropsies have been conducted by 2021 on stranded marine mammals in Indonesia [19]. Of those necropsies, 14 were conducted on East Kalimantan with bycatch as the concluding circumstances of death of four cases (Kreb, pers.comm.). One necropsy of a short-finned pilot whale (Globicephala macrorhynchus) was conducted in East Nusa Tenggara, concluding that the whale died of asphyxiation due to obstruction of the upper airway upon consuming a 1 m long oilfish (Ruvettus pretiosus) [19]. Attempts to discuss the results of necropsies in Indonesia (to answer the question on why the marine mammals were stranded) is not a focus on this paper. However, with a better understanding of the spatial and temporal patterns of the stranding events, efforts to rescue these animals in the country can still be improved, thus, answering the question of how we can help these animals.
Cetacean stranding events in Indonesia were first recorded by the Dutch naturalists during the Dutch colonial era [3,4]. Amongst these rare records were the stranding of a humpback whale on 12 April 1863 in Pekalongan, north Java [4]; 52 presumed short-finned pilot whales in Lhokseumawe, Aceh in 1901/1902; 27 unidentified cetaceans in Nias, Aceh in 1914; 55 short-finned pilot whales in Madura Strait, East Java on 2 January 1923; and a Cuvier’s beaked whale (Ziphius cavirostris) stranded in north Java in 1925/1926 [3]. However, the recording of marine mammal stranding events in modern-era Indonesia only started in the early 2000s, when some researchers (some of them are authors of this paper) independently collated news of marine mammal stranding events from online and offline sources [20].
In October 2012, the stranding of 51 short-finned pilot whales in Sabu-Raijua, East Nusa Tenggara propelled discussion on the need for a national stranding network. In early 2013, the Indonesian government through the Ministry of Marine Affairs and Fisheries (MMAF) started the national stranding network initiative. At the same time, the independent researchers mentioned above launched the already curated stranding data online under the name of Whale Stranding Indonesia (heretofore abbreviated as “WSI”, www.whalestrandingindonesia.com (accessed on 1 February 2022)). Since then, the database has been operating in service of the government-led stranding network initiative.
Between 1995 and 2021, the voluntary database contained at least 638 recorded marine mammal (including the dugongs) stranding events. To improve our stranding response management, we need to understand the spatial and temporal patterns of general stranding events in Indonesia. To that end, we ask the following questions:
- (1)
- Are there spatial hotspots of cetacean strandings, and if yes, where are they?
- (2)
- Are there spatio-temporal trends of cetacean strandings, and if yes, how?
We limit our examination to the cetacean subset of the stranding data due to the expertise of the authors. Thus, we work with 568 cetacean-specific data points from our general database. This study is a part of a larger project on the conservation planning of the cetaceans in Savu Sea (East Nusa Tenggara). The project was under COREMAP-CTI World Bank Program funded by ICCTF (Indonesia Climate Change Trust Fund) Bappenas which is funded by ICCTF (the Indonesian Climate Change Trust Fund) Bappenas for the COREMAP-CTI World Bank program.
2. Materials and Methods
2.1. Study Area and Dataset
The study area of this paper is the entire territory of Indonesia and its 34 provinces (Figure 1). The Archipelago of Indonesia is located between the Indian Ocean and the Pacific Ocean, thus, parts of Indonesia’s waters experience influences from both oceans. Indonesia has the second-longest coastline (108,000 km [21]) in the world after Canada (202,080 km [22]). The inner Indonesian waters have seabeds ranging from very shallow waters of less than 50 m at the western Sunda shelf to more than 3000 m deep, particularly in the eastern Sahul shelf. Many parts of the Archipelago have strong currents, e.g., the waters around the Komodo National Park in East Nusa Tenggara (Figure 1, at the western tip of province #23). The waters between Bali (Figure 1, province #2) and Lombok (Figure 1, at the western tip of province #22) also have strong currents due to the presence of the Indonesian Throughflow that flows through the Lombok Strait towards the Indian Ocean [23]. Consequently, many parts of the Indonesian waters have high productivities, inviting the cetaceans to utilise the areas. The Archipelago has more than 17,500 islands [21], many of them in remote areas in eastern Indonesia, thus, presenting challenges to the rapid response to any stranding events.
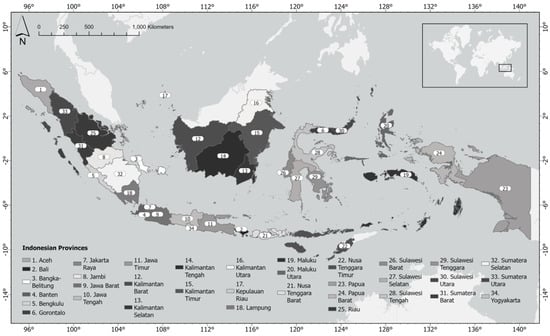
Figure 1.
The provinces in Indonesia. Please consult this map to understand the whereabouts of certain stranding hotspot locations throughout this paper; subsequent maps will not contain provincial names for visual clarity. At the end of June 2022, the Province of Papua (#23) was divided into three new provinces, but no shapefiles were yet available to update #23 into three new provinces as of the end of July 2022.
Based on the stranding event codification of Geraci and Lounsbury [24], the majority (81.83%) of the cetacean stranding events in Indonesia were of codes 1–3, i.e., they were either alive (Code 1, 19.05%), freshly dead (Code 2, 23.1%), or in the early phase of decomposition (Code 3, 39.68%). About 18% of the events were of Code 4 (advance decomposition) and 0.18% were of Code 5 (mummified or skeleton), while the rest of the data points have no sufficient information for coding. Since this paper is not an attempt to explain the covariates or predictors of a stranding event (which would require only codes 1 or 2 specimens for precision), we included events involving all stranding codes in our analysis.
Our dataset contains 26 years of data (1995 to 2021) collected by Whale Stranding Indonesia from various sources acknowledged in the Whale Stranding Indonesia website. The information caused reporting bias because most data points were based on reports instead of generated by observers actively scanning beaches regularly for stranding events. Since more reporting has been observed as the stranding network became more active, we considered the need to divide the analysis between a period when the stranding network had not been established and a period following its establishment.
In 2012, prior to the online publication of the Whale Stranding Indonesia database, the efforts to collect data were intensified. In the same year, the MMAF gathered stakeholders to establish the national stranding network in the following year of 2013. Therefore, we split the analytical periods to pre-2012 (i.e., 1995–2011) and 2012 onwards (2012–2021) to correct for biased sampling effort. We did not combine the two periods into an “all-years model” because of the very different data collection efforts between the two periods. We had a total of 568 data points of cetacean stranding events, 124 from 1995 to 2011 and 444 from 2012 to 2021. We used R ver. 4.2.1. in RStudio to calculate the monthly variabilities of stranding events in Indonesia with Kruskal–Wallis tests.
We also split the models into single stranding events and mass stranding events because the efforts to rescue these two stranding events can be very different due to the number of animals involved. Here, single stranding events are defined as events with only one stranded animal or a pair of mother and calf, and mass stranding events as events with more than one stranded animal, excluding a pair of mother and calf [24]. Despite this division, there were four stranding events involving four Cuvier’s beaked whales (Ziphius cavirostris) in north Bali on 8 August 2015 that were grouped as single stranding events, but these whales actually stranded at the same day around the same time in mid-morning local time. Therefore, these four particular events will be examined in the Discussion when considering our mass stranding models.
2.2. Spatial and Temporal Analyses
All strandings were plotted using ArcGIS 10.6 (ESRI Inc., Redlands, CA, USA) to investigate the spatio-temporal patterns of the stranding events. Following Betty et al. [25], several distance classes were tested (increments from 5, 15, 20, 50, 100, 150, and 200 km) to understand if stranding locations were dispersed, random, or clustered, resulting in 50 km as the best distance class for subsequent analyses. A kernel density analysis [26,27] was used to examine the spatial pattern of stranding marine mammals in Indonesia with a distance of 50 km. We then used the Global Moran’s Index spatial autocorrelation highest z-score (an indication of clustering patterns [28]) to decide the fixed distance bands for pre-2012 (200 km) and 2012 onward (100 km), in order to eventually evaluate the spatial autocorrelation patterns of our stranding events for each model.
We conducted a cluster analysis [29] to determine if hotspot locations for individual and mass stranding events correspond to locations with high incidents in both time periods. The term ‘hot spot’, an area that has high occurrences compared to neighbouring locations, has been widely used, from biodiversity, to crime and emergency reports, forestry loss, disease outbreaks and marine mammal strandings studies [30,31,32,33]. By applying the fixed distance bands, the ‘Optimized Hot Spot’ geoprocessing tool used the Getis-Ord Gi* statistics to identify the areas of significant hotspots in three confidence intervals (90% CI, 95% CI, and 99% CI).
By using the ‘Emerging Hot Spot’ geoprocessing tool, we were able to investigate the spatio-temporal patterns of marine mammal strandings at each location. The ‘Emerging hot spot’ tool has been used to identify the hotspots and baseline stranding patterns of marine mammals globally, which can lead to improving the surveillance and monitoring and informing management [25,34,35,36]. This tool can combine both the Getis-Ord Gi* statistics, which can be used to identify the degree of spatial patterns, and the Mann–Kendall statistics [25], which investigate the temporal trends across both pre-2012 and 2012 onwards. A Network Common Data Form (netCDF) file was formed to create a space–time cube, which aggregated the stranding data points throughout Indonesia with a resolution of 50 km and neighbourhood distances 200 km and 100 km for pre-2012 and 2012 onwards, respectively. This space–time cube assigned latitude and longitude coordinates (X, Y) and the time in year (Z).
First, the tool generated the Getis-Ord Gi* statistic, which measured the concentration of the spatial trends that made up the hotspots and cold spots (counts of marine mammal strandings) relative to neighbouring bins within the space–time cube. The Getis-Ord Gi* statistic gave a z-score for each bin in the space–time cube. The Gi* statistics were measured, and the total value was compared between neighbouring bins and the overall sum of all bins.
Significant positive z-scores, or standard deviations, translate to hotspots [37]. The higher the z-score or standard deviation value, the more intense the hotspot, and vice versa for cold spots. The null hypothesis was no spatial clusters of marine mammal stranding events, with the expected sum of zero. A z-score with a value greater than 1.96 or less than −1.96 inferred significant hotspots or cold spots of marine mammal stranding events at a significance level of p < 0.05.
Once the Gi* was computed, a Mann–Kendall trends test was used to assess the temporal trends for the two time series with the given z-scores from the previous Gi* statistic. This test investigated correlations between the Getis-Ord Gi* values from every bin with data that represented an independent time series, where each time series was compared to the subsequent time series [28]. Each pair was compared over the two time series, in order to generate the Mann–Kendall statistic with the associated z-score and p-value for every bin [32]. The null hypothesis was that there were no trends within the marine mammal strandings events over time, with the expected sum of zero. The observed sum (based on the variance from the bin time series and the number of time steps) was then compared to the expected sum, or zero, to determine the statistical significance of the difference (p < 0.05).
3. Results
3.1. Descriptive Statistics of the Stranding Events
More stranding events were recorded from 2012 onwards (Figure 2). The highest number of stranding events were observed in March and August, while December had the lowest observed number of stranding events (Figure 3). There was no significant difference amongst these observations (Kruskal–Wallis chi-squared = 3.8947, df = 11, p-value = 0.9729). Of the 568 stranding events, 92.4% (525 events) were single strandings, while the remaining (7.6%, 43 events) were of mass stranding events. The re-floating rate for single stranding events in Indonesia was ~74.8%, but it dropped to only 52.03% for the mass stranding events. More than 52% of the total number of mass stranding individuals ended up dead during the rescue events.
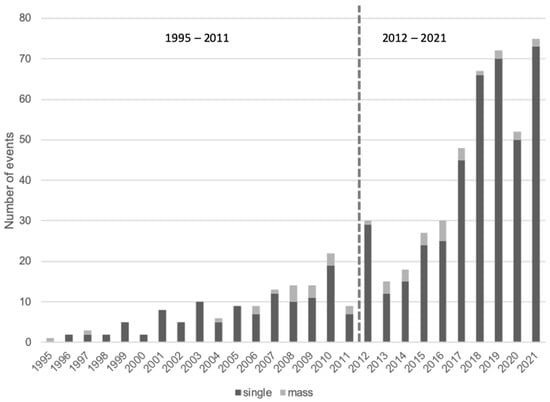
Figure 2.
The annual trend of cetacean stranding events in Indonesia.
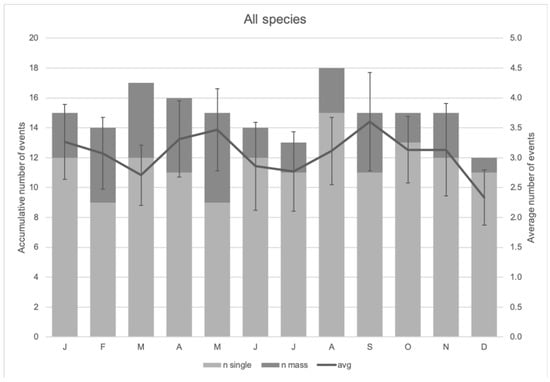
Figure 3.
The monthly trend of cetacean stranding events in Indonesia.
At least 26 species were observed in the 1995–2021 stranding events. A table of species-specific stranding events is available in the Supplementary Material, containing a recap of the number of events per species (Table S1). Out of the 568 cetacean stranding events, 16.02% of them (91 events) were unidentified species, but the number of uncertain cases increased when including unidentified baleen whales (two events, 0.35%) and unidentified delphinids (two events, 0.35%) to the total of 16.7%. The Irrawaddy dolphins (Orcaella brevirostris, mostly along the Mahakam River, East Kalimantan (province #16 in Figure 1) were the most-frequently recorded stranded species with 102 events (17.95%), followed by sperm whales (Physeter macrocephalus, 75 events, 13.20%), short-finned pilot whales (31 events, 5.46%) and spinner dolphins (Stenella longirostris, 29 events, 5.11%).
Figure 4 describes the spatial stranding patterns of six selected cetacean species in the country. Some of the species were selected because they were considered threatened in the IUCN Red List (i.e., the Irrawaddy dolphin (EN, IUCN Red List ver, 3.1), the Indo-Pacific finless porpoise (Neophocaena phocaenoides, VU, IUCN Red List ver. 3.1), and sperm whale (VU, IUCN Red List ver. 3.1)). The Bryde’s whale (LC, IUCN Red List ver. 3.1) was selected because it is a large whale of public interest. The Cuvier’s beaked whale (LC, IUCN Red List ver. 3.1) was selected for its propensity to be influenced by man-made sonar [10,38]. The short-finned pilot whale (LC, IUCN Red List ver. 3.1) was selected for its propensity to mass strandings in the country, thus, requiring a lot of resources to rescue. No differentiation between single and mass stranding events were made in Figure 4. However, some subsequent figures have segregations between single and mass stranding events.
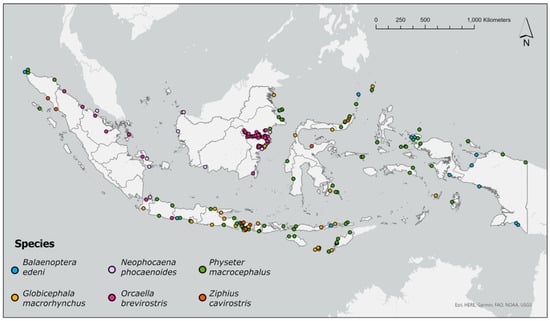
Figure 4.
Species-specific cetacean strandings of six species in Indonesia 1995–2021. The stranding distributions of other species are available in the http://www.whalestrandingindonesia.com/map.html (accessed on 1 February 2022).
In terms of the spatial distributions of these six species (Figure 5), the Irrawaddy dolphins were mostly stranded along the Mahakam River in East Kalimantan, as well as at the southern shores of the same province. Sperm whale and short-finned pilot whale stranding events were relatively evenly distributed throughout Indonesia. The Indo-Pacific finless porpoise stranding events were mostly observed in the western parts of the country (e.g., the eastern waters of Sumatra and some areas in Kalimantan), although a stranding event of this species was confirmed in the eastern waters of Madura (see Figure 1, province #11), thus, adding the location to the list of confirmed home range of this species. Most stranding events for Bryde’s whales were observed in the eastern part of the country, notably West Papua and Papua (Figure 1, provinces #7 and #24). Cuvier’s beaked whale stranding events were absent from Kalimantan. Instead, this species stranded on the western coast of Aceh (Figure 1, province #1), some locations in Bali (Figure 1, province #2), Central Sulawesi (Figure 1, province #28) and North Sulawesi (Figure 1, province #30).
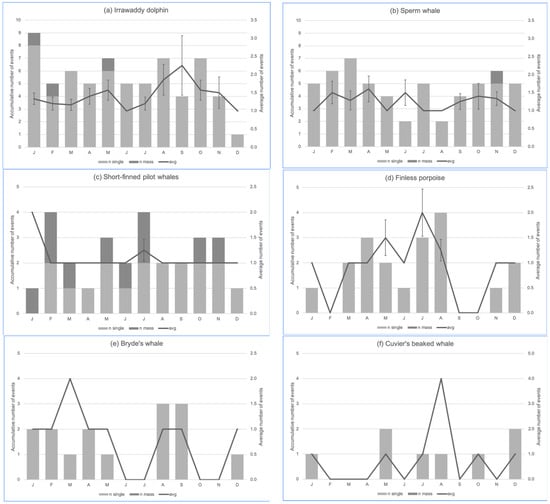
Figure 5.
The monthly trend of stranding events of the six most frequently stranded cetaceans in Indonesia: (a) the Irrawaddy dolphin (Orcaella brevirostris, n = 102), (b) sperm whale (Physeter macrocephalus, n = 75), (c) short-finned pilot whale (Globicephala macrorhynchus, n = 31), (d) Indo-Pacific finless porpoise (Neophocaena phocaenoides, n = 24), (e) Bryde’s whale (Balaenoptera edeni, n = 16), (f) Cuvier’s beaked whale (Ziphius cavirostris, n = 11). Some species had stranding data with no associated months; thus, the cumulative counts may be less than the total events for each species. Bryde’s whale and Cuvier’s beaked whale charts have no measure of dispersion because se = 0.
In terms of species stranding occurrence per month (Figure 5), the Irrawaddy dolphin, sperm whale and short-finned pilot whale have been observed to strand at any given month of the year. The Indo-Pacific finless porpoise stranding events were mostly recorded in the dry season (typically starting in late March or April and ending in October in Indonesia), whereas records of Bryde’s whale and Cuvier’s beaked whale stranding events were scattered across the year. The August trend in the Cuvier’s beaked whale chart (Figure 5f) was attributed to four single stranding events in north Bali on the same day (8 August 2015) in mid-morning, thus, it can be considered a quasi-mass stranding event. There was no significant difference amongst these observations at the 0.05 level of significance. The monthly variability of the Bryde’s whale stranding events was approaching the 0.05 level of significance (Kruskal–Wallis chi-squared = 14, df = 7, p-value = 0.05118), but the number of events ranged only between 1 and 3 per month.
3.2. RQ1: Kernel Density Analysis
Figure 6 and Figure 7 describe the kernel density analyses towards the stranding events of 1995–2011 and 2012–2021 events, respectively. For 1995–2011, the upstream Mahakam River in East Kalimantan and Bali had the highest number of stranding events with up to 12.24 cases per 50 km2) (Figure 6). For the same period, Donggala in Central Sulawesi had the highest number of total stranded individuals (up to 56.96 individuals per 50 km2) (Supplementary Material Figure S1). In general, stranding events were mostly detected in Java, Bali, Nusa Tenggara (Figure 1, provinces #22 and 23), East Kalimantan and Raja Ampat in West Papua and several sporadic locations in Sumatra and Sulawesi.
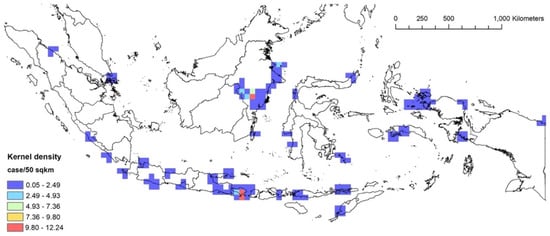
Figure 6.
The kernel density analysis of the 1995–2011 stranding events based on the number of cases.
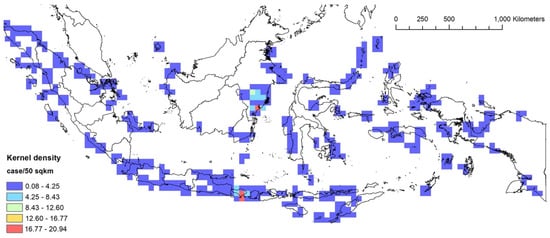
Figure 7.
The kernel density analysis of the 2012–2021 stranding events based on the number of cases.
For the following period of 2012–2021, Bali and East Kalimantan had the highest number of stranding events with up to 20.94 cases per 50 km2 (Figure 7). For the same period, Madura in East Java had the highest number of total stranded individuals (up to 47.15 individuals per 50 km2, followed by Sabu-Raijua in East Nusa Tenggara (up to 37.75 individuals per 50 km2) (Supplementary Material Figure S2). In general, stranding events were detected in almost all major islands, indicating that the stranding detection efforts have improved compared to the previous period.
3.3. RQ1: Spatial Hotspot Analysis
Figure 8, Figure 9 and Figure 10 describe the spatial hotspot analyses of the stranding events of 1995–2011 and 2012–2021 events. Between 1995 and 2011, Bali was the hotspot location with the highest confidence level for single stranding events, followed by East Kalimantan (Figure 8). For the same period, Donggala in Central Sulawesi was the hotspot location for mass stranding events (Supplementary Material Figure S3). Between 2012 and 2021, East Kalimantan and Bali remained the hotspot locations for single stranding events (Figure 9). For the same period, Madura Strait in East Java (Figure 1, province #12) and Sabu Raijua in East Nusa Tenggara became the hotspot locations for mass stranding events (Figure 10). Overall, stranding events were recorded in more dispersed locations in 2012–2021 compared to the previous period.
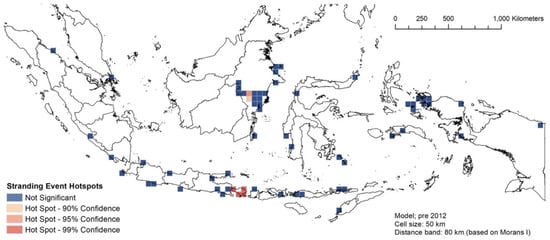
Figure 8.
The spatial hotspot analysis of single stranding events of 1995–2011.
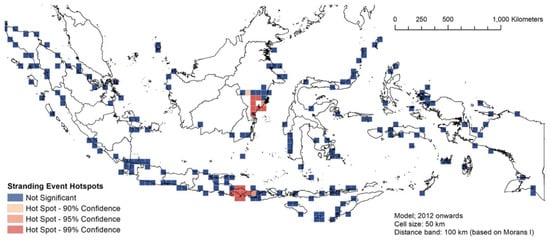
Figure 9.
The spatial hotspot analysis of single stranding events of 2012–2021.
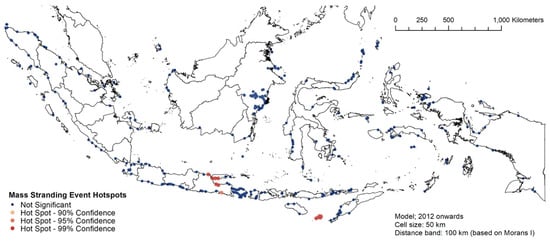
Figure 10.
The spatial hotspot analysis of mass stranding events of 2012–2021.
3.4. RQ2: Temporal Hotspot Analysis
Between 1995 and 2011, Bali and the surrounding waters (including East Java and the western and northern part of Lombok) were recorded as consecutive and sporadic hotspots, meaning that the stranding events here were quite periodic (Supplementary Material Figure S4). For the same period, no pattern was detected from East Kalimantan. One new hotspot location emerged at the Riau Islands, while the upper Savu Sea in East Nusa Tenggara had one consecutive hotspot.
Between 2012 and 2021, the southern coast of East Kalimantan became the consecutive hotspot, East Java and the eastern part of Bali became the sporadic hotspot, while the western part of Bali retained consecutive hotspot status (Figure 11). No considerable new hotspot locations were detected in this period.
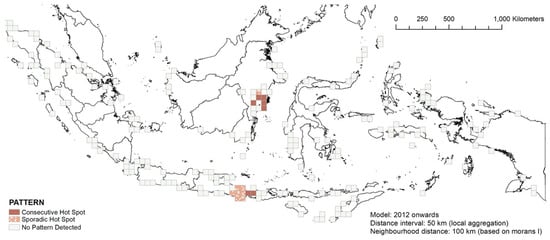
Figure 11.
The space–time hotspot patterns of the 2012–2021 stranding events.
Between 1995 and 2011, many places in Java, Nusa Tenggara and the southern part of Sulawesi experienced a 90–95% increase in stranding events, while Bali, East Java and Raja Ampat in West Papua experienced the highest increase in stranding events (99%, Supplementary Material Figure S5). The northern tip of North Sulawesi was the only place experiencing a downward trend in this period (Supplementary Material Figure S5). For the latter period, while some places in Sumatra and Java experienced a downward trend, upward trends were also observed in many places, chief among them Bali and East Kalimantan (Figure 12).
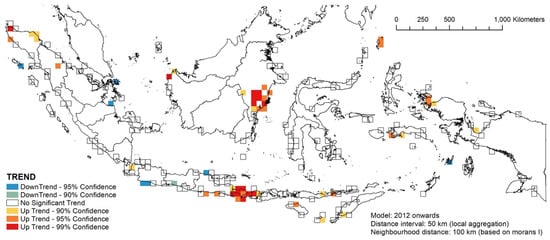
Figure 12.
The temporal hotspot patterns of the 2012–2021 stranding events.
3.5. Notes on Methodological Caveats
Our analyses in this paper have several methodological caveats. The major caveat is that our result was a reflection of reporting efforts instead of observers directly surveying the coasts on possible stranding events. Consequently, the models were very sensitive to human errors linked to reporting efforts. Our model parameters (including the cell size of 50 km) may have been influenced by this bias. Another consequence of this bias is that we cannot conclude that locations with no or few stranding events are locations that are “safe” for the cetaceans, at least when stranding is concerned. In particular, we need to be mindful of the “blank spots” at remote locations, or locations with insufficient telecommunication networks. To improve future analyses, more attempts must be made to minimise the bias. For instance, regular scans of beaches with public access would create a more even coverage of possible stranding observations.
The second caveat for our analyses is that the stranding coordinates might be either of the following: (1) the real stranding event coordinates (taken from GPS or high-resolution location screen shots from mobile phones); or (2) estimated stranding coordinates based on news and descriptions from informants. Nevertheless, due to the large scale of our study area (the country is the whole study area), we are confident that our coordinates, coarse as they are, still generated reliable results. To improve future analyses, data recorders need to ensure that all stranding locations are taken with GPS at the location where the animals strand and at the time of the event. In the absence of a GPS, zoomed-in locations of future stranding events must be screen-shot with additional geo-spatial information, such as “the stranding event is on the beach, xx km south of the coastal road of [insert name], between [insert name of a feature such as a bay] and [insert name of another feature]”. In addition, news from remote locations should be properly recorded, even with just approximate coordinates (see the following paragraph) and any descriptions of the stranding event.
The third caveat in the analyses is the inaccuracy of species identification during any stranding events. A large proportion of the stranding specimens are unidentified species, partly due to the advanced decomposition of the carcasses upon discovery. However, at times, the carcasses were still considerably fresh (up to code 3). Yet, the absence of good quality photographs rendered species identification impossible or incorrect, leading to an incorrect understanding of the stranding event. To increase the likelihood of correct species identifications in the future, media and members of the public must be educated in the proper ways of taking photographs (inter alia, taking clear images of the dorsal fin, head, snout, etc., as well as taking the photographs in a good lighting condition, with measurements whenever possible).
4. Discussion
4.1. Species-Specific and Location-Specific Findings
Out of the 34 cetacean species in Indonesia, 26 of them (76.5%) have been found to strand at any given time at any location in the country. The species-specific analysis of some of these species showed that the Irrawaddy dolphins, sperm whales and the Indo-Pacific finless porpoises are regular stranders. This is a cause for concern because these species are considered threatened in the IUCN Red List. For instance, the mean annual mortality of the Critically Endangered Mahakam River population of Irrawaddy dolphins was 4.3 dolphins of a minimum estimated population size of 75 and 62, respectively, in 2007 and 2021 [39] (also unpublished data Kreb, 2022). The annual average bycatch rate of three individuals per year exceeds the PBR of 0.12 by twenty-five times for this population (unpublished data Kreb, 2022), thus, urging direct mitigation actions. Although excluded from the data analysis of this paper, the bycatch of an Indo-Pacific finless porpoise in eastern Madura in East Java (Mustika, unpublished data) indicates a previously unknown distribution range of this elusive and vulnerable species. From a logistical perspective, the repeated strandings of short-finned pilot whales (many of them were mass stranding events) is a point of concern due to the massive rescue efforts required to re-float the animals.
Another species of interest is the Cuvier’s beaked whales. This deep-diving species [40,41] was recorded to strand individually at four adjacent locations (~10–20 km apart) along the northern coast of Bali on 8 August 2015. Although internal bleedings were observed in one of the four Cuvier’s beaked whales, no conclusive findings were made due to the absence of necropsy on these specimens. However, owing to our conclusion that Bali is considered a spatial stranding hotspot, more efforts are needed to ensure the next Cuvier’s beaked whale stranding is handled appropriately. This effort should include conducting necropsies when applicable to understand whether man-made sonar was responsible for the stranding of this species, as shown by research in other parts of the world [10,38].
Our analyses shed light on how more stranding events were recorded from 2012 onwards compared to the previous period, widening the detection coverage from just some main islands in Indonesia to almost all provinces in Indonesia. Dedicating more time to record stranding events through the online database, as well as increasing the awareness of members of the public regarding reporting stranding events, might have contributed to this trend. However, care must be applied in interpreting these results. The presence of active NGOs, individuals or government agencies in certain locations (such as the case of Irrawaddy dolphins in East Kalimantan or Bali in general) might result in higher numbers of reported cases compared to areas with less active institutions and/or individuals, vis-à-vis a systematic beach monitoring regime to minimize reporting bias [42].
Although it is understandable to equate the stranding hotspots with areas of high cetacean abundance, we do not think such an approach is applicable to Indonesia. Generally speaking, with the exception of East Kalimantan, we have no baseline data on cetacean population dynamics and residency in the country. The general residency and migratory pattern of cetaceans in the country are not yet well studied, but there are emerging patterns of migratory cetaceans (e.g., the pygmy blue whales Balaenoptera musculus brevicauda that migrate between Banda Sea and Australia, [43,44], and Mustika et al.’s unpublished data]) and some areas with year-round residency (e.g., the spinner dolphins Stenella longirostris north of Bali [45]).
Location-wise, East Kalimantan and Bali seem to be the hotspots of single stranding events in Indonesia. Raja Ampat in West Papua was also a highly significant hotspot (99% CI) for 1995–2011, but the significance declined slightly to 95% in 2012–2021 (that is, there was a slight decline in the number of strandings per 50 km2). The trends in these three locations might have the same confounding factor as stated in the previous paragraph, which resulted in the uneven distribution of observations and response efforts. Comparatively speaking, stranding events in Bali are somewhat more manageable than stranding events in East Kalimantan or Raja Ampat (West Papua).
East Java (in particular, Madura Strait) and the islands of Sabu and Raijua in East Nusa Tenggara seem to be the hotspots for mass stranding events. In relation to the Madura Strait, the fact that another mass stranding happened in the same locality in 1923 corroborates the notion that Madura Strait is an important stranding hotspot to manage, and more efforts should be dedicated to ensure that this location has sufficient numbers of first responders and veterinarians.
4.2. Management Implications
To date, there have been some insights into the trends or management of marine mammal stranding events in developing countries such as Brazil, Chile, India, Mexico and, to some extent, Indonesia [20,31,42,46,47]. Our paper contributes to that growing body of knowledge and provides an update on the progress of the marine mammal stranding management in the country. Our analyses can improve the current Indonesia ocean policy and management support for marine mammal conservation. Direct policy benefits include: the implementation of a marine mammal conservation plan (established by the Ministry of Marine Affairs and Fisheries in 2018) and marine spatial planning, by highlighting areas that are highly susceptible to strandings and, therefore, need more attention. We hope that the identification of such areas can at least increase the chance of survival of stranded cetaceans, particularly for mass stranding events, given that on average only half of the number of stranded individuals in mass stranding events survived. In addition, locations with regular stranding events, regardless of whether they are hotspots or not, can also serve as baseline data for future stranding analysis studies, for these locations can provide time series data on marine mammal stranding trends.
Several locations in Indonesia that need more efforts during stranding events have been identified in this paper, notably East Java (particularly Madura Strait) and Sabu-Raijua (East Nusa Tenggara), as they have been identified as stranding hotspots. Therefore, more training workshops in these locations are needed to improve the stranding responses. Specifically, training to improve the stranding response to mass stranding events and/or large whale stranding events is needed, because of the difficulty in ensuring the survival rate of these two events.
However, the difference of scale matters. For instance, although Bali and East Kalimantan are both stranding hotspots, Bali is much smaller, hence, it is easier to manage than East Kalimantan. Similarly, although East Java and the Sabu-Raijua Islands in East Nusa Tenggara are both mass stranding hotspots, the locations in East Java are much easier to reach than the remote islands of Sabu-Raijua. Therefore, better preparations should also be allocated to remote areas, for instance, the remote locations of East Nusa Tenggara and upstream of the Mahakam River in East Kalimantan.
Although hotspot locations do need to be alert to stranding events, non-hotspot locations would also benefit from better preparations, including more training workshops. “Cold spot” locations such as the remote areas of the Kei Islands in Maluku need to be examined further. These areas might have stranding events, yet they remain undetected due to the lack of telecommunication facilities.
A more proactive plan in responding to stranding cases would increase the chance of survival of stranded cetaceans. Stranding alert systems need to be improved as well. Although social media (e.g., Instagram, Facebook, Twitter) and invited-only applications (e.g., WhatsApp groups) have been used quite frequently, a more dedicated team to, for instance, scan news for stranding-related events, would improve network response and add to the data collection efforts.
In response to the need to improve the management of stranding cases in remote areas, more in-depth training workshops for data collection during stranding events (for all codes and including necropsies) are needed. For necropsy in particular, the fact that the country has only conducted necropsies on around 5% of the total stranding cases underscores the need for more necropsies in the future. Detailed response protocols for any stranding event that are divided into protocols for live stranding events and protocols for dead stranding events (including necropsies) are also needed.
In this paper, we did not attempt to answer the perennial question of why these animals stranded, neither did we attempt to understand the predictors or covariates of these stranding events; the last issue is reserved for another working paper. Nevertheless, to gain a deeper understanding of the associations between stranding covariates, we need more necropsies in the future, particularly on code 2 and 3 specimens. The low number of necropsies in Indonesia has been attributed to the lack of veterinarians and the late notifications of events; thus, the veterinarians could not reach the locations in time. Therefore, necropsy training workshops for veterinarians and marine biologists in Indonesia are a necessity to improve the likelihood of proper necropsies being conducted on the appropriate specimens. In the absence of necropsies, essential genetic and histopathological samples should at least be collected. Since storage is an issue for a country as large as Indonesia, creating the capacity to properly preserve the samples for analysis in laboratories is critical. In addition, a good pathology team with access to affordable examination tools and tests for developing countries is a very valuable factor in improving the conservation of the wild cetacean populations in the country [48,49].
The lack of funding, insufficient knowledge and training for first responders and veterinarians, limited equipment and the lack of systematic data collection have been identified as obstacles to effective marine mammal stranding management in other developing countries [31,42,46,47]. Our analysis of the available stranding data in Indonesia concurs with that list, but several more issues have also been identified above. As for the funding, we are heartened by the fact that the Indonesian government has been able to regularly conduct first responder training workshops in various locations. However, to improve the stranding management in the country, more consistent funding would be very beneficial. The results of this paper should be able to add to the list of locations of future training workshops, both for first responders and veterinarians.
In closing, the cetacean conservation is a “new thing” in Indonesia; hence, many conservation management frameworks in the country have limited programs to address this issue. Additionally, in some areas such as capture fishery or sea transportation, cetacean conservation is not incorporated as a part of their management plans. However, the increasing focus on stranding events has drawn more attention to this neglected taxon, thus, generating a renewed awareness that we need to better understand cetacean (and general marine mammal) stranding events. We believe that this paper contributes to this cause.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oceans3040034/s1, Table S1: The recap of species-specific stranding events in Indonesia (1995–2021); Figure S1: The kernel density analysis of the 1995–2011 stranding events based on the number of stranded individuals; Figure S2: The kernel density analysis of the 2012–2021 stranding events based on the number of stranded individuals; Figure S3: The spatial hotspot analysis of mass stranding events of 1995–2011; Figure S4: The space-time hotspot patterns of the 1995–2011 stranding events; Figure S5: The temporal hotspot patterns of the 1995–2011 stranding events.
Author Contributions
Conceptualization, P.L.K.M., K.K.H., M.I.H.P., A.S. and F.A.; methodology, P.L.K.M., K.K.H., M.I.H.P., A.S. and F.A.; software, K.K.H. and M.I.H.P.; validation, P.L.K.M., K.K.H., A.S., I.M.J.R. and D.K.; formal analysis, P.L.K.M., K.K.H., M.I.H.P., A.S. and F.A.; investigation, P.L.K.M., K.K.H., M.I.H.P., A.S., I.M.J.R., M.O.P., F.S.P. and D.K.; resources, P.L.K.M., I.M.J.R., M.O.P. and F.S.P.; data curation, P.L.K.M., K.K.H., I.M.J.R., M.O.P., F.S.P. and D.K.; writing—original draft preparation, P.L.K.M. and K.K.H.; writing—review and editing, P.L.K.M., K.K.H., M.I.H.P., A.S., I.M.J.R., F.A., F.S.P. and D.K.; visualization, K.K.H. and M.I.H.P.; supervision, P.L.K.M.; project administration, I.M.J.R. and M.O.P.; funding acquisition, P.L.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by ICCTF (the Indonesian Climate Change Trust Fund) Bappenas for the COREMAP-CTI World Bank program with contract number ICCTF: 003/SPK/PPK-06-14/PH-ICCTF/08/2020.
Institutional Review Board Statement
Ethical review and approval were waived for this study because stranding events were not events with prior-designs. In addition, the data required from the stranding events were only coordinates and species, thus requiring minimum interactions with the animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Stranding data can be found at www.whalestrandingindonesia.com accessed on 1 February 2022.
Acknowledgments
We thank the Ministry of Marine Affairs and Fisheries (MMAF) and the Ministry of Environment and Forestry (MEF) for spear-heading the marine mammal stranding initiatives in Indonesia. We thank the members of the national stranding network, inter alia: the BKKPN Kupang, BPSPL Padang, BPSPL Pontianak, BPSPL Denpasar, BPSPL Makasar, LKKPN Pekanbaru, LPSPL Serang and LPSPL Sorong of the MMAF; the Natural Resources Conservation Agency of the MEF; the Indonesian Navy; the Indonesian National Police; WWF Indonesia; the Jakarta Animal Aid Network; Yayasan Konservasi RASI (the Conservation Foundation for Rare Aquatic Species of Indonesia); IAM Flying Vet; Westerlaken Foundation; TCEC (Turtle Conservation and Education Center); Nusa Dua Reef Foundation; the Misool Baseftin Foundation; as well as the many communities all over Indonesia who were willing to spend time, energy and at times financial resources for the stranded marine mammals. We thank Rushan Bin Abdul Rahman of James Cook University Singapore for the R consultation. K. K. High’s time was partially supported by a National Science Foundation Research Traineeship Grant (Award No. DGE-16333) through the Estuary and Ocean Science Center at the San Francisco State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mustika, P.L.K.; Sadili, D.; Sunuddin, A.; Kreb, D.; Hadi, S.; Ramli, I.; Suprapti, D.; Ratha, J.; Lazuardi, E.; Rasdiana, H.; et al. Rencana Aksi Nasional (RAN) Konservasi Cetacea Indonesia 2016–2020 (the Cetacean National Plan of Action (NPOA) for Indonesia 2016–2020); Ministry of Marine Affairs and Fisheries: Jakarta, Indonesia, 2016; p. 88. [Google Scholar]
- Beasley, I.; Jedensjö, M.; Wijaya, G.M.; Anamiato, J.; Kahn, B.; Kreb, D. Observations on Australian Humpback Dolphins (Sousa sahulensis) in Waters of the Pacific Islands and New Guinea. In Humpback Dolphins (Sousa spp.): Current Status and Conservation, Part 2: Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 219–271. [Google Scholar]
- Rudolph, P.; Smeenk, C.; Leatherwood, S. Preliminary checklist of cetacea in the Indonesian Archipelago and adjacent waters. Zool. Verh. 1997, 312, 3–48. [Google Scholar]
- Slijper, E.J.; Utrecht, W.L.V.; Naaktgeboren, C. Remarks on the Distribution and Migration of Whales, Based on Observations from Netherlands Ships. Bijbragen Tot Djerkunde 1964, 34, 3–93. [Google Scholar] [CrossRef]
- Walker, W.A.; Coe, J.M. Survey of marine debris ingestion by odontocete cetaceans. In Proceedings of the Second International Conference on Marine Debris, Honolulu, HI, USA, 2–7 April 1989; pp. 2–7. [Google Scholar]
- Leeney, R.H.; Amies, R.; Broderick, A.C.; Witt, M.J.; Loveridge, J.; Doyle, J.; Godley, B.J. Spatio-temporal analysis of cetacean strandings and bycatch in a UK fisheries hotspot. Biodivers. Conserv. 2008, 17, 2323–2338. [Google Scholar] [CrossRef]
- Waereebeek, K.V.; Baker, A.; Félix, F.; Gedamke, J.; Iñiguez, M.; Sanino, G.P.; Secchi, E.R.; Sutaria, D.; Helden, A.V.; Wang, Y. Vessel collisions with small cetaceans worldwide and with large whales in the Southern Hemisphere, an initial assessment. Lat. Am. J. Aquat. Mamm. 2007, 6, 43–69. [Google Scholar] [CrossRef]
- Martineau, D.; Lemberger, K.; Dallaire, A.; Labelle, P.; Lipscomb, T.P.; Michel, P.; Mikaelian, I. Cancer in Wildlife, a Case Study: Beluga from the St. Lawrence Estuary, Quebec, Canada. Environ. Health Perspect. 2002, 110, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; ARbelo, M.; Deaville, R.; Patterson, I.A.P.; Castro, P.; Baker, J.R.; Degollada, E.; Ross, H.M.; Herraez, P.; Pocknell, A.M.; et al. Gas-bubble lesions in stranded cetaceans. Nature 2003, 425, 575–576. [Google Scholar] [CrossRef]
- Cox, T.M.; Ragen, T.J.; Read, A.J.; Vos, E.; Baird, R.W.; Balcomb, K.; Barlow, J.; Caldwell, J.; Cranford, T.; Crum, L.; et al. Understanding the impact of anthropogenic sound on beaked whales. J. Cetacean Resour. Manag. 2006, 7, 177–187. [Google Scholar]
- Tyack, P.L.; Zimmer, W.M.; Moretti, D.; Southall, B.L.; Claridge, D.E.; Durban, J.W.; Clark, C.W.; D′Amico, A.; DiMarzio, N.; Jarvis, S. Beaked whales respond to simulated and actual navy sonar. PLoS ONE 2011, 6, e17009. [Google Scholar] [CrossRef]
- Forney, K.A.; Southall, B.L.; Slooten, E.; Dawson, S.; Read, A.J.; Baird, R.W.; Brownell, R.L., Jr. Nowhere to go: Noise impact assessments for marine mammal populations with high site fidelity. Endanger. Species Res. 2017, 32, 391–413. [Google Scholar] [CrossRef]
- Vanselow, K.H.; Ricklefs, K. Are solar activity and sperm whale Physeter macrocephalus strandings around the North Sea related? J. Sea Res. 2005, 53, 319–327. [Google Scholar] [CrossRef]
- Vanselow, K.H.; Ricklefs, K.; Colijn, F. Solar Driven Geomagnetic Anomalies and Sperm Whales (Physeter macrocephalus) Strandings around the North Sea: An Analysis of Long Term Datasets. Open Mar. Biol. J. 2009, 3, 89–94. [Google Scholar] [CrossRef]
- Vanselow, K.H.; Jacobsen, S.; Hall, C.; Garthe, S. Solar storms may trigger sperm whale strandings: Explanation approaches for multiple strandings in the North Sea in 2016. Int. J. Astrobiol. 2018, 17, 336–344. [Google Scholar] [CrossRef]
- Fossi, M.C.; Baini, M.; Simmonds, M.P. Cetaceans as ocean health indicators of marine litter impact at global scale. Front. Environ. Sci. 2020, 8, 255. [Google Scholar] [CrossRef]
- Gulland, F.; Hall, A.J. Is marine mammal health deteriorating? Trends in the global reporting of marine mammal disease. EcoHealth 2007, 4, 135–150. [Google Scholar] [CrossRef]
- Obusan, M.C.M.; Aragones, L.V.; Salibay, C.C.; Siringan, M.A.T.; Rivera, W.L. Occurrence of human pathogenic bacteria and Toxoplasma gondii in cetaceans stranded in the Philippines: Providing clues on ocean health status. Aquat. Mamm. 2015, 41, 149. [Google Scholar] [CrossRef]
- Mustika, P.L.; High, K.K.; Ratha, I.M.J.; Siko, M.M.; Acebes, J.M.; Makin, R.M.; Meo, M.S.; d’Alxandro, E.; Didok, P.R.E. First Record of Predation on an Oilfish (Ruvettus pretiosus) and a Previously Unknown Cephalopod Prey (Thysanoteuthis rhombus) by a Short-finned Pilot Whale (Globicephala macrorhynchus) in East Nusa Tenggara, Indonesia. Aquat. Mamm. 2022; in press. [Google Scholar]
- Mustika, P.L.K.; Hutasoit, P.; Madusari, C.C.; Purnomo, F.S.; Setiawan, A.; Tjandra, K.; Prabowo, W.E. Whale strandings in Indonesia, including the first record of a humpback whale (Megaptera novaeangliae) in the Archipelago. Raffles Bull. Zool. 2009, 57, 199–206. [Google Scholar]
- Badan Informasi Geospasial. Atlas Geospasial Indonesia: Fisik dan Lingkungan Alam; Badan Informasi Geospasial (BIG): Jakarta, Indonesia, 2020. [Google Scholar]
- Nag, O.S. Countries with the Longest Coastline. Available online: https://www.worldatlas.com/articles/countries-with-the-most-coastline.html (accessed on 2 May 2022).
- Ningsih, N.S.; Rakhmaputeri, N.; Harto, A.B. Upwelling variability along the southern coast of Bali and in Nusa Tenggara waters. Ocean. Sci. J. 2013, 48, 49–57. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Fuide for Strandings; Texas A&M University Sea Grant College Program: College Station, TX, USA, 1993. [Google Scholar]
- Betty, E.L.; Bollard, B.; Murphy, S.; Ogle, M.; Hendriks, H.; Orams, M.B.; Stockin, K.A. Using emerging hot spot analysis of stranding records to inform conservation management of a data-poor cetacean species. Biodivers. Conserv. 2020, 29, 643–665. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology 1989, 70, 164. [Google Scholar] [CrossRef]
- Augé, A.A.; Otley, H.; Rendell, N.; Frans, V.F. Spatial distribution of cetacean strandings in the Falkland Islands to define monitoring opportunities. J. Cetacean Res. Manag. 2018, 19, 1–7. [Google Scholar]
- Getis, A.; Ord, J. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zu, Z.; Lu, J. Traffic crash evolution characteristic analysis and spatiotemporal hotspot identification of urban road intersections. Sustainability 2018, 11, 160. [Google Scholar] [CrossRef]
- Dudhat, S.; Pande, A.; Nair, A.; Mondal, I.; Srinivasan, M.; Sivakumar, K. Spatio-temporal analysis identifies marine mammal stranding hotspots along the Indian coastline. Sci. Rep. 2022, 12, 4128. [Google Scholar] [CrossRef]
- Harris, N.L.; Goldman, E.; Gabris, C.; Nordling, J.; Minnemeyer, S.; Ansari, S.; Lippmann, M.; Bennett, L.; Raad, M.; Hansen, M. Using spatial statistics to identify emerging hot spots of forest loss. Environ. Res. Lett. 2017, 12, 024012. [Google Scholar] [CrossRef]
- Purwanto, P.; Utaya, S.; Handoyo, B.; Bachri, S.; Astuti, I.S.; Utomo, K.S.B.; Aldianto, Y.E. Spatiotemporal analysis of COVID-19 spread with emerging hotspot analysis and space–time cube models in East Java, Indonesia. ISPRS Int. J. Geo-Inf. 2021, 10, 133. [Google Scholar] [CrossRef]
- Bass, C.A. Emerging hotspot analysis of Florida manatee (Trichechus manatus latirostris) mortality (1974–2012). Master’s Thesis, Nova Southeastern University, Davies, FL, USA, October 2017. [Google Scholar]
- Olson, J.K.; Aschoff, J.; Goble, A.; Larson, S.; Gaydos, J.K. Maximizing surveillance through spatial characterization of marine mammal stranding hot spots. Mar. Mammal Sci. 2020, 36, 1083–1096. [Google Scholar] [CrossRef]
- Falcone, E.A.; Schorr, G.S.; Watwood, S.L.; DeRuiter, S.L.; Zerbini, A.N.; Andrews, R.D.; Morrissey, R.P.; Moretti, D.J. Diving behaviour of Cuvier′s beaked whales exposed to two types of military sonar. R. Soc. Open Sci. 2017, 4, 170629. [Google Scholar] [CrossRef]
- Caldas de Castro, M.; Singer, B.H. Controlling the false discovery rate: A new application to account for multiple and dependent tests in local statistics of spatial association. Geogr. Anal. 2006, 38, 180–208. [Google Scholar] [CrossRef]
- Bernaldo de Quirós, Y.; Fernandez, A.; Baird, R.; Brownell, R., Jr.; Aguilar de Soto, N.; Allen, D.; Arbelo, M.; Arregui, M.; Costidis, A.; Fahlman, A. Advances in research on the impacts of anti-submarine sonar on beaked whales. Proc. R. Soc. B 2019, 286, 20182533. [Google Scholar] [CrossRef] [PubMed]
- Kreb, D.; Reeves, R.R.; Thomas, P.O.; Braulik, G.T.; Smith, B.D. Establishing Protected Areas for Asian Freshwater Cetaceans: Final Workshop Report for a Workshop Titled “Freshwater Cetaceans as Flagship Species for Integrated River Conservation Management” (Samarinda, Indonesia, 19–24 October 2009); Conservation Foundation for Rare Aquatic Species of Indonesia: Samarinda, Indonesia, 2010; p. 47. [Google Scholar]
- Schorr, G.S.; Falcone, E.A.; Moretti, D.J.; Andrews, R.D. First long-term behavioral records from Cuvier’s beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS ONE 2014, 9, e92633. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.M.; Quick, N.J.; Cioffi, W.R.; Baird, R.W.; Webster, D.L.; Foley, H.J.; Swaim, Z.T.; Waples, D.M.; Bell, J.T.; Read, A.J. Diving behaviour of Cuvier’s beaked whales (Ziphius cavirostris) off Cape Hatteras, North Carolina. R. Soc. Open Sci. 2019, 6, 181728. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; Siciliano, S.; Emin-Lima, R.; Martins, B.M.L.; Sousa, M.E.M.; Giarrizzo, T.; Silva Júnior, J.d.S.e. Stranding survey as a framework to investigate rare cetacean records of the north and north-eastern Brazilian coasts. ZooKeys 2017, 688, 111–134. [Google Scholar] [CrossRef]
- Möller, L.M.; Attard, C.R.; Bilgmann, K.; Andrews-Goff, V.; Jonsen, I.; Paton, D.; Double, M.C. Movements and behaviour of blue whales satellite tagged in an Australian upwelling system. Sci. Rep. 2020, 10, 21165. [Google Scholar] [CrossRef]
- Sahri, A.; Jak, C.; Putra, M.I.H.; Murk, A.J.; Andrews-Goff, V.; Double, M.C.; Van Lammeren, R.J. Telemetry-based home range and habitat modelling reveals that the majority of areas important for pygmy blue whales are currently unprotected. Biol. Conserv. 2022, 272, 109594. [Google Scholar] [CrossRef]
- Mustika, P.L.K.; Birtles, A.; Everingham, Y.; Marsh, H. Evaluating the potential disturbance from dolphin watching in Lovina, north Bali, Indonesia. Mar. Mammal Sci. 2015, 31, 808–817. [Google Scholar] [CrossRef]
- Gómez-Hernández, G.; Leyva-Aguilera, J.C.; Delhumeau-Rivera, S.; Seingier, G.; Elorriaga-Verplancken, F.R.; Heckel, G. Marine mammal stranding response in Mexico: Lessons learned. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 841–852. [Google Scholar] [CrossRef]
- Alvarado-Rybak, M.; Toro, F.; Escobar-Dodero, J.; Kinsley, A.C.; Sepúlveda, M.A.; Capella, J.; Azat, C.; Cortés-Hinojosa, G.; Zimin-Veselkoff, N.; Mardones, F.O. 50 years of cetacean strandings reveal a concerning rise in Chilean Patagonia. Sci. Rep. 2020, 10, 9511. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar]
- Arbelo, M.; de Los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernández, A. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999−2005). Dis. Aquat. Org. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).