Temporal and Spatial Evaluation of Mono(2-ethylhexyl) Phthalate (MEHP) Detection in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dolphin Community and Study Location
2.2. Phthalate Exposure Assessment
2.3. Photo-Identification Records
2.4. Temporal Analysis
2.5. Spatial Analysis
3. Results
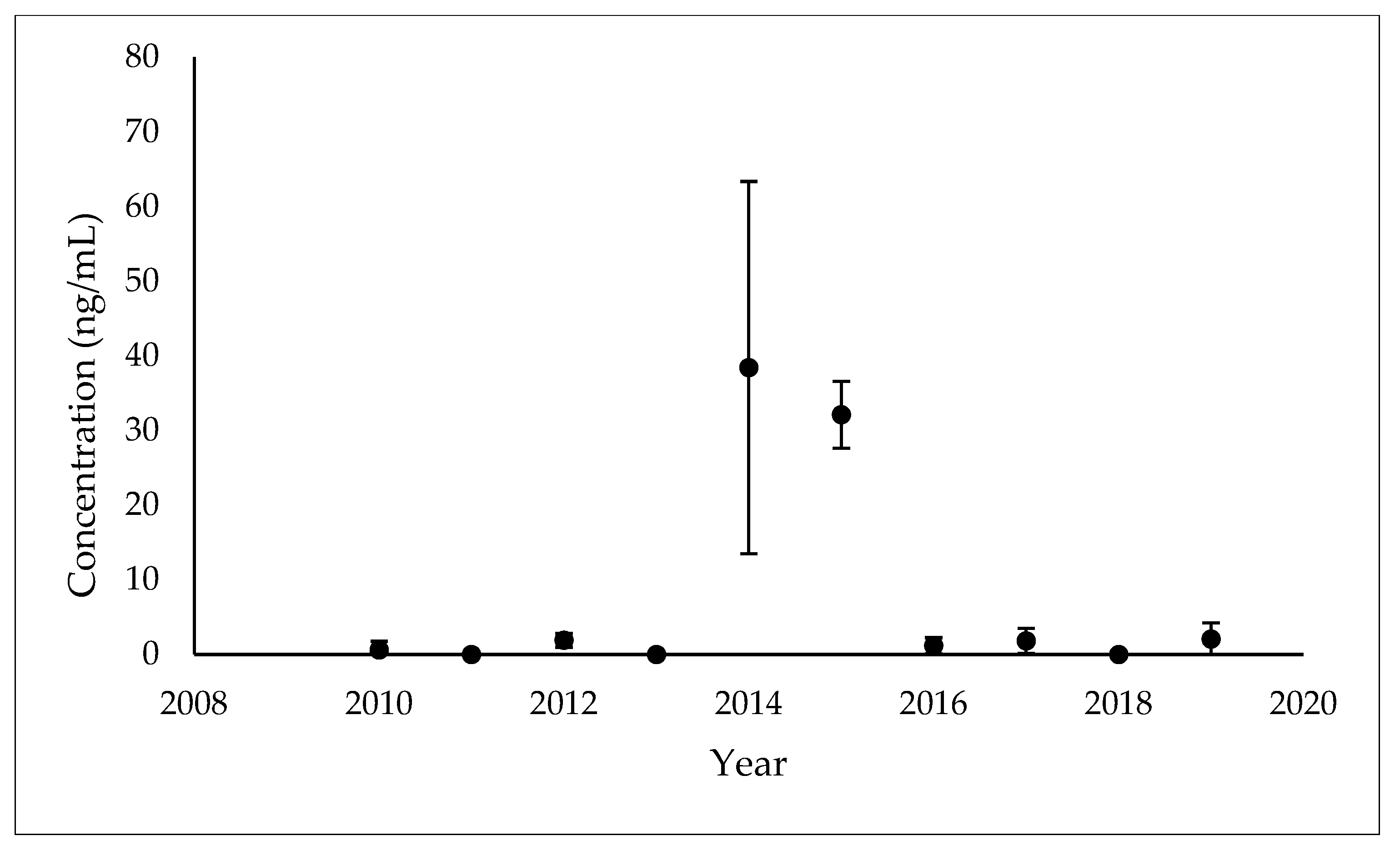
3.1. Temporal Patterns of MEHP Concentrations
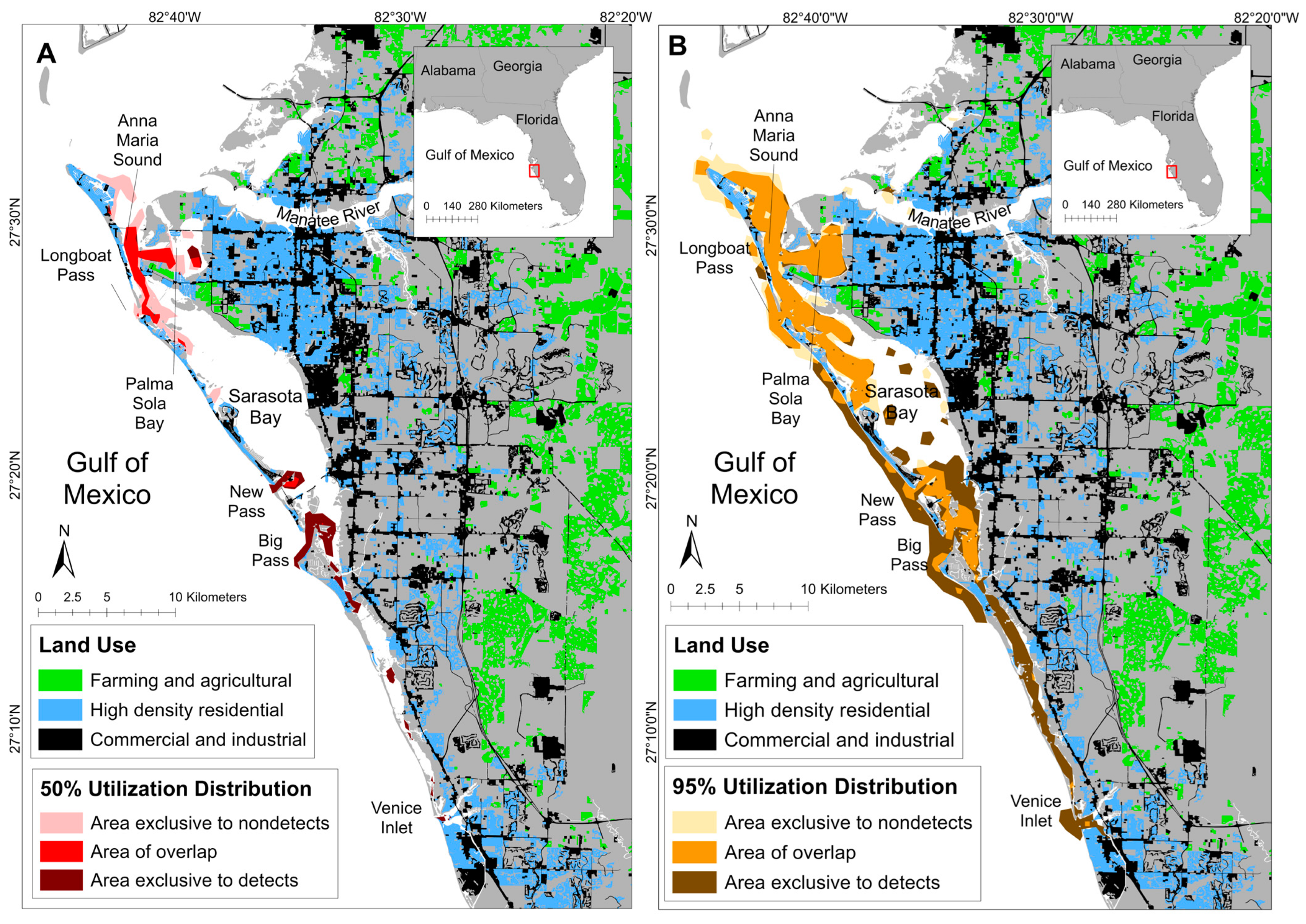
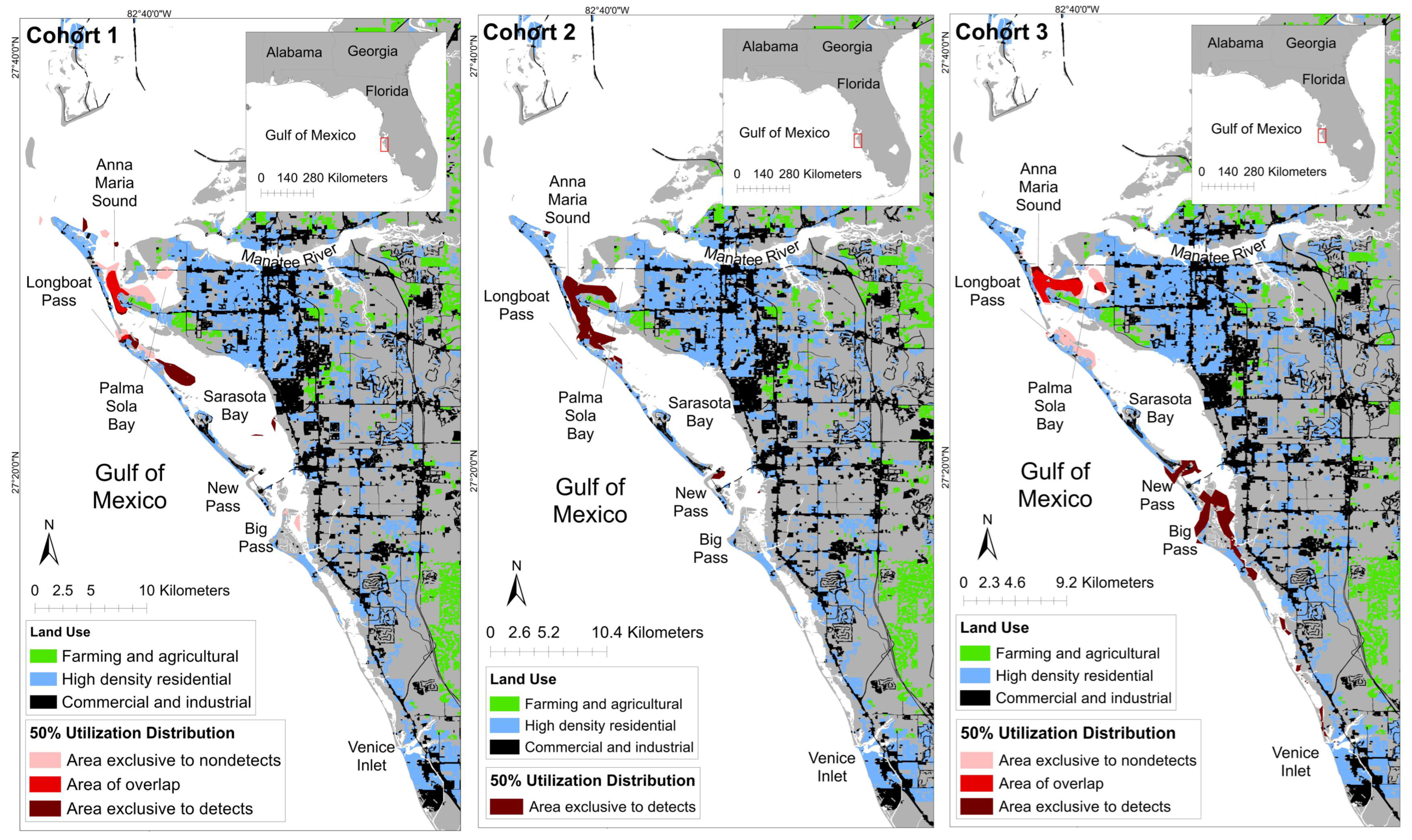
3.2. Spatial Patterns of MEHP Concentrations
4. Discussion
4.1. Overall Findings
4.2. Spatial Findings
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- EPA. Phthalates Action Plan; EPA: Sydney, Australia, 2012; pp. 1–16. [Google Scholar]
- Lowell Center for Sustainable Production (LCSP). Phthalates and Their Alternatives: Health and Environmental Concerns; LCSP: Lowell, MA, USA, 2011. [Google Scholar]
- ATSDR. Toxicological Profile for Di(2-Ethylhexyl)Phthalate (DEHP) Draft for Public Comment; ATSDR: Atlanta, GA, USA, 2019.
- ATSDR. Toxicological Profile for Di-n-Butyl Phthalate; ATSDR: Atlanta, GA, USA, 2001.
- CDC. Biomonitoring Summary; CDC: Atlanta, GA, USA, 2017.
- NTP-CERHR. Expert Panel Report on the Reproductive and Developmental Toxicity of Methanol. Reprod. Toxicol. 2004, 18, 303–390. [Google Scholar] [CrossRef] [PubMed]
- Gani, K.M.; Rajpal, A.; Kazmi, A.A. Contamination Level of Four Priority Phthalates in North Indian Wastewater Treatment Plants and Their Fate in Sequencing Batch Reactor Systems. Environ. Sci. Process. Impacts 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, J.B.; Gao, M.; Shi, G.Z.; Fu, X.J.; Cai, P.; Lv, Y.F.; Guo, Z.B.; Shan, C.Q.; Yang, Z.B.; et al. Occurrence and Distribution of Phthalate Esters in Freshwater Aquaculture Fish Ponds in Pearl River Delta, China. Environ. Pollut. 2019, 245, 883–888. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.; Choi, K.; Zoh, K.D. Distribution of Phthalate Esters in Air, Water, Sediments, and Fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef]
- Weizhen, Z.; Xiaowei, Z.; Peng, G.; Ning, W.; Zini, L.; Jian, H.; Zheng, Z. Distribution and Risk Assessment of Phthalates in Water and Sediment of the Pearl River Delta. Environ. Sci. Pollut. Res. 2020, 27, 12550–12565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Zhang, H.H.; Zhang, J.; Wang, Q.W.; Yang, G.P. Occurrence, Distribution, and Ecological Risks of Phthalate Esters in the Seawater and Sediment of Changjiang River Estuary and Its Adjacent Area. Sci. Total Environ. 2018, 619–620, 93–102. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, W.; Chen, L.; Wu, L.; Christie, P.; Liu, W. Toxicity of Phthalate Esters to Lettuce (Lactuca sativa) and the Soil Microbial Community under Different Soil Conditions. PLoS ONE 2018, 13, e0208111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Liu, T.; Xie, E.; Liu, M.; Ding, A.; Zhang, B.T.; Li, X.; Zhang, D. Partition and Fate of Phthalate Acid Esters (PAEs) in a Full-Scale Horizontal Subsurface Flow Constructed Wetland Treating Polluted River Water. Water 2020, 12, 865. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, Q.; He, W.; Xu, F. Phthalate Esters (PAEs) in Atmospheric Particles around a Large Shallow Natural Lake (Lake Chaohu, China). Sci. Total Environ. 2019, 687, 297–308. [Google Scholar] [CrossRef]
- Teil, M.J.; Blanchard, M.; Chevreuil, M. Atmospheric Fate of Phthalate Esters in an Urban Area (Paris-France). Sci. Total Environ. 2006, 354, 212–223. [Google Scholar] [CrossRef]
- Blair, J.D.; Ikonomou, M.G.M.G.; Kelly, B.C.; Surridge, B.; Gobas, F.A.P.C.P.C. Ultra-Trace Determination of Phthalate Ester Metabolites in Seawater, Sediments, and Biota from an Urbanized Marine Inlet by LC/ESI-MS/MS. Environ. Sci. Technol. 2009, 43, 6262–6268. [Google Scholar] [CrossRef]
- Brock, J.W.; Bell, J.M.; Guillette, L.J. Urinary Phthalate Metabolites in American Alligators (Alligator Mississippiensis) from Selected Florida Wetlands. Arch. Environ. Contam. Toxicol. 2016, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Large Filter Feeding Marine Organisms as Indicators of Microplastic in the Pelagic Environment: The Case Studies of the Mediterranean Basking Shark (Cetorhinus Maximus) and Fin Whale (Balaenoptera Physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Padula, V.; Beaudreau, A.H.; Hagedorn, B.; Causey, D. Plastic-Derived Contaminants in Aleutian Archipelago Seabirds with Varied Foraging Strategies. Mar. Pollut. Bull. 2020, 158, 111435. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Occurrence and Air-Sea Exchange of Phthalates in the Arctic. Environ. Sci. Technol. 2007, 41, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Arfaeinia, H.; Fazlzadeh, M.; Taghizadeh, F.; Saeedi, R.; Spitz, J.; Dobaradaran, S. Phthalate Acid Esters (PAEs) Accumulation in Coastal Sediments from Regions with Different Land Use Configuration along the Persian Gulf. Ecotoxicol. Environ. Saf. 2019, 169, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, J.; Zhang, A.; Liu, W.; Cheng, W. Occurrence of Phthalate Esters in Sediments in Qiantang River, China and Inference with Urbanization and River Flow Regime. J. Hazard. Mater. 2013, 248–249, 142–149. [Google Scholar] [CrossRef]
- Hu, X.; Gu, Y.; Huang, W.; Yin, D. Phthalate Monoesters as Markers of Phthalate Contamination in Wild Marine Organisms. Environ. Pollut. 2016, 218, 410–418. [Google Scholar] [CrossRef]
- Martins, K.; Hagedorn, B.; Ali, S.; Kennish, J.; Applegate, B.; Leu, M.; Epp, L.; Pallister, C.; Zwollo, P. Tissue Phthalate Levels Correlate With Changes in Immune Gene Expression in a Population of Juvenile Wild Salmon. Arch. Environ. Contam. Toxicol. 2016, 71, 35–47. [Google Scholar] [CrossRef]
- Salvaggio, A.; Tiralongo, F.; Krasakopoulou, E.; Marmara, D.; Giovos, I.; Crupi, R.; Messina, G.; Lombardo, B.M.; Marzullo, A.; Pecoraro, R.; et al. Biomarkers of Exposure to Chemical Contamination in the Commercial Fish Species Lepidopus Caudatus(Euphrasen, 1788): A Particular Focus on Plastic Additives. Front. Physiol. 2019, 10, 905. [Google Scholar] [CrossRef] [Green Version]
- Valton, A.S.; Serre-Dargnat, C.; Blanchard, M.; Alliot, F.; Chevreuil, M.; Teil, M.J. Determination of Phthalates and Their By-Products in Tissues of Roach (Rutilus Rutilus) from the Orge River (France). Environ. Sci. Pollut. Res. 2014, 21, 12723–12730. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Cau, A.; Renzi, M.; Badini, S.; Grazioli, E.; Perra, G.; Focardi, S.E. Phthalates and Perfluorinated Alkylated Substances in Atlantic Bluefin Tuna (Thunnus Thynnus) Specimens from Mediterranean Sea (Sardinia, Italy): Levels and Risks for Human Consumption. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2016, 51, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rian, M.B.; Vike-Jonas, K.; Gonzalez, S.V.; Ciesielski, T.M.; Venkatraman, V.; Lindstrøm, U.; Jenssen, B.M.; Asimakopoulos, A.G. Phthalate Metabolites in Harbor Porpoises (Phocoena Phocoena) from Norwegian Coastal Waters. Environ. Int. 2020, 137, 105525. [Google Scholar] [CrossRef] [PubMed]
- Baini, M.; Martellini, T.; Cincinelli, A.; Campani, T.; Minutoli, R.; Panti, C.; Finoia, M.G.; Fossi, M.C. First Detection of Seven Phthalate Esters (PAEs) as Plastic Tracers in Superficial Neustonic/Planktonic Samples and Cetacean Blubber. Anal. Methods 2017, 9, 1512–1520. [Google Scholar] [CrossRef]
- Dziobak, M.K.; Wells, R.S.; Pisarski, E.C.; Wirth, E.F.; Hart, L.B. Demographic Assessment of Mono(2-ethylhexyl) Phthalate (MEHP) and Monoethyl Phthalate (MEP) Concentrations in Common Bottlenose Dolphins (Tursiops Truncatus) from Sarasota Bay, FL, USA. GeoHealth 2021, 5, e2020GH000348. [Google Scholar] [CrossRef]
- Hart, L.B.; Dziobak, M.K.; Pisarski, E.C.; Wirth, E.F.; Wells, R.S. Sentinels of Synthetics—A Comparison of Phthalate Exposure between Common Bottlenose Dolphins (Tursiops Truncatus) and Human Reference Populations. PLoS ONE 2020, 15, e0240506. [Google Scholar] [CrossRef]
- Hart, L.B.; Beckingham, B.; Wells, R.S.; Alten Flagg, M.; Wischusen, K.; Moors, A.; Kucklick, J.; Pisarski, E.; Wirth, E. Urinary Phthalate Metabolites in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, FL, USA. GeoHealth 2018, 2, 313–326. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate Release from Plastic Fragments and Degradation in Seawater. Environ. Sci. Technol. 2019, 53, 166–175. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The Influence of Phthalates and Bisphenol A on the Obesity Development and Glucose Metabolism Disorders. Endocrine; Humana Press Inc.: Totova, NJ, USA, 2017; pp. 666–681. [Google Scholar] [CrossRef]
- Parks, L.G.P.; Ostby, J.S.; Lambright, C.R.; Abbott, B.D.; Klinefelter, G.R.; Barlow, N.J.; Gray Jr, L.E. The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicol. Sci. 2000, 58, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Sainath, S.B.; Reddy, P.S. Prenatal Di-n-Butyl Phthalate Exposure Alters Reproductive Functions at Adulthood in Male Rats. Environ. Toxicol. 2014, 29, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Jarmołowicz, S.; Demska-Zakeś, K.; Zakeś, Z. Impact of Di-n-Butyl Phthalate on Reproductive System Development in European Pikeperch (Sander Lucioperca). Acta Vet. Brno 2013, 82, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Engel, S.M.; Miodovnik, A.; Canfield, R.L.; Zhu, C.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal Phthalate Exposure Is Associated with Childhood Behavior and Executive Functioning. Environ. Health Perspect. 2010, 118, 565–571. [Google Scholar] [CrossRef]
- Engel, S.M.; Zhu, C.; Berkowitz, G.S.; Calafat, A.M.; Silva, M.J.; Miodovnik, A.; Wolff, M.S. Prenatal Phthalate Exposure and Performance on the Neonatal Behavioral Assessment Scale in a Multiethnic Birth Cohort. Neurotoxicology 2009, 30, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine Disruptors and Childhood Social Impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Whyatt, R.M.; Liu, X.; Rauh, V.A.; Calafat, A.M.; Just, A.C.; Hoepner, L.; Diaz, D.; Quinn, J.; Adibi, J.; Perera, F.P.; et al. Maternal Prenatal Urinary Phthalate Metabolite Concentrations and Child Mental, Psychomotor, and Behavioral Development at 3 Years of Age. Environ. Health Perspect. 2012, 120, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Yolton, K.; Xu, Y.; Strauss, D.; Altaye, M.; Calafat, A.M.; Khoury, J. Prenatal Exposure to Bisphenol A and Phthalates and Infant Neurobehavior. Neurotoxicol. Teratol. 2011, 33, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Dirinck, E.; Dirtu, A.C.; Geens, T.; Covaci, A.; Van Gaal, L.; Jorens, P.G. Urinary Phthalate Metabolites Are Associated with Insulin Resistance in Obese Subjects. Environ. Res. 2015, 137, 419–423. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, C.H.; Liang, W.Y.; Li, S.S.; Huang, H.B.; Kuo, P.L. Early Phthalates Exposure in Pregnant Women Is Associated with Alteration of Thyroid Hormones. PLoS ONE 2016, 11, e0159398. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.S. Social Structure and Life History of Bottlenose Dolphins Near Sarasota Bay, Florida: Insights from Four Decades and Five Generations. In Primates and Cetaceans; Yamagiwa, J., Karczmarski, L., Eds.; Springer: Tokyo, Japan, 2014; pp. 149–172. [Google Scholar] [CrossRef]
- Flannery, M.S. Tampa and Sarasota Bays: Watersheds and Tributaries; US Department of Commerce: Washington, DC, USA, 1989.
- SBEP. Sarasota Bay Estuary Program; SBEP: Sarasota, FL, USA, 2014. [Google Scholar]
- Davis, R.A. Morphodynamics of the West-Central Florida Barrier System: The Delicate Balance between Wave- and Tide-Domination. In Coastal Lowlands: Geology and Geotechnology; van der Linden, W.J.M., Cloetingh, S.A.P.L., Kaasschieter, J.P.K., van de Graaff, W.J.E., Vandenberghe, J., van der Gun, J.A.M., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 225–235. [Google Scholar] [CrossRef]
- Wells, R.S. Learning from Nature: Bottlenose Dolphin Care and Husbandry. Zoo Biol. 2009, 28, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.S.; McHugh, K.A.; Douglas, D.C.; Shippee, S.; McCabe, E.B.; Barros, N.B.; Phillips, G.T. Evaluation of Potential Protective Factors against Metabolic Syndrome in Bottlenose Dolphins: Feeding and Activity Patterns of Dolphins in Sarasota Bay, Florida. Front. Endocrinol. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Office of Economic and Demographic Research (EDR). Sarasota County; EDR: Tallahassee, FL, USA, 2019. [Google Scholar]
- USF. Sarasota Bay Watershed. Available online: https://www.sarasota.wateratlas.usf.edu/watershed/geography.asp?wshedid=5&wbodyatlas=watershed (accessed on 14 February 2021).
- Scholes, L.; Faulkner, H.; Tapsell, S.; Downward, S. Urban Rivers as Pollutant Sinks and Sources: A Public Health Concern for Recreational River Users? Water Air Soil Pollut. Focus 2008, 8, 543–553. [Google Scholar] [CrossRef]
- Tarr, J.A. The Search for the Ultimate Sink: Urban Pollution in Historical Perspective; University of Akron Press: Akron, OH, USA, 1984; Volume 51. [Google Scholar] [CrossRef]
- Lacy, R.C.; Wells, R.S.; Scott, M.D.; Allen, J.B.; Barleycorn, A.A.; Urian, K.W.; Hofmann, S. Assessing the Viability of the Sarasota Bay Community of Bottlenose Dolphins. Front. Mar. Sci. 2021, 8, 788086. [Google Scholar] [CrossRef]
- McHugh, K.A.; Allen, J.B.; Barleycorn, A.A.; Wells, R.S. Natal Philopatry, Ranging Behavior, and Habitat Selection of Juvenile Bottlenose Dolphins in Sarasota Bay, Florida. J. Mammal. 2011, 92, 1298–1313. [Google Scholar] [CrossRef]
- Wells, R.S.; Rhinehart, H.L.; Hansen, L.J.; Sweeney, J.C.; Townsend, F.I.; Stone, R.; Casper, D.R.; Scott, M.D.; Hohn, A.A.; Rowles, T.K. Bottlenose Dolphins as Marine Ecosystem Sentinels: Developing a Health Monitoring System. EcoHealth 2004, 1, 246–254. [Google Scholar] [CrossRef]
- Wells, R.S.; Scott, M.D. Bottlenose Dolphin Tursiops Truncatus (Montagu, 1821). Handb. Mar. Mamm. 1999, 6, 137–182. [Google Scholar]
- Würsig, B.; Würsig, M. The Photographic Determination of Group Size, Composition, and Stability of Coastal Porpoises (Tursiops Truncatus). Science 1977, 198, 755–756. [Google Scholar] [CrossRef]
- Urian, K.; Gorgone, A.; Read, A.; Balmer, B.; Wells, R.S.; Berggren, P.; Durban, J.; Eguchi, T.; Rayment, W.; Hammond, P.S. Recommendations for Photo-Identification Methods Used in Capture-Recapture Models with Cetaceans. Mar. Mammal Sci. 2015, 31, 298–321. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.S. Identification Methods. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K., Eds.; Academic Press/Elsevier: San Diego, CA, USA, 2018; pp. 503–509. [Google Scholar]
- Balmer, B.; Zolman, E.; Rowles, T.; Smith, C.; Townsend, F.; Fauquier, D.; George, C.; Goldstein, T.; Hansen, L.; Quigley, B.; et al. Ranging Patterns, Spatial Overlap, and Association with Dolphin Morbillivirus Exposure in Common Bottlenose Dolphins (Tursiops Truncatus) along the Georgia, USA Coast. Ecol. Evol. 2018, 8, 12890–12904. [Google Scholar] [CrossRef] [Green Version]
- Yordy, J.E.; Wells, R.S.; Balmer, B.C.; Schwacke, L.H.; Rowles, T.K.; Kucklick, J.R. Life History as a Source of Variation for Persistent Organic Pollutant (POP) Patterns in a Community of Common Bottlenose Dolphins (Tursiops Truncatus) Resident to Sarasota Bay, FL. Sci. Total Environ. 2010, 408, 2163–2172. [Google Scholar] [CrossRef]
- Wells, R.S.; Tornero, V.; Borrell, A.; Aguilar, A.; Rowles, T.K.; Rhinehart, H.L.; Hofmann, S.; Jarman, W.M.; Hohn, A.A.; Sweeney, J.C. Integrating Life-History and Reproductive Success Data to Examine Potential Relationships with Organochlorine Compounds for Bottlenose Dolphins (Tursiops Truncatus) in Sarasota Bay, Florida. Sci. Total Environ. 2005, 349, 106–119. [Google Scholar] [CrossRef]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary Levels of Seven Phthalate Metabolites in the U.S. Population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 112, 331–338. [Google Scholar] [CrossRef]
- Högberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate Diesters and Their Metabolites in Human Breast Milk, Blood or Serum, and Urine as Biomarkers of Exposure in Vulnerable Populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Tyson, R.B.; Wells, R.S. Sarasota Bay/Little Sarasota Bay Bottlenose Dolphin Abundance, Estimates 2015; US Department of Commerce: Silver Spring, MD, USA, 2016. [CrossRef]
- Genuis, S.J.; Beesoon, S.; Lobo, R.A.; Birkholz, D. Human Elimination of Phthalate Compounds: Blood, Urine, and Sweat (BUS) Study. Sci. World J. 2012, 2012, 615068. [Google Scholar] [CrossRef] [Green Version]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The Environmental Fate of Phthalate Esters: A Literature Review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Hauser, R.; Meeker, J.D.; Park, S.; Silva, M.J.; Calafat, A.M. Temporal Variability of Urinary Phthalate Metabolite Levels in Men of Reproductive Age. Environ. Health Perspect. 2004, 112, 1734–1740. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.L.; Britton, J.A.A.; Calafat, A.M.M.; Ye, X.; Silva, M.J.J.; Reidy, J.A.A.; Galvez, M.P.P.; Brenner, B.L.L.; Wolff, M.S.S. Temporal Variability in Urinary Concentrations of Phthalate Metabolites, Phytoestrogens and Phenols among Minority Children in the United States. Environ. Res. 2008, 106, 257–269. [Google Scholar] [CrossRef]
- Helsel, D.R. Nondetects and Data Analysis: Statistics for Censored Environmental Data; Helsel, D.R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hites, R.A. Correcting for Censored Environmental Measurements. Environ. Sci. Technol. 2019, 53, 11059–11060. [Google Scholar] [CrossRef] [Green Version]
- Helsel, D.R. Much Ado about next to Nothing: Incorporating Nondetects in Science. Ann. Occup. Hygiene. 2009, 54, 257–262. [Google Scholar] [CrossRef]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups Using Dunn’s Test. Stata J. Promot. Commun. Stat. Stata 2015, 15, 292–300. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, C. An Introduction to Using GIS in Marine Biology: Supplementary Workbook Four—Investigating Home Ranges of Individual Animals; Pictish Beast Publications PSLS: Glasgow, UK, 2013. [Google Scholar]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Routledge: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Kie, J.G. A Rule-Based Ad Hoc Method for Selecting a Bandwidth in Kernel Home-Range Analyses. Anim. Biotelemetry 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Urinary Phthalate Metabolites and Their Biotransformation Products: Predictors and Temporal Variability among Men and Women. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 376–385. [Google Scholar] [CrossRef]
- Sakhi, A.K.; Sabaredzovic, A.; Cequier, E.; Thomsen, C. Phthalate Metabolites in Norwegian Mothers and Children: Levels, Diurnal Variation and Use of Personal Care Products. Sci. Total Environ. 2017, 599–600, 1984–1992. [Google Scholar] [CrossRef]
- SWFWMD. Land Use Land Cover 2017. Available online: https://data-swfwmd.opendata.arcgis.com/datasets/land-use-land-cover-2017 (accessed on 15 February 2021).
- Zeng, F.; Cui, K.; Xie, Z.; Wu, L.; Luo, D.; Chen, L.; Lin, Y.; Liu, M.; Sun, G. Distribution of Phthalate Esters in Urban Soils of Subtropical City, Guangzhou, China. J. Hazard. Mater. 2009, 164, 1171–1178. [Google Scholar] [CrossRef]
- Codling, G.; Yuan, H.; Jones, P.D.; Giesy, J.P.; Hecker, M. Metals and PFAS in Stormwater and Surface Runoff in a Semi-Arid Canadian City Subject to Large Variations in Temperature among Seasons. Environ. Sci. Pollut. Res. 2020, 27, 18232–18241. [Google Scholar] [CrossRef]
- Teil, M.-J.; Blanchard, M.; Dargnat, C.; Larcher-Tiphagne, K.; Chevreuil, M. Occurrence of Phthalate Diesters in Rivers of the Paris District (France). Hydrol. Process. 2007, 21, 2515–2525. [Google Scholar] [CrossRef]
- Sarasota County. Unknown Water Resource: Geography and Land Use. Available online: http://www.sarasota.wateratlas.usf.edu/watershed/geography.asp?wshedid=0&wbodyatlas=watershed#impervious (accessed on 3 April 2020).
- Björklund, K.; Cousins, A.P.; Strömvall, A.M.; Malmqvist, P.A.; Palm, A.; Strömvall, A.M.; Malmqvist, P.A. Science of the Total Environment Phthalates and Nonylphenols in Urban Runoff: Occurrence, Distribution and Area Emission Factors. Sci. Total Environ. 2009, 407, 4665–4672. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, C.F.; Dong, C.D. Distribution of Phthalate Esters in Sediments of Kaohsiung Harbor, Taiwan. Soil Sediment Contam. 2013, 22, 119–131. [Google Scholar] [CrossRef]
- The City of Sarasota’s Utilities Department. The Utilities Support Document; The City of Sarasota’s Utilities Department: Sarasota, FL, USA, 2017.
- Miller, K. Pressure from EPA Results in Bee Ridge Water Reclamation Facility Overhaul. Catalyst, 26 March 2020. [Google Scholar]
- Rodriguez, N. Sarasota County Commission Addresses Wastewater Spills. Herald-Tribune. Sarasota, 29 April 2019. [Google Scholar]
- Clara, M.; Windhofer, G.; Hartl, W.; Braun, K.; Simon, M.; Gans, O.; Scheffknecht, C.; Chovanec, A. Occurrence of Phthalates in Surface Runoff, Untreated and Treated Wastewater and Fate during Wastewater Treatment. Chemosphere 2010, 78, 1078–1084. [Google Scholar] [CrossRef]
- Ajay, K.; Behera, D.; Bhattacharya, S.; Mishra, P.K.; Ankit, Y.; Anoop, A. Distribution and Characteristics of Microplastics and Phthalate Esters from a Freshwater Lake System in Lesser Himalayas. Chemosphere 2021, 283, 131132. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and Fate of Microplastics in Urban Areas: A Focus on Paris Megacity. In Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2018; Volume 58, pp. 69–83. [Google Scholar] [CrossRef] [Green Version]
- Leads, R.R.; Weinstein, J.E. Occurrence of Tire Wear Particles and Other Microplastics within the Tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- McEachern, K.; Alegria, H.; Kalagher, A.L.; Hansen, C.; Morrison, S.; Hastings, D. Microplastics in Tampa Bay, Florida: Abundance and Variability in Estuarine Waters and Sediments. Mar. Pollut. Bull. 2019, 148, 97–106. [Google Scholar] [CrossRef]
- Luo, W.; Su, L.; Craig, N.J.; Du, F.; Wu, C.; Shi, H. Comparison of Microplastic Pollution in Different Water Bodies from Urban Creeks to Coastal Waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Iribarne, O.; Gagliardini, D.A.; Lasta, C.; Daleo, P. The Role of the Río de La Plata Bottom Salinity Front in Accumulating Debris. Mar. Pollut. Bull. 2003, 46, 197–202. [Google Scholar] [CrossRef]
- EPA. Proposed Designation of Di-Ethylhexyl Phthalate (DEHP) (1,2-Benzene-Dicarboxylic Acid, 1,2-Bis (2-Ethylhexyl) Ester) (CASRN 117-81-7) as a High-Priority Substance for Risk Evaluation; EPA: Sydney, Australia, 2019. [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals Update; CDC: Atlanta, GA, USA, 2019.
- Federal Register. Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates. Final Rule. Fed. Regist. 2017, 82, 49938–49982. [Google Scholar]
- CPSC. Memorandum to Michael Babich from Kent Carlson Regarding the Toxicity Review of Di(2-Ethylhexyl) Phthalate (DEHP); CPSC: Bethesda, MD, USA, 2010.
- Du, P.; Zhou, Z.; Huang, H.; Han, S.; Xu, Z.; Bai, Y.; Li, X. Estimating Population Exposure to Phthalate Esters in Major Chinese Cities through Wastewater-Based Epidemiology. Sci. Total Environ. 2018, 643, 1602–1609. [Google Scholar] [CrossRef]
- Thurston, S.W.; Mendiola, J.; Bellamy, A.R.; Levine, H.; Wang, C.; Sparks, A.; Redmon, J.B.; Drobnis, E.Z.; Swan, S.H. Phthalate Exposure and Semen Quality in Fertile US Men. Andrology 2016, 4, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are Baleen Whales Exposed to the Threat of Microplastics? A Case Study of the Mediterranean Fin Whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Rhind, S.M.; Kyle, C.E.; Mackie, C.; Yates, K.; Duff, E.I. Geographic Variation in Tissue Accumulation of Endocrine Disrupting Compounds (EDCs) in Grazing Sheep. Environ. Pollut. 2011, 159, 416–422. [Google Scholar] [CrossRef]
- Kubanek, J.; Faulkner, D.J.; Andersen, R.J. Geographic Variation and Tissue Distribution of Endogenous Terpenoids in the Northeastern Pacific Ocean Dorid Nudibranch Cadlina Luteomarginata: Implications for the Regulation of de Novo Biosynthesis. J. Chem. Ecol. 2000, 26, 377–389. [Google Scholar] [CrossRef]
- Calafat, A.M.; McKee, R.H. Integrating Biomonitoring Exposure Data into the Risk Assessment Process: Phthalates [Diethyl Phthalate and Di(2-Ethylhexyl) Phthalate] as a Case Study. Environ. Health Perspect. 2006, 114, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, H.; Skakkebæk, N.E.; Andersson, A.M. Metabolism of Phthalates in Humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of Seven Urinary Phthalate Metabolites in a Human Reference Population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef]
- Horne, J.S.; Garton, E.O. Likelihood Cross-Validation Versus Least Squares Cross-Validation for Choosing the Smoothing Parameter in Kernel Home-Range Analysis. J. Wildl. Manag. 2006, 70, 641–648. [Google Scholar] [CrossRef]

| Total Samples | Retained Samples | ||||
|---|---|---|---|---|---|
| Sampling Year (n = 69) | MEHP Detects (n) | % Total | Mean (s.d.) Detects and Nondetects (ng/mL) | Sampling Year 4 (n = 51) | Geometric Mean and 95% Confidence Interval 4 (ng/mL) |
| 2010 (n = 9) | 3 | 33.00% | 0.61 (1.17) 2 | 2010 (n = 1) | - |
| 2011 (n = 4) | 0 | - | - | 2011 (n = 0) | - |
| 2012 (n = 10) | 7 | 70.00% | 1.91 (0.94) 1 | 2012 (n = 2) | - |
| 2013 (n = 1) | 0 | - | - | 2013 (n = 0) | - |
| 2014 (n = 8) | 8 | 100.00% | 38.42 (24.94) 3 | 2014 (n = 7) | 19.35 (3.94–95.02) |
| 2015 (n = 5) | 5 | 100.00% | 32.12 (4.47) 3 | 2015 (n = 5) | 31.90 (26.90–37.82) |
| 2016 (n = 8) | 3 | 37.50% | 1.16 (1.10) 2 | 2016 (n = 2) | - |
| 2017 (n = 10) | 6 | 60.00% | 1.83 (1.67) 1 | 2017 (n = 5) | 2.03 (0.76–5.40) |
| 2018 (n = 6) | - | - | - | 2018 (n = 0) | - |
| 2019 (n = 8) | 7 | 87.50% | 2.06 (2.18) 1 | 2019 (n = 7) | 1.40 (0.48–4.05) |
| Temporal Group | Mean and Standard Deviation (ng/mL) | Percent > LOD (95% CI) | Pairwise Comparisons of Percent Detect between Temporal Groups | |
|---|---|---|---|---|
| Cohort 2 | Cohort 3 | |||
| Cohort 1 (n = 10) | 0.43 (0.79) 1 | 30.00 (1.60–58.40) | p = 0.0016 | p = 0.0059 |
| Cohort 2 (n = 12) | 33.71 (18.43) 2 | 100.00 (-) | - | p = 0.0059 |
| Cohort 3 (n = 29) | 1.24 (1.69) 1 | 51.72 (23.45–54.86) | - | - |
| Detection Status | Total Area (km2; 95% UD) | Total Area: % of Ranging Pattern Overlap (95% UD) | Core Area (km2; 50% UD) | Core Area: % of Ranging Pattern Overlap (50% UD) |
|---|---|---|---|---|
| Detect | 108.79 | 55.78% | 17.77 | 45.00% |
| Nondetect | 79.17 | 76.65% | 19.67 | 40.66% |
| Overlap | 60.69 | - | 8.00 | - |
| Temporal Grouping | Detection Status | Core Area (km2; 50% UD) | Core Area: % of Ranging Pattern Overlap (50% UD) |
|---|---|---|---|
| Cohort 1 | Detect | 4.89 | 62.64% |
| Nondetect | 6.50 | 69.03% | |
| Overlap | 2.92 | - | |
| Cohort 2 | Detect | 11.09 | 100% |
| Nondetect | N/A | N/A | |
| Overlap | N/A | N/A | |
| Cohort 3 | Detect | 12.70 | 69.03 |
| Nondetect | 5.26 | 48.01 | |
| Overlap | 5.70 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziobak, M.K.; Balmer, B.C.; Wells, R.S.; Pisarski, E.C.; Wirth, E.F.; Hart, L.B. Temporal and Spatial Evaluation of Mono(2-ethylhexyl) Phthalate (MEHP) Detection in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Oceans 2022, 3, 231-249. https://doi.org/10.3390/oceans3030017
Dziobak MK, Balmer BC, Wells RS, Pisarski EC, Wirth EF, Hart LB. Temporal and Spatial Evaluation of Mono(2-ethylhexyl) Phthalate (MEHP) Detection in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Oceans. 2022; 3(3):231-249. https://doi.org/10.3390/oceans3030017
Chicago/Turabian StyleDziobak, Miranda K., Brian C. Balmer, Randall S. Wells, Emily C. Pisarski, Ed F. Wirth, and Leslie B. Hart. 2022. "Temporal and Spatial Evaluation of Mono(2-ethylhexyl) Phthalate (MEHP) Detection in Common Bottlenose Dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA" Oceans 3, no. 3: 231-249. https://doi.org/10.3390/oceans3030017







