Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef
Abstract
:1. Introduction
2. Materials and Methods
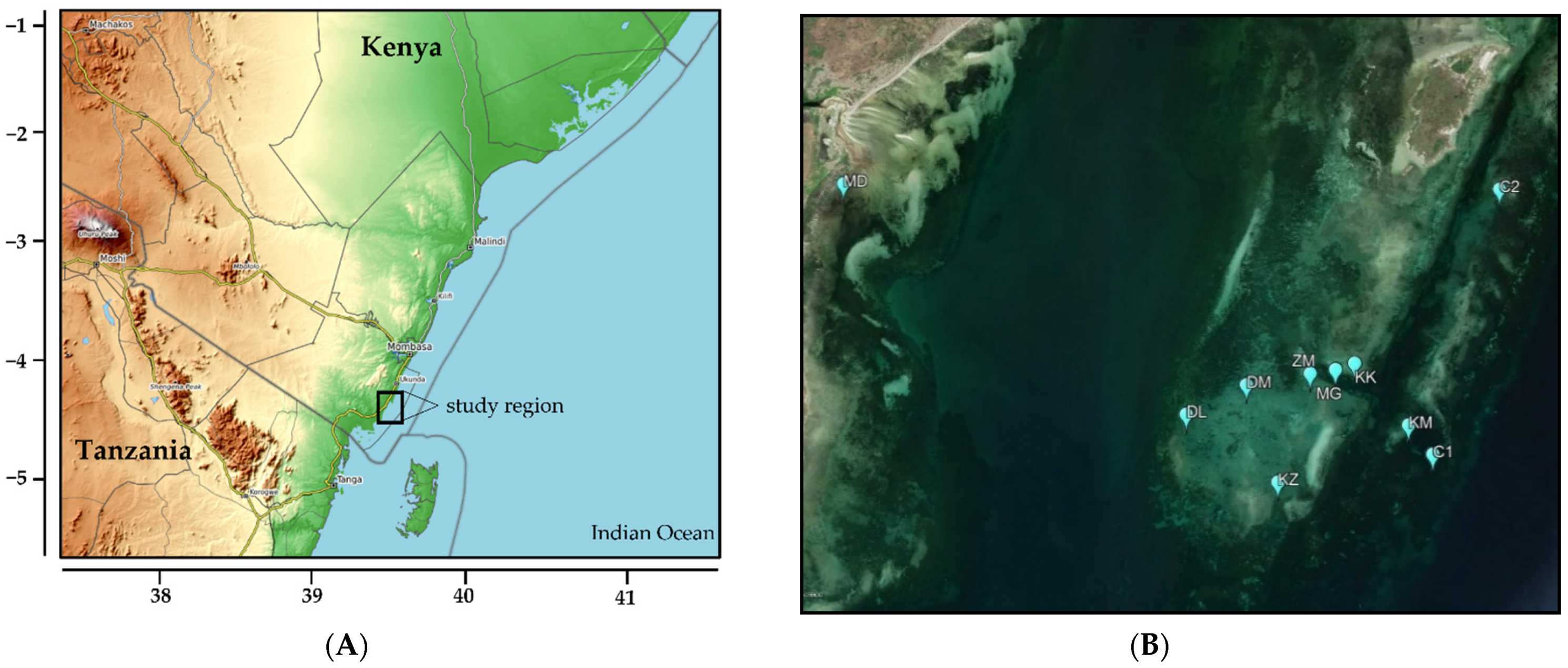
2.1. Study Region and Sites
2.2. Monitoring of Coral Reef Fishes and Corals
2.3. Benthic Analyses
2.4. Statistical Analysis
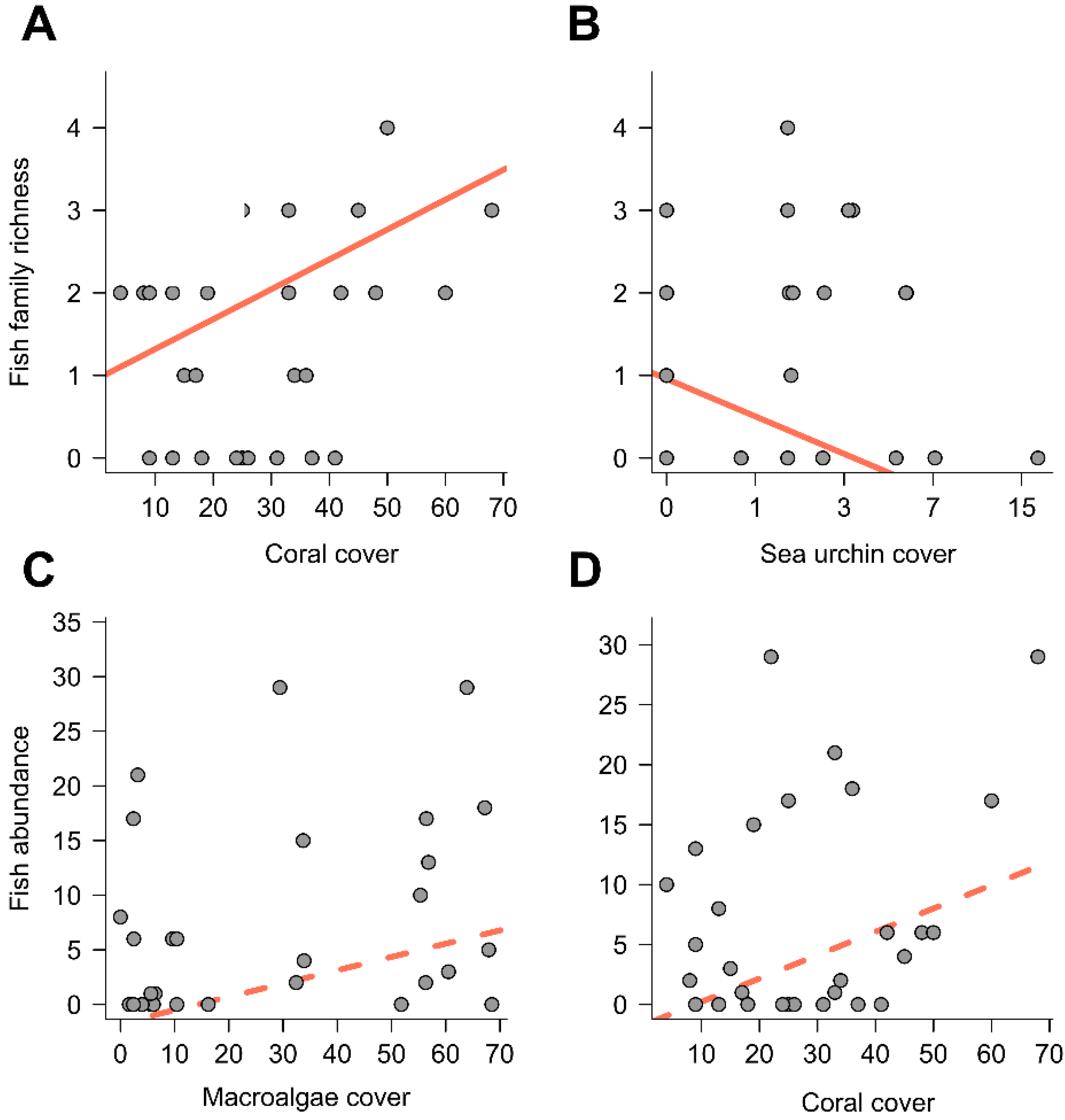
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, C.M.; McClean, C.J.; Veron, J.E.N.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [Green Version]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, Australia, 2000; Volume 1. [Google Scholar]
- Ogden, J.C.; Lobel, P.S. The role of herbivorous fishes and urchins in coral reefs communities. Environ. Biol. Fishes 1978, 3, 49–63. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Obura, D.O.; Grimsditch, G. Resilience Assessment of Coral Reefs—Assessment Protocol for Coral Reefs, Focusing on Coral Bleaching and Thermal Stress; IUCN: Gland, Switzerland, 2009; p. 70. [Google Scholar]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Knowlton, N.; Jackson, J.B.C. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008, 6, e54. [Google Scholar] [CrossRef] [Green Version]
- Maynard, J.A.; Marshall, P.A.; Parker, B.; Mcleod, E.; Ahmadia, G.; Van Hooidonk, R.; Planes, S.; Williams, G.J.; Raymundo, L.; Beeden, R.; et al. A Guide to Assessing Coral Reef Resilience for Decision Support; UN Environment: Nairobi, Kenya, 2017. [Google Scholar]
- Tebbett, S.B.; Bellwood, D.R. Functional links on coral reefs: Urchins and triggerfishes, a cautionary tale. Mar. Environ. Res. 2018, 141, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, F.; Bianchi, C.N.; Lasagna, R.; Morri, C.; Russo, G.F.; Sandulli, R. Corals in high diversity reefs resist human impact. Ecol. Indic. 2016, 70, 106–113. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Global change and coral reefs: Impacts on reefs, economies and human cultures. Glob. Chang. Biol. 1996, 2, 547–558. [Google Scholar] [CrossRef]
- Magris, R.A.; Grech, A.; Pressey, R.L. Cumulative human impacts on coral reefs: Assessing risk and management implications for Brazilian reefs. Diversity 2018, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Nepote, E.; Bianchi, C.N.; Chiantore, M.; Morri, C.; Montefalcone, M. Pattern and intensity of human impact on coral reefs depend on depth along the reef profile and on the descriptor adopted. Estuar. Coast. Shelf Sci. 2016, 178, 86–91. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bonaldo, R.M.; Fox, R.J.; Bellwood, D.R. Sediments and herbivory as sensitive indicators of coral reef degradation. Ecol. Soc. 2016, 21, 21. [Google Scholar] [CrossRef] [Green Version]
- McClanahan, T.R.; Muthiga, N.; Mangi, S. Coral and algal changes after the 1998 coral bleaching: Interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 2001, 19, 380–391. [Google Scholar] [CrossRef]
- Safaie, A.; Silbiger, N.J.; McClanahan, T.R.; Pawlak, G.; Barshis, D.J.; Hench, J.L.; Rogers, J.S.; Williams, G.J.; Davis, K.A. High-frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 2018, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Coral bleaching—How and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef]
- Rudi, E.; Iskandar, T.; Fadli, N.; Hidayati. Effects of coral bleaching on reef fish fisheries at Sabang. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Connolly, S.R.; Tanner, J. Environmental and geometric constraints on Indo-Pacific coral reef biodiversity. Ecol. Lett. 2005, 8, 643–651. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Shafir, S.H. Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia 1990, 83, 362–370. [Google Scholar] [CrossRef]
- Bahartan, K.; Zibdah, M.; Ahmed, Y.; Israel, A.; Brickner, I.; Abelson, A. Macroalgae in the coral reefs of Eilat (Gulf of Aqaba, Red Sea) as possible indicator of reef degradation. Mar. Pollut. Bull. 2010, 60, 759–764. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Uku, J.N.; Machano, H. Effect of macroalgal reduction on coral-reef fish in the Watamu Marine National Park, Kenya. Mar. Freshw. Res. 2002, 53, 223–231. [Google Scholar] [CrossRef]
- Hill, J.; Wilkinson, C. Methods for Ecological Monitoring of Coral Reefs—A Resource for Managers; Australian Institute of Marine Science: Townsville, Australia, 2004; p. 117. [Google Scholar]
- Green, A.L.; Bellwood, D.R. Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience—A Practical Guide for Reef Managers in the Asia Pacific Region; IUCN working group on Climate Change and Coral Reefs: Gland, Switzerland, 2009; p. 70. [Google Scholar]
- Humphries, A.T.; McClanahan, T.R.; McQuaid, C.D. Algal turf consumption by sea urchins and fishes is mediated by fisheries management on coral reefs in Kenya. Coral Reefs 2020, 39, 1137–1146. [Google Scholar] [CrossRef]
- McManus, J.W.; Polsenberg, J.F. Coral-algal phase shifts on coral reefs: Ecological and environmental aspects. Prog. Oceanogr. 2004, 60, 263–279. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral-macroalgal phase shifts or reef resilience: Links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Reverter, M.; Jackson, M.; Daraghmeh, N.; von Mach, C.; Milton, N. 11-yr of coral community dynamics in reefs around Dahab (Gulf of Aqaba, ed Sea): The collapse of urchins and rise of macroalgae and cyanobacterial mats. Coal Reefs 2020, 39, 1605–1618. [Google Scholar] [CrossRef]
- Rempel, H.S.; Bodwin, K.N.; Ruttenberg, B.I. Impacts of parrotfish predation on a major reef-building coral: Quantifying healing rates and thresholds of coral recovery. Coral Reefs 2020, 39, 1441–1452. [Google Scholar] [CrossRef]
- Roach, N.F.; Little, M.; Arts, M.G.I.; Huckeba, J.; Haas, A.F.; George, E.E.Q.R.A.; Cobián-Güemes, A.; Naliboff, D.S.; Silveira, C.; Vermeij, M.J.A.; et al. A multiomic analysis of in situ coral-turf algal interactions. Proc. Natl. Acad. Sci. USA 2020, 117, 13588–13595. [Google Scholar] [CrossRef]
- Nozawa, Y.; Lin, C.; Meng, P. Sea urchins (diadematids) promote coral recover via recruitment on Taiwanese reefs. Coral Reefs 2020, 39, 1199–1207. [Google Scholar] [CrossRef]
- Leahy, S.M.; Russ, G.R.; Abesamis, R.A. Primacy of bottom-up effects on a butterflyfish assemblage. Mar. Freshw. Res. 2016, 67, 1175–1185. [Google Scholar] [CrossRef]
- Soule, D.F.e.a. Marine Organisms as Indicators; Springer: New York, NY, USA, 1988. [Google Scholar]
- Reese, E.S. Predation on corals by fishes of the family Chaetodontidae: Implications for conservation and management of coral reef ecosystems. Bull. Mar. Sci. 1981, 31, 594–604. [Google Scholar]
- Chabanet, P.; Adjeroud, M.; Andrèfouet, S.; Bozec, Y.M.; Ferraris, J.; Garcia-Charton, J.; Schrimm, M. Human-induced physical disturbances and their indicators on coral reef habitats: A multi-scale approach. Aquat. Living Resour. 2005, 18, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Noonan, K.R.; Childress, M.J. Association of butterflyfishes and stony coral tissue loss disease in the Florida Keys. Coral Reefs 2020, 39, 1581–1590. [Google Scholar] [CrossRef]
- Nicolet, K.J.; Chong-Seng, K.M.; Pratchett, M.S.; Willis, B.L.; Hoogenboom, M.O. Predation scars may influence host susceptibility to pathogens: Evaluating the role of corallivores as vectors of coral disease. Sci. Rep. 2018, 8, 5258. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.M.; Ezzat, L.; Burkepile, D.E. Corallivory in the anthropocene: Interactive effects of anthropogenic stressors and corallivory on coral reefs. Front. Mar. Sci. 2019, 5, 525. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P.; Connell, J.H. Multiple stressors on coral reefs: A long-term perspective. Limnol. Oceanogr. 1999, 44, 932–940. [Google Scholar] [CrossRef]
- Bell, J.D.; Galzin, R. Influence of live coral on coral reef fish communities. Mar. Ecol. Prog. Ser. 1984, 15, 265–274. [Google Scholar] [CrossRef]
- Chabanet, P.; Ralambondrainy, H.; Amanieu, M.; Faure, G.; Galzin, R. Relationships between coral reef substrata and fish. Coral Reefs 1997, 19, 93–102. [Google Scholar] [CrossRef]
- Done, T.J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 1992, 247, 121–132. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Glaesel, H.; Rubens, J.; Kiambo, R. The effects of traditional fisheries management on fisheries yields and the coral-reef ecosystem of southern Kenya. Environ. Conserv. 1997, 24, 105–120. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Van Pottelbergh, I.; Kairo, J.G.; Cannicci, S.; Koedam, N. Human-impacted mangroves in Gazi (Kenya): Predicting future vegetation based on retrospective remote sensing, social surveys, and tree distribution. Mar. Ecol. Prog. Ser. 2004, 272, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Cowburn, B.; Samoilys, M.A.; Obura, D. The current status of coral reefs and their vulnerability to climate change and multiple human sresses in the Comoros Archipelago, Western India. MArine Pollut. Bull. 2018, 133, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Lieske, E.; Myers, R. Coral Reef Fishes; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel interference—Understanding AIC and BIC in model selection. Sociol. Methoads Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Brooker, R.M.; Munday, P.L.; Mcleod, I.M. Habitat preferences of a corallivorous reef fish: Predation risk versus food quality. Coral Reefs 2013, 32, 613–622. [Google Scholar] [CrossRef]
- Smith, J.E.; Brainard, R.; Carter, A.; Grillo, S.; Edwards, C.; Harris, J.; Lewis, L.; Obura, D.; Rohwer, F.; Sala, E.; et al. Re-evaluating the health of coral reef communities: Baselines and evidence for human impacts across the central Pacific. Proc. R. Soc. B 2016, 283, 20151985. [Google Scholar] [CrossRef] [PubMed]
- Lieske, E.; Myers, R. Korallenriff-Führer Rotes Meer; Franckh-Kosmos Verlags GmbH & Co. KG: Stuttgart, Germany, 2010; Volume 2. [Google Scholar]
- Roberts, C.M.; Ormond, R. Habitat complexity and coral reef diversity and abundance on Red Sea fringing reefs. Mar. Ecol. Prog. Ser. 1987, 41, 1–8. [Google Scholar] [CrossRef]
- Fowler, A.J. Spatial and temporal patterns of distribution and abundance of chaetodontid fishes at One Tree Reef, southern GBR. Mar. Ecol. Prog. Ser. 1990, 64, 39–53. [Google Scholar] [CrossRef]
- Sebens, K.P. Biodiversity of coral reefs: What are we losing and why? Am. Zool. 1994, 34, 115–133. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Hay, M.E. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. USA 2008, 105, 16201–16206. [Google Scholar] [CrossRef] [Green Version]
- McClanahan, T.R.; Kamukuru, A.T.; Muthiga, N.A.; Gilagabher Yebio, M.; Obura, D. Effect of sea urchin reductions to algae, coral and fish populations. Conserv. Biol. 1996, 10, 136–154. [Google Scholar] [CrossRef]
- Sammarco, P.W. Echinoid grazing as a structuring force in coral communities: Whole reef manipulations. J. Exp. Mar. Biol. Ecol. 1982, 61, 31–35. [Google Scholar] [CrossRef]
- Carpenter, R.C. Mass mortality of Diadema antillarum: 1. long-term effects on sea urchin population dynamics and coral reef algal communitites. Mar. Biol. 1990, 104, 67–77. [Google Scholar] [CrossRef]
- Hughes, T.P.; Reed, D.C.; Boyle, M.J. Herbivory on coral reefs: Community structure following mass mortalities of sea urchins. J. Exp. Mar. Biol. Ecol. 1987, 113, 39–59. [Google Scholar] [CrossRef]
- McClanahan, T.R. A coral reef ecosystem-fisheries model: Impacts of fishing intensity and catch selection on reef structure and process. Ecol. Model. 1995, 80, 1–19. [Google Scholar] [CrossRef]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–2009. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A. Changes in Kenyan coral reef community structure and function due to exploitation. Hydrobiologia 1988, 166, 269–276. [Google Scholar] [CrossRef]
- Carreiro-Silva, M.; McClanahan, T.R. Echinoid bioerosion and herbivory on Kenyan coral reefs: The role of protection from fishing. J. Exp. Mar. Biol. Ecol. 2001, 262, 133–153. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.J.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B.L. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Littler, M.M.; Littler, D.S. Assessment of coral reefs using herbivory/nutrient assays and indicator groups of benthic primary producers: A critical synthesis, proposed protocols. and critique of management strategies. Aquat. Conserv. Mar. Freschwater Ecosyst. 2007, 17, 195–215. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Collier, C.J.; Henderson, G.M.; McKenzie, L.J. Tropical seagrass meadows modify seawater carbon chemistry: Implications for coral reefs impacted by ocean acidification. Environ. Res. Lett. 2012, 7, 7. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, I.E.; Olsen, Y.S.; Ramajo, L.; Basoo, L.; Steckbauer, A.; Moore, T.S.; Howard, J.; Duarte, C.M. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 2014, 11, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Nanami, A. Spatial distribution and feeding substrate of butterflyfishes (family Chaetodontidae) on an Okinawan coral reef. PeerJ 2020, 8, e9666. [Google Scholar] [CrossRef] [PubMed]
- Hoey, A.S.; Pratchett, M.S.; Cvitanovic, C. High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southermist coral reef assemblages. PLoS ONE 2011, 6, e25824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. Adv. Mar. Biol. 1998, 34, 203–352. [Google Scholar]
- Luckhurst, B.; Luckhurst, K. Estimating total abundance of a large temperate reef fish using visual strip-transect. Mar. Biol. 1987, 96, 469–478. [Google Scholar]
- Cole, A.J.; Pratchett, M.S.; Jones, G.P. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish. 2008, 9, 286–307. [Google Scholar] [CrossRef]
- Darling, E.S.; Graham, N.A.J.; Januchowski-Hartley, F.A.; Nash, K.L.; Pratchett, M.S.; Wilson, S.K. Relationship between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 2017, 36, 561–575. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClanahan, T.R. Kenyan coral reef lagoon fish: Effects of fishing, substrate complexity, and sea urchins. Coral Reefs 1994, 13, 231–241. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Nugues, M.M.; Mwachireya, S. Fish and sea urchin herbivory and competition in Kenyan coral reef lagoons: The role of reef management. J. Exp. Mar. Biol. Ecol. 1994, 184, 237–254. [Google Scholar] [CrossRef]
- McClanahan, T.R. Recovery of a coral reef keystone predator, Balistapus undulatus, in East African marine parks. Biol. Conserv. 2000, 94, 191–198. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Obura, D. Status of Kenyan coral reefs. Coast. Manag. 1995, 23, 57–76. [Google Scholar] [CrossRef]
- Watson, M.; Righton, D.; Austin, T.; Ormond, R. The effects of fishing on coral reef fish abundance and diversity. J. Mar. Biol. Assoc. United Kingd. 1996, 76, 229–233. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Mwaguni, S.; Muthiga, N.A. Management of the Kenyan coast. Ocean. Coast. Manag. 2005, 48, 901–931. [Google Scholar] [CrossRef]

| Chale 1 | Chale 2 | Doa Lower | Doa Upper | Kijamba Mkandi | Kiziwa Kule | Kukuni | Mikingamo | Mikingamo 2 | Mwandamo | |
|---|---|---|---|---|---|---|---|---|---|---|
| Acanthuridae | 4.0 ± 2.6 | 0.0 | 0.0 | 0.0 | 18 ± 4.0 | 0.7 ± 1.2 | 6.0 ± 7.9 | 2.0 ± 1.7 | 6.0 ± 5.3 | 3.0 ± 1.7 |
| Chaetodontidae | 4.3 ± 3.1 | 0.7 ± 1.2 | 0.0 | 0.3 ± 0.6 | 2.0 ± 1.7 | 1.0 ± 1.7 | 7.7 ± 7.1 | 2.0 ± 1.7 | 5.0 ± 5.0 | 1.7 ± 1.2 |
| Ephippidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 ± 1.7 | 0.0 |
| Lutjanidae | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 ± 2.3 | 0.0 | 1.0 ± 1.7 | 0.0 | 0.0 | 0.7 ± 1.2 |
| Pomacanthidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.6 |
| Scaridae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.6 | 0.0 | 1.3 ± 1.2 | 0.0 |
| Siganidae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 ± 0.6 | 0.3 ± 0.6 |
| Chale 1 | Chale 2 | Doa Lower | Doa Upper | Kijamba Mkandi | Kiziwa Kule | Kukuni | Mikingamo | Mikingamo 2 | Mwandamo | |
|---|---|---|---|---|---|---|---|---|---|---|
| Acanthastrea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 | 0.67 ± 0.58 |
| Acropora | 0.00 | 4.33 ± 1.15 | 2.00 ± 1.00 | 4.00 ± 2.00 | 14.00 ± 6.56 | 3.00 ± 1.73 | 1.33 ± 1.53 | 3.33 ± 3.06 | 2.00 | 7.33 ± 7.77 |
| Astreopora | 0.00 | 1.00 ± 1.73 | 3.00 ± 1.00 | 3.67 ± 2.52 | 0.00 | 0.33 ± 0.58 | 1.00 ± 1.73 | 1.00 ± 1.00 | 1.33 ± 1.73 | 0.33 ± 0.58 |
| Diploastrea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 | 0.00 | 0.00 |
| Echinopora | 0.33 ± 0.58 | 1.33 ± 0.58 | 1.33 ± 1.53 | 0.00 | 0.33 ± 0.58 | 0.00 | 1.67 ± 2.08 | 0.00 | 1.00 ± 1.73 | 3.33 ± 4.04 |
| Favia | 0.67 ± 1.15 | 5.33 ± 1.53 | 0.00 | 0.00 | 0.67 ± 0.58 | 0.33 ±0.58 | 0.00 | 2.00 ± 2.65 | 0.00 | 1.33 ± 1.15 |
| Favites | 0.00 | 1.33 ± 1.15 | 1.33 ± 1.15 | 0.33 ± 0.58 | 1.00 ± 1.73 | 1.67 ± 2.08 | 0.33 ± 0.58 | 2.67 ± 1.53 | 0.67 ± 0.58 | 0.67 ± 0.58 |
| Fungia | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 ± 5.20 | 0.00 | 3.33 ± 2.52 | 1.00 ± 1.73 | 0.00 | 0.33 ± 0.58 |
| Galaxea | 2.33 ± 1.15 | 1.67 ± 1.53 | 0.67 ± 1.15 | 1.00 ± 1.00 | 19.33 ± 8.08 | 0.33 ± 0.58 | 2.33 ± 2.08 | 0.33 ± 0.58 | 0.67 ± 0.58 | 0.00 |
| Goniastrea | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Goniopora | 1.67 ± 1.53 | 2.67 ± 1.15 | 1.00 ± 1.00 | 0.00 | 0.00 | 0.00 | 0.33 ± 0.58 | 4.67 ± 1.53 | 2.33 ± 3.21 | 0.67 ± 1.15 |
| Hydnophora | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 | 0.33 ± 0.58 | 0.67 ± 0.58 | 0.67 ± 0.58 | 0.33 ± 0.58 | 0.00 | 0.00 |
| Leptoria | 0.00 | 2.67 ± 1.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 |
| Lobophyillia | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Millepora | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Montipora | 2.0 ± 1.73 | 1.33 ± 1.53 | 0.67 ± 0.58 | 0.00 | 1.00 ± 1.73 | 0.33 ± 0.58 | 0.33 ± 0.58 | 1.67 ± 1.15 | 2.00 ± 1.00 | 0.00 |
| Pavona | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 | 0.00 | 0.00 | 0.67 ± 1.15 | 1.00 ± 1.00 | 0.00 | 3.00 ± 2.00 |
| Platygyra | 0.00 | 1.33 ± 1.15 | 0.67 ± 0.58 | 0.00 | 0.67 ± 1.15 | 0.67 ± 0.58 | 1.00 ± 1.15 | 2.67 ± 0.58 | 0.00 | 2.67 ± 3.29 |
| Plesiastrea | 0.00 | 1.00 ± 1.00 | 0.67 ± 0.58 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.33 ± 0.58 | 0.00 |
| Pocillopora | 0.00 | 1.00 | 0.33 ± 0.58 | 1.33 ± 0.58 | 12.67 ± 3.21 | 1.67 ± 2.08 | 1.67 ± 1.15 | 0.67 ± 1.15 | 0.67 ± 0.58 | 7.00 ± 6.00 |
| Porites (Branching) | 0.00 | 0.00 | 0.33 ± 0.58 | 0.00 | 1.33 ± 1.15 | 0.00 | 0.00 | 0.00 | 0.67 ± 1.15 | 4.33 ± 5.13 |
| Porites (Massive) | 0.00 | 4.67 ± 0.58 | 16.67 ± 1.53 | 10.67 ± 4.73 | 0.00 | 1.33 ± 1.15 | 0.67 ± 0.58 | 17.33 ± 8.08 | 16.00 ± 9.17 | 4.33 ± 2.52 |
| Seriatopora | 0.00 | 0.00 | 0.00 | 0.33 ± 0.58 | 0.33 ± 0.58 | 0.00 | 0.00 | 0.33 ± 0.58 | 0.33 ± 0.58 | 0.00 |
| Stylophora | 0.00 | 0.00 | 0.00 | 0.67 ± 0.58 | 0.00 | 0.00 | 3.00 ± 1.73 | 4.33 ± 5.86 | 1.00 ± 1.00 | 0.00 |
| Symphilla | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Chale 1 | Chale 2 | Doa Lower | Doa Upper | Kijamba Mkandi | Kiziwa Kule | Kukuni | Mikingamo 1 | Mikingamo 2 | Mwandamo | |
|---|---|---|---|---|---|---|---|---|---|---|
| AHAL | 0.27 ± 0.46 | 0.00 | 0.27 ± 0.46 | 0.00 | 0.27 ± 0.46 | 0.81 ± 1.40 | 0.57 ± 0.96 | 0.00 | 1.39 ± 2.41 | 0.00 |
| AMAC | 56.15 ± 0.76 | 19.67 ± 11.41 | 2.68 ± 1.21 | 5.50 ± 1.31 | 62.51 ± 5.52 | 62.74 ± 9.48 | 41.20 ± 16.85 | 5.91 ± 3.57 | 3.74 ± 1.68 | 14.76 ± 17.35 |
| ATRF | 33.27 ± 1.89 | 51.48 ± 15.07 | 32.45 ± 8.47 | 46.41 ± 16.83 | 14.67 ± 2.46 | 18.63 ± 8.59 | 26.97 ± 20.59 | 63.63 ± 5.91 | 54.50 ± 13.95 | 28.31 ± 7.56 |
| BS | 0.00 | 0.00 | 0.00 | 0.56 ± 0.96 | 0.00 | 0.00 | 3.56 ± 3.38 | 0.28 ± 0.48 | 0.00 | 0.00 |
| HC | 0.53 ± 0.92 | 4.32 ± 1.89 | 14.22 ± 0.37 | 2.18 ± 1.72 | 10.73 ± 6.05 | 0.53 ± 0.92 | 8.33 ± 3.58 | 1.34 ± 1.66 | 10.47 ± 6.78 | 16.28 ± 8.56 |
| DC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.40 ± 2.42 | 0.00 | 0.00 | 0.00 |
| URC | 0.00 | 1.34 ± 1.24 | 3.77 ± 3.08 | 2.53 ± 2.28 | 1.89 ± 1.68 | 0.27 ± 0.46 | 1.88 ± 3.25 | 8.31 ± 8.37 | 2.22 ± 0.97 | 0.53 ± 0.92 |
| RDC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.35 ± 5.80 | 2.70 ± 4.68 | 0.00 | 0.00 |
| RUB | 0.00 | 0.54 ± 0.94 | 7.78 ± 1.71 | 3.89 ± 3.94 | 4.84 ± 4.47 | 3.49 ± 1.22 | 0.00 | 4.83 ± 5.24 | 10.46 ± 13.95 | 12.10 ± 10.67 |
| SND | 5.39 ± 5.19 | 15.94 ± 7.01 | 28.53 ± 13.15 | 16.45 ± 8.69 | 0.00 | 7.03 ± 3.27 | 4.63 ± 7.34 | 10.04 ± 9.87 | 15.04 ± 7.34 | 7.37 ± 7.03 |
| SGR | 3.06 ± 5.29 | 5.07 ± 2.31 | 8.91 ± 6.99 | 21.66 ± 20.03 | 4.27 ± 3.78 | 6.22 ± 0.88 | 9.23 ± 9.29 | 0.00 | 0.80 ± 1.39 | 16.03 ± 13.00 |
| SC | 1.33 ± 1.22 | 1.62 ± 1.41 | 1.39 ± 2.41 | 0.83 ± 1.44 | 0.81 ± 0.80 | 0.27 ± 0.46 | 0.00 | 0.27 ± 0.46 | 0.58 ± 0.50 | 1.33 ± 2.31 |
| UNID/OT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 ± 0.50 | 0.00 | 0.80 ± 1.39 | 3.29 ± 1.67 |
| OBS/TWS | 1.33 ± 0.46 | 0.80 ± 0.80 | 1.07 ± 0.46 | 2.93 ± 1.67 | 0.53 ± 0.46 | 1.33 ± 0.92 | 2.67 ± 1.22 | 1.07 ± 1.22 | 1.87 ± 1.67 | 1.07 ± 0.46 |
| Response | AMAC | ATRF | FABUN | FFM | Lg1(INV) | SGR | R2 | |
|---|---|---|---|---|---|---|---|---|
| CGR | −0.5676 ** | −0.3010 . | 146.1 | 0.3581 | ||||
| −0.8815 ** | −0.4119 | −0.4850 * | 146.2 | 0.4139 | ||||
| −0.5195 ** | 147.3 | 0.2698 | ||||||
| CA | 0.3333 * | 0.3908 * | 0.5389 ** | 247.0 | 0.3573 | |||
| −0.1330 | 0.4886 ** | 248.6 | 0.3211 | |||||
| 0.3968 * | 0.6091 ** | 249.3 | 0.3702 |
| Response | AMAC | ATRF | CA | Lg1(INV) | SGR | R2 | |
|---|---|---|---|---|---|---|---|
| FFR | 0.4747 * | −0.4291 * | 98.4 | 0.21930 | |||
| 0.4238 * | −0.4498 * | −0.2056 | 99.8 | 0.25690 | |||
| 0.2809 | 100.6 | 0.07892 | |||||
| FA | 0.3562. | 0.3557. | 217.2 | 0.20680 | |||
| −0.3186 . | −0.3737 . | 218.8 | 0.16360 | ||||
| 0.2908 | 218.8 | 0.08457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditzel, P.; König, S.; Musembi, P.; Peters, M.K. Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef. Oceans 2022, 3, 1-14. https://doi.org/10.3390/oceans3010001
Ditzel P, König S, Musembi P, Peters MK. Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef. Oceans. 2022; 3(1):1-14. https://doi.org/10.3390/oceans3010001
Chicago/Turabian StyleDitzel, Pia, Sebastian König, Peter Musembi, and Marcell K. Peters. 2022. "Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef" Oceans 3, no. 1: 1-14. https://doi.org/10.3390/oceans3010001
APA StyleDitzel, P., König, S., Musembi, P., & Peters, M. K. (2022). Correlation between Coral Reef Condition and the Diversity and Abundance of Fishes and Sea Urchins on an East African Coral Reef. Oceans, 3(1), 1-14. https://doi.org/10.3390/oceans3010001







