Influence of the Seasonal Thermocline on the Vertical Distribution of Larval Fish Assemblages Associated with Atlantic Bluefin Tuna Spawning Grounds
Abstract
1. Introduction
2. Materials and Methods
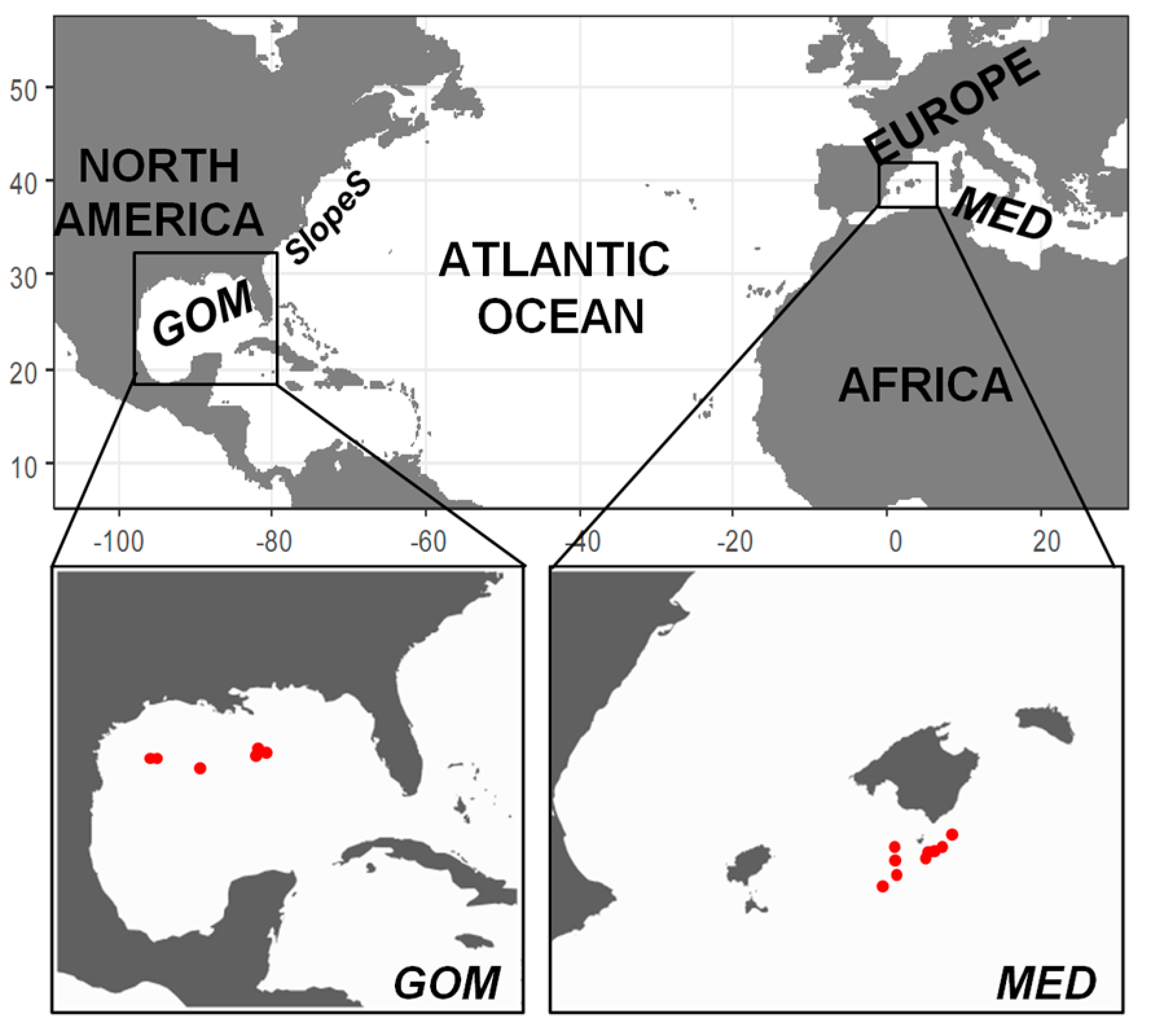
2.1. Field Sampling
2.2. Description of Assemblages: Taxa and Groups by Adult Habitat
2.3. Influence of Environment on the Vertical Distribution of the Assemblages
3. Results
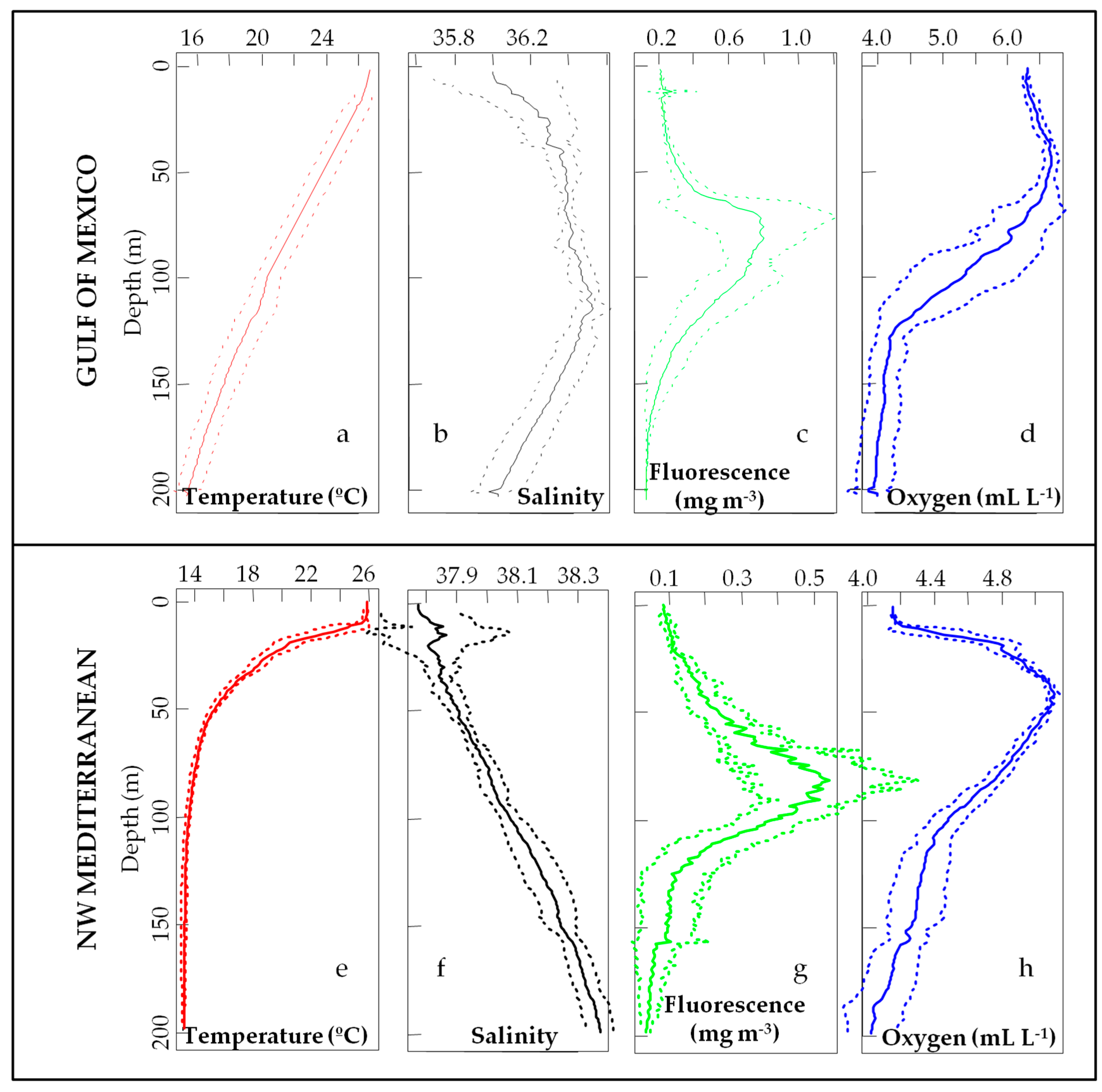
3.1. Environmental Scenario
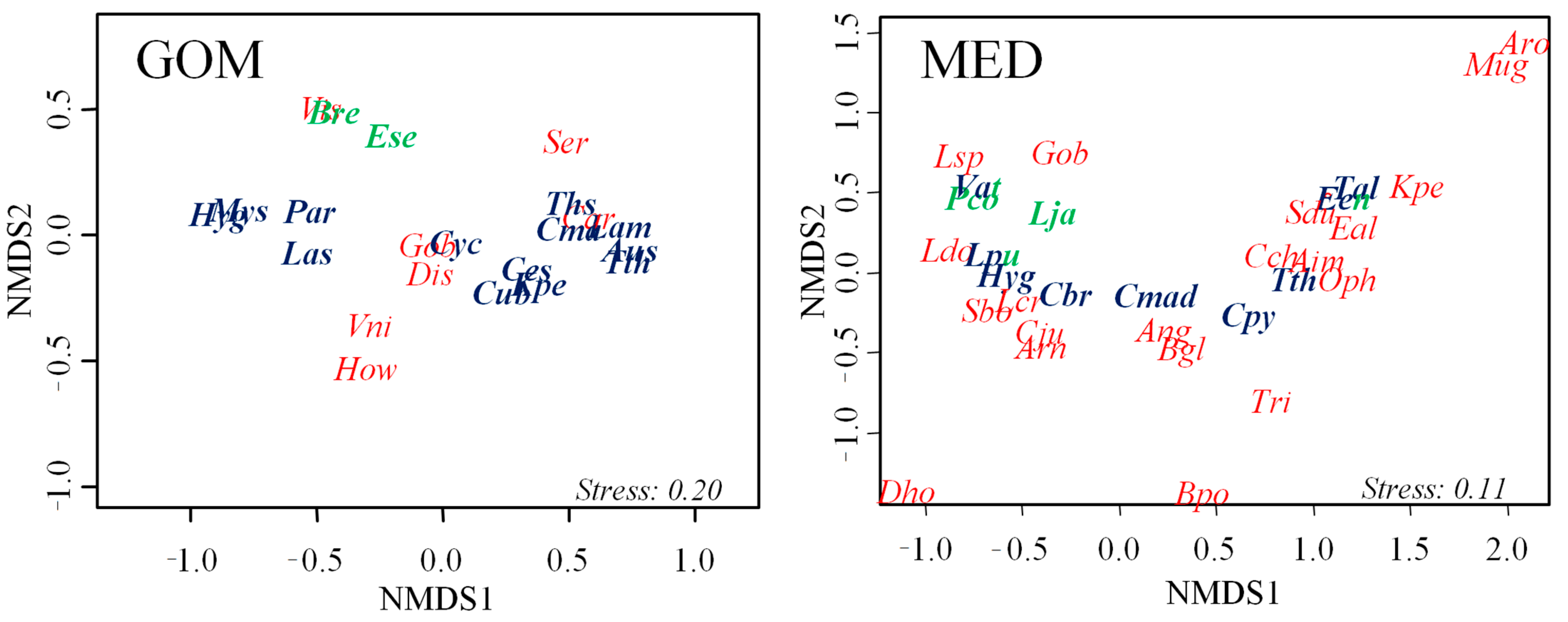
3.2. Description of Assemblages: Taxa and Groups by Adult Habitat
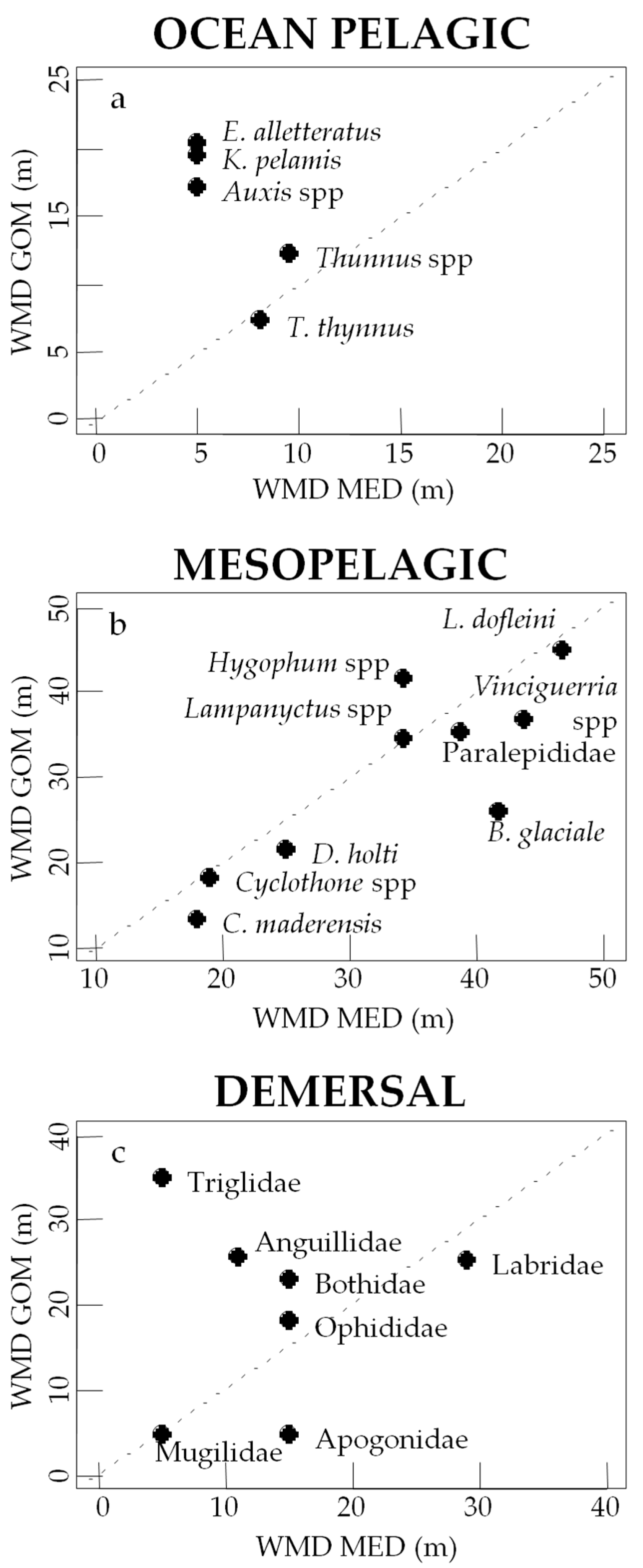
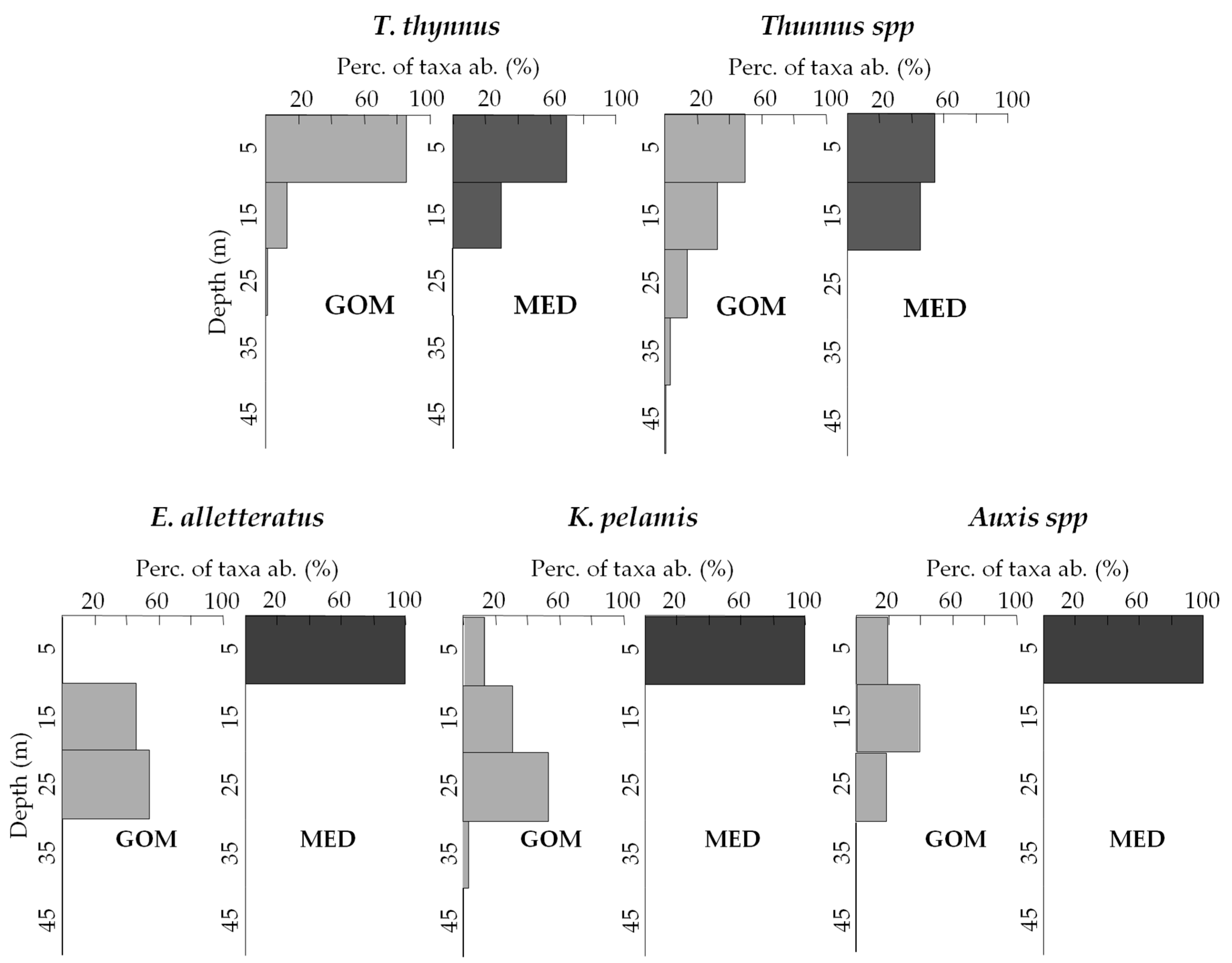
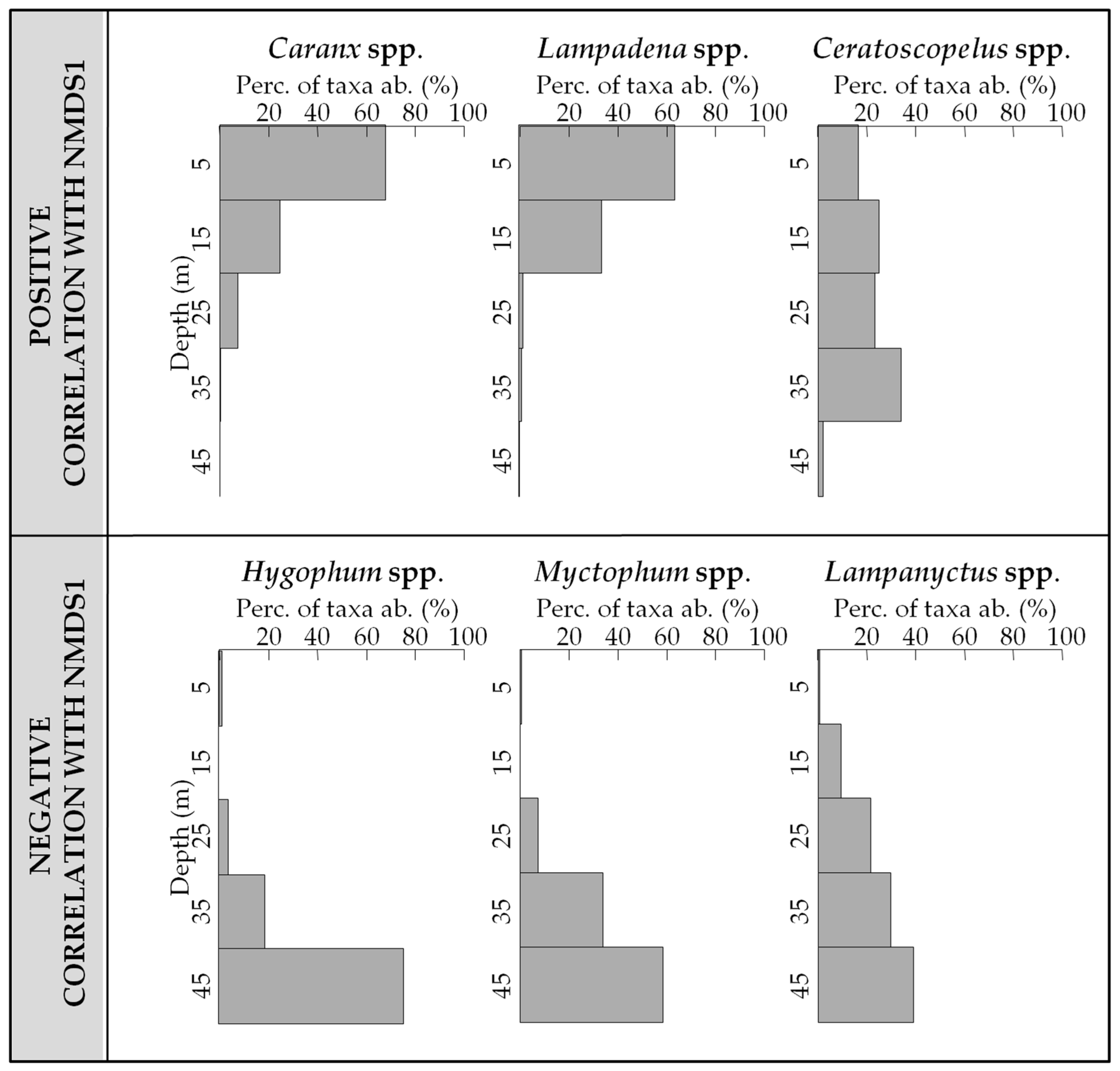
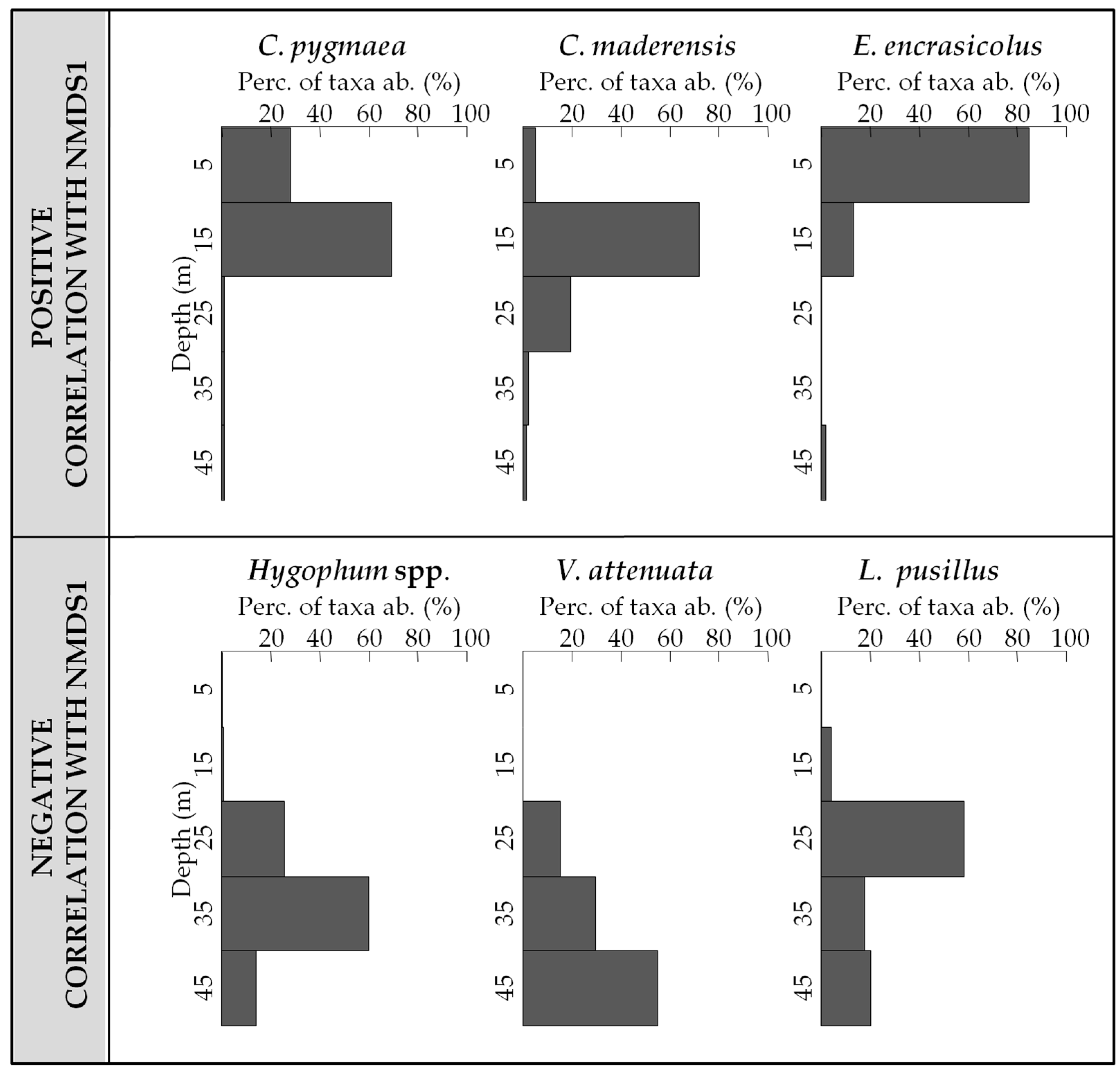
3.3. Vertical Distribution of Assemblages
3.4. Influence of Environment on the Vertical Distribution of the Assemblages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loreau, M. Linking biodiversity and ecosystems: Towards a unifying ecological theory. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Maureaud, A.; Andersen, K.H.; Zhang, L.; Lindegren, M. Trait-based food web model reveals the underlying mechanisms of biodiversity-ecosystem functioning relationships. J. Anim. Ecol. 2020, 89, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Kim, H.J.; Watson, W.; Di Lorenzo, E.; Sugihara, G. Climate-driven changes in abundance and distribution of larvae of oceanic fishes in the southern California region. Glob. Chang. Biol. 2009, 15, 2137–2152. [Google Scholar] [CrossRef]
- Leis, J.M. Are Larvae of Demersal Fishes Plankton or Nekton? Adv. Mar. Biol. 2006, 51, 58–141. [Google Scholar]
- Olivar, M.P.; Contreras, T.; Hulley, P.A.; Emelianov, M.; López-Pérez, C.; Tuset, V.; Castellón, A. Variation in the diel vertical distributions of larvae and transforming stages of oceanic fishes across the tropical and equatorial Atlantic. Prog. Oceanogr. 2018. [Google Scholar] [CrossRef]
- Reygondeau, G.; Maury, O.; Beaugrand, G.; Fromentin, J.M.; Fonteneau, A.; Cury, P. Biogeography of tuna and billfish communities. J. Biogeogr. 2012, 39, 114–129. [Google Scholar] [CrossRef]
- Olson, R.J.; Young, J.W.; Ménard, F.; Potier, M.; Allain, V.; Goñi, N.; Logan, J.M.; Galván-Magaña, F. Bioenergetics, Trophic Ecology, and Niche Separation of Tunas. Adv. Mar. Biol. 2016, 74, 199–344. [Google Scholar] [CrossRef] [PubMed]
- Tawa, A.; Kodama, T.; Sakuma, K.; Ishihara, T.; Ohshimo, S. Fine-scale horizontal distributions of multiple species of larval tuna off the Nansei Islands, Japan. Mar. Ecol. Prog. Ser. 2020, 636, 123–137. [Google Scholar] [CrossRef]
- Llopiz, J.K.; Hobday, A.J. A global comparative analysis of the feeding dynamics and environmental conditions of larval tunas, mackerels, and billfishes. Deep Res. Part II Top. Stud. Oceanogr. 2015, 113, 113–124. [Google Scholar] [CrossRef]
- Richardson, D.E.; Marancik, K.E.; Guyon, J.R.; Lutcavage, M.E.; Galuardi, B.; Lam, C.H.; Walsh, H.J.; Wildes, S.; Yates, D.A.; Hare, J.A. Discovery of a spawning ground reveals diverse migration strategies in Atlantic bluefin tuna (Thunnus thynnus). Proc. Natl. Acad. Sci. USA 2016, 113, 3299–3304. [Google Scholar] [CrossRef]
- McGowan, M.F.; Richards, W.J. Bluefin tuna, Thunnus thynnus, larvae in the Gulf Stream off the southeastern United States: Satellite and shipboard observations of their environment. Fish. Bull. 1989, 87, 615–631. [Google Scholar]
- Cowen, R.K.; Paris, C.B.; Srinivasan, A. Scaling of Connectivity in Marine Populations. Science 2006, 311, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.J.; Hare, J.A.; Fey, D.P. Evaluating diel, ontogenetic and environmental effects on larval fish vertical distribution using generalized additive models for location, scale and shape. Fish. Oceanogr. 2009. [Google Scholar] [CrossRef]
- Richardson, D.E.; Llopiz, J.K.; Guigand, C.M.; Cowen, R.K. Larval assemblages of large and medium-sized pelagic species in the Straits of Florida. Prog. Oceanogr. 2010, 86, 8–20. [Google Scholar] [CrossRef]
- Rooker, J.R.; Simms, J.R.; David Wells, R.J.; Holt, S.A.; Holt, G.J.; Graves, J.E.; Furey, N.B. Distribution and habitat associations of billfish and swordfish larvae across mesoscale features in the gulf of Mexico. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.O.; Jenkins, G.P.; Young, J.W. Patterns of horizontal distribution of the larvae of southern bluefin (Thunnus maccoyii) and other tuna in the Indian Ocean. J. Plankton Res. 1990, 12, 1295–1314. [Google Scholar] [CrossRef]
- Boehlert, G.W.; Mundy, B.C. Vertical and onshore-offshore distributional patterns of tuna larvae in relation to physical habitat features. Mar. Ecol. Prog. Ser. 1994, 107, 1–14. [Google Scholar] [CrossRef]
- Schaefer, K.M. Assessment of skipjack tuna (Katsuwonus pelamis) spawning activity in the eastern Pacific Ocean. Fish. Bull. 2001, 99, 343–350. [Google Scholar]
- Reglero, P.; Tittensor, D.P.D.; Álvarez-Berastegui, D.; Aparicio-González, A.; Worm, B. Worldwide distributions of tuna larvae: Revisiting hypotheses on environmental requirements for spawning habitats. Mar. Ecol. Prog. Ser. 2014, 501, 207–224. [Google Scholar] [CrossRef]
- Muhling, B.A.; Lamkin, J.T.; Alemany, F.; García, A.; Farley, J.; Ingram, G.W.; Berastegui, D.A.; Reglero, P.; Carrion, R.L. Reproduction and Larval Biology in Tunas, and the Importance of Restricted Area Spawning Grounds. Rev. Fish Biol. Fish. 2017, 27. [Google Scholar] [CrossRef]
- Torres, A.P.; Reglero, P.; Balbin, R.; Urtizberea, A.; Alemany, F. Coexistence of larvae of tuna species and other fish in the surface mixed layer in the NW Mediterranean. J. Plankton Res. 2011. [Google Scholar] [CrossRef]
- Muhling, B.A.; Reglero, P.; Ciannelli, L.; Alvarez-Berastegui, D.; Alemany, F.; Lamkin, J.T.; Roffer, M.A. Comparison between environmental characteristics of larval bluefin tuna Thunnus thynnus habitat in the Gulf of Mexico and western Mediterranean Sea. Mar. Ecol. Prog. Ser. 2013, 486, 257–276. [Google Scholar] [CrossRef]
- Pruzinsky, N.M.; Milligan, R.J.; Sutton, T.T. Pelagic Habitat Partitioning of Late-Larval and Juvenile Tunas in the Oceanic Gulf of Mexico. Front. Mar. Sci. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Margulies, D.; Suter, J.M.; Hunt, S.L.; Olson, R.J.; Scholey, V.P.; Wexler, J.B.; Nakazawa, A. Spawning and early development of captive yellowfin tuna (Thunnus albacares). Fish. Bull. 2007, 105, 249–265. [Google Scholar]
- Reglero, P.; Ortega, A.; Balbín, R.; Abascal, F.J.; Medina, A.; Blanco, E.; de la Gándara, F.; Alvarez-Berastegui, D.; Hidalgo, M.; Rasmuson, L.; et al. Atlantic bluefin tuna spawn at suboptimal temperatures for their offspring. Proc. R. Soc. B Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Satoh, K. Horizontal and vertical distribution of larvae of Pacific bluefin tuna Thunnus orientalis in patches entrained in mesoscale eddies. Mar. Ecol. Prog. Ser. 2010, 404, 227–240. [Google Scholar] [CrossRef]
- Reglero, P.; Blanco, E.; Alemany, F.; Ferrá, C.; Alvarez-Berastegui, D.; Ortega, A.; De La Gándara, F.; Aparicio-González, A.; Folkvord, A. Vertical distribution of Atlantic bluefin tuna Thunnus thynnus and bonito Sarda sarda larvae is related to temperature preference. Mar. Ecol. Prog. Ser. 2018, 594, 231–243. [Google Scholar] [CrossRef]
- Kodama, T.; Ohshimo, S.; Tawa, A.; Furukawa, S.; Nohara, K.; Takeshima, H.; Chiba, S.N.; Ishihara, T.; Sawai, E.; Kawazu, M.; et al. Vertical distribution of larval Pacific bluefin tuna, Thunnus orientalis, in the Japan Sea. Deep Res. Part II Top. Stud. Oceanogr. 2020, 175. [Google Scholar] [CrossRef]
- Álvarez, I.; Rodríguez, J.M.; Catalán, I.A.; Hidalgo, M.; Álvarez-Berastegui, D.; Balbín, R.; Aparicio-González, A.; Alemany, F. Larval fish assemblage structure in the surface layer of the northwestern Mediterranean under contrasting oceanographic scenarios. J. Plankton Res. 2015, 37. [Google Scholar] [CrossRef]
- Rodriguez, J.M.M.; Alvarez, I.; Lopez-Jurado, J.L.L.; Garcia, A.; Balbin, R.; Alvarez-Berastegui, D.; Torres, A.P.P.; Alemany, F. Environmental forcing and the larval fish community associated to the Atlantic bluefin tuna spawning habitat of the Balearic region (Western Mediterranean), in early summer 2005. Deep Res. Part I Oceanogr. Res. Pap. 2013, 77, 11–22. [Google Scholar] [CrossRef][Green Version]
- Alemany, F.; Quintanilla, L.; Velez-Belchí, P.; García, A.; Cortés, D.; Rodríguez, J.M.; Fernández de Puelles, M.L.; González-Pola, C.; López-Jurado, J.L. Characterization of the spawning habitat of Atlantic bluefin tuna and related species in the Balearic Sea (western Mediterranean). Prog. Oceanogr. 2010, 86, 21–38. [Google Scholar] [CrossRef]
- Alemany, F.; Deudero, S.; Morales-Nin, B.; López-Jurado, J.L.; Jansà, J.; Palmer, M.; Palomera, I. Influence of physical environmental factors on the composition and horizontal distribution of summer larval fish assemblages off Mallorca island (Balearic archipelago, western Mediterranean). J. Plankton Res. 2006, 28, 473–487. [Google Scholar] [CrossRef]
- Muhling, B.A.; Lamkin, J.T.; Richards, W.J. Decadal-scale responses of larval fish assemblages to multiple ecosystem processes in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 450, 37–53. [Google Scholar] [CrossRef]
- Ingram, G.W.; Alvarez-Berastegui, D.; Reglero, P.; Balbín, R.; García, A.; Alemany, F. Incorporation of habitat information in the development of indices of larval bluefin tuna (Thunnus thynnus) in the Western Mediterranean Sea (2001–2005 and 2012–2013). Deep Res. Part II Top. Stud. Oceanogr. 2017. [Google Scholar] [CrossRef]
- Alemany, F. Ictioplancton del Mar Balear; Universitat de les Illes Balears: Palma, Spain, 1997. [Google Scholar]
- Rodríguez, J.M.; Alemany, F.; García, A. A Guide to the Eggs and Larvae of 100 Common Western Mediterranean Sea Bony Fish Species; FAO: Rome, Italy, 2017. [Google Scholar]
- Fortier, L.; Leggett, W.C. Vertical migrations and transport of larval fish in a partially mixed estuary. Can. J. Fish. Aquat. Sci. 1983, 40, 1543–1555. [Google Scholar] [CrossRef]
- Durazo-Arvizu, R.; Gaxiola-Castro, G. Dinámica Del Ecosistema Pelágico Frente a Baja California 1997–2007. In Diez Años de Investigaciones Mexicanas de La Corriente de California; Durazo-Arvizu, R., Gaxiola-Castro, G., Eds.; NE/CICESE/UABC/SEMARNAT: Ensenada, Mexico, 2010. [Google Scholar]
- Goring, S.; Pellatt, M.G.; Lacourse, T.; Walker, I.R.; Mathewes, R.W. A new methodology for reconstructing climate and vegetation from modern pollen assemblages: An example from British Columbia. J. Biogeogr. 2009, 36, 626–638. [Google Scholar] [CrossRef]
- Muenchow, J.; Feilhauer, H.; Bräuning, A.; Rodríguez, E.F.; Bayer, F.; Rodríguez, R.A.; Von Wehrden, H. Coupling ordination techniques and GAM to spatially predict vegetation assemblages along a climatic gradient in an ENSO-affected region of extremely high climate variability. J. Veg. Sci. 2013, 24, 1154–1166. [Google Scholar] [CrossRef]
- Siddon, E.C.; Duffy-Anderson, J.T.; Mueter, F.J. Community-level response of fish larvae to environmental variability in the southeastern Bering Sea. Mar. Ecol. Prog. Ser. 2011, 426, 225–239. [Google Scholar] [CrossRef][Green Version]
- Hidalgo, M.; Reglero, P.; Álvarez-Berastegui, D.; Torres, A.P.A.P.; Álvarez, I.; Rodriguez, J.M.J.M.; Carbonell, A.; Zaragoza, N.; Tor, A.; Goñi, R.; et al. Hydrographic and biological components of the seascape structure the meroplankton community in a frontal system. Mar. Ecol. Prog. Ser. 2014, 505. [Google Scholar] [CrossRef]
- Keller, S.; Hidalgo, M.; Álvarez-Berastegui, D.; Bitetto, I.; Casciaro, L.; Cuccu, D.; Esteban, A.; Garofalo, G.; Gonzalez, M.; Guijarro, B.; et al. Demersal cephalopod communities in the Mediterranean: A large-scale analysis. Mar. Ecol. Prog. Ser. 2017, 584, 105–118. [Google Scholar] [CrossRef]
- Field, J.; Clarke, K.; Warwick, R. A Practical Strategy for Analysing Multispecies Distribution Patterns. Mar. Ecol. Prog. Ser. 1982, 8, 37–52. [Google Scholar] [CrossRef]
- Kock, N.; Lynn, G.S. Lateral collinearity and misleading results in variance-based SEM: An illustration and recommendations. J. Assoc. Inf. Syst. 2012, 13, 546–580. [Google Scholar] [CrossRef]
- Reglero, P.; Balbín, R.; Abascal, F.J.; Medina, A.; Alvarez-Berastegui, D.; Rasmuson, L.; Mourre, B.; Saber, S.; Ortega, A.; Blanco, E.; et al. Pelagic habitat and offspring survival in the eastern stock of Atlantic bluefin tuna. ICES J. Mar. Sci. 2019, 76, 549–558. [Google Scholar] [CrossRef]
- Matsumoto, W.M. Description and Distribution of Larvae of Four Species of Tuna. Fish. Bull. 1958, 58, 31–72. [Google Scholar]
- Strasburg, D. Estimates of larval tuna abundance in the central Pacific. Fish. Bull. US Fish Wildl. Serv. 1960, 60, 231–255. [Google Scholar]
- Ueyanagi, S. Observations on the distribution of tuna larvae in the Indo-Pacific Ocean with emphasis on the delineation of the spawning areas of the albacore Thunnus alalunga. Bull. Far Seas Fish. Res. Lab. 1969, 2, 177–219. [Google Scholar]
- Cornic, M.; Smith, B.L.; Kitchens, L.L.; Alvarado Bremer, J.R.; Rooker, J.R. Abundance and habitat associations of tuna larvae in the surface water of the Gulf of Mexico. Hydrobiologia 2018, 806, 29–46. [Google Scholar] [CrossRef]
- Reglero, P.; Ciannelli, L.; Alvarez-Berastegui, D.; Balbín, R.; López-Jurado, J.L.; Alemany, F. Geographically and environmentally driven spawning distributions of tuna species in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 2012, 463, 273–284. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Hirota, Y.; Ishida, M. Distribution patterns of larval myctophid fish assemblages in the subtropical-tropical waters of the western North Pacific. Fish. Oceanogr. 2004, 13, 267–282. [Google Scholar] [CrossRef]
- Hopkins, T.L. The vertical distribution of zooplankton in the eastern Gulf of Mexico. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1982, 29, 1069–1083. [Google Scholar] [CrossRef]
- Olivar, M.P.; Sabatés, A.; Alemany, F.; Balbín, R.; Fernández de Puelles, M.L.; Torres, A.P.P. Diel-depth distributions of fish larvae off the Balearic Islands (western Mediterranean) under two environmental scenarios. J. Mar. Syst. 2014, 138, 127–138. [Google Scholar] [CrossRef]
- Hare, J.A.; Govoni, J.J. Comparison of average larval fish vertical distributions among species exhibiting different transport pathways on the southeast United States continental shelf. Fish. Bull. 2005, 103, 728–736. [Google Scholar]
- Habtes, S.; Muller-Karger, F.E.; Roffer, M.A.; Lamkin, J.T.; Muhling, B.A. A comparison of sampling methods for larvae of medium and large epipelagic fish species during spring SEAMAP ichthyoplankton surveys in the Gulf of Mexico. Limnol. Oceanogr. Methods 2014, 12, 86–101. [Google Scholar] [CrossRef]
- Vollset, K.W.; Catalán, I.A.; Fiksen, Ø.; Folkvord, A. Effect of food deprivation on distribution of larval and early juvenile cod in experimental vertical temperature and light gradients. Mar. Ecol. Prog. Ser. 2013, 475, 191–201. [Google Scholar] [CrossRef]
- Blanco, E.; Reglero, P.; Ortega, A.; de la Gándara, F.; Fiksen, Ø.; Folkvord, A. The effects of light, darkness and intermittent feeding on the growth and survival of reared Atlantic bonito and Atlantic bluefin tuna larvae. Aquaculture 2017, 479, 233–239. [Google Scholar] [CrossRef]
- Laevastu, T.; Hayes, M.L. Fisheries Oceanography and Ecology; Fishing News Books Ltd.: Surrey, UK, 1981. [Google Scholar]
- Reglero, P.; Ortega, A.; Blanco, E.; Fiksen, T.; Viguri, F.J.; de la Gándara, F.; Seoka, M.; Folkvord, A. Size-related differences in growth and survival in piscivorous fish larvae fed different prey types. Aquaculture 2014, 433, 94–101. [Google Scholar] [CrossRef]
- Catalán, I.A.; Dunand, A.; Álvarez, I.; Alós, J.; Colinas, N.; Nash, R.D.M. An evaluation of sampling methodology for assessing settlement of temperate fish in seagrass meadows. Mediterr. Mar. Sci. 2014, 15. [Google Scholar] [CrossRef]
| Family | Taxa | NMDS Code | AH | GOM | MED | ||||
|---|---|---|---|---|---|---|---|---|---|
| TA (i × 103 m−3) | TA (%) | WMD (m) | TA (i × 103 m−3) | TA (%) | WMD (m) | ||||
| Acropomatidae | Acropomatidae NI | D | 20.9 | 0.12 | 38.7 | ||||
| Anguilliformes | Anguilliformes | Ang | D | 8.5 | 0.05 | 25.6 | 79.1 | 0.03 | 10.95 |
| Apogonidae | Apogon imberbis | Aim | D | 42.8 | 0.02 | 15.00 | |||
| Apogonidae | Apogon spp | D | 20.6 | 0.11 | 5.0 | ||||
| Bothidae | Arnoglossus spp | Arn | D | 43.3 | 0.02 | 35.00 | |||
| Bothidae NI | D | 36.9 | 0.20 | 28.3 | |||||
| Bothus podas | Bpo | D | 43.9 | 0.02 | 15.00 | ||||
| Bothus spp | D | 66.2 | 0.36 | 23.1 | |||||
| Engyophrys senta | Ese | D | 75.7 | 0.42 | 24.7 | ||||
| Trichopsetta spp | D | 52.2 | 0.29 | 28.0 | |||||
| Bramidae | Bramidae NI | OP | 17.4 | 0.10 | 18.2 | ||||
| Bregmacerotidae | Bregmaceros spp | Bre | CP | 443.8 | 2.44 | 34.3 | |||
| Carangidae | Caranx spp | Car | CP | 1796.8 | 9.87 | 9.0 | |||
| Elagatis bipinnulata | CP | 43.9 | 0.24 | 15.0 | |||||
| Selar crumenophthalmus | CP | 28.4 | 0.16 | 10.6 | |||||
| Seriola durmerilii | Sdu | CP | 42.8 | 0.02 | 15.00 | ||||
| Ceratioidei | Ceratioidei NI | B | 66.3 | 0.36 | 29.5 | ||||
| Chlorophthalmidae | Chlorophthalmus agassizi | D | 30.2 | 0.17 | 24.1 | ||||
| Chlorophthalmus brasiliensis | D | 21.9 | 0.12 | 18.5 | |||||
| Congridae | Congridae NI | D | 50.3 | 0.28 | 24.6 | ||||
| Coryphaenidae | Coryphaena hippurus | OP | 58.3 | 0.32 | 8.0 | ||||
| Cynoglossidae | Symphurus spp | D | 41.3 | 0.23 | 24.8 | ||||
| Engraulidae | Engraulis encrasicolus | Een | CP | 2579.7 | 1.04 | 7.05 | |||
| Gempylidae | Diplospinus multistriatus | M | 39.7 | 0.22 | 44.1 | ||||
| Epinnula magistralis | M | 26.8 | 0.15 | 24.0 | |||||
| Gempylidae | M | 18.0 | 0.10 | 45.0 | |||||
| Gempylus serpens | M | 36.0 | 0.20 | 10.2 | |||||
| Gobiidae | Gobiidae | Gob | D | 290.2 | 1.59 | 26.6 | 46.5 | 0.02 | 45.00 |
| Gonostomatidae | Cyclothone spp | Cyc | M | 3266.8 | 17.94 | 18.2 | |||
| Gonostomatidae NI | M | 29.5 | 0.16 | 29.4 | |||||
| Cyclothone braueri | Cbr | M | 77407.8 | 31.08 | 25.31 | ||||
| Cyclothone pygmaea | Cpy | M | 4560.6 | 1.83 | 12.75 | ||||
| Lestidiops jayakari | Lja | B | 778.2 | 0.31 | 41.80 | ||||
| Lestidiops sphyrenoides | Lsp | B | 121.9 | 0.05 | 48.28 | ||||
| Howellidae | Howella spp | How | B | 100.6 | 0.55 | 27.7 | |||
| Labridae | Coris julis | Cju | D | 397.2 | 0.16 | 28.99 | |||
| Lutjanidae | Pristipomoides aquilonaris | D | 58.2 | 0.32 | 15.3 | ||||
| Mugilidae | Mugilidae NI | Mug | D | 45.1 | 0.02 | 5.00 | |||
| Myctophidae | Benthosema glaciale | Bgl | M | 142.4 | 0.06 | 41.79 | |||
| Benthosema spp | M | 31.4 | 0.17 | 26.1 | |||||
| Ceratoscopelus maderensis | Cma | M | 558.4 | 3.07 | 13.5 | 24786.9 | 9.95 | 17.84 | |
| Ceratoscopelus spp | Ces | M | 823.7 | 4.52 | 23.0 | ||||
| Diaphus holti | Dho | M | 42.7 | 0.02 | 25.00 | ||||
| Diaphus spp | Dis | M | 4671.0 | 25.65 | 21.6 | ||||
| Diogenichthys atlanticus | M | 32.5 | 0.18 | 40.2 | |||||
| Hygophum spp | Hyg | M | 196.1 | 1.08 | 41.7 | 68444.3 | 27.48 | 34.17 | |
| Lampadena spp | Lam | M | 335.3 | 1.84 | 9.0 | ||||
| Lampanyctus crocodilus | Lcr | M | 1474.5 | 0.59 | 35.67 | ||||
| Lampanyctus pusillus | Lpu | M | 5026.7 | 2.02 | 32.94 | ||||
| Lampanyctus spp | Las | M | 313.2 | 1.72 | 34.7 | ||||
| Lobianchia dofleini | Ldo | M | 204.7 | 0.08 | 46.68 | ||||
| Myctophidae NI | M | 64.4 | 0.35 | 35.4 | |||||
| Myctophum punctatum | M | 39.6 | 0.02 | 55.00 | |||||
| Myctophum spp | Mys | M | 303.9 | 1.67 | 39.9 | ||||
| Notolychnus valdiviae | M | 22.0 | 0.12 | 32.9 | |||||
| Symbolophorus veranyi | M | 40.6 | 0.02 | 55.00 | |||||
| Nettastomatidae | Nettastomatidae NI | B | 26.1 | 0.14 | 33.1 | ||||
| Nomeidae | Psenes spp | OP | 19.9 | 0.11 | 32.0 | ||||
| Ophichthidae | Ophichthidae | D | 22.9 | 0.13 | 25.0 | ||||
| Ophichthidae | Ophichthus gomesi | D | 20.4 | 0.11 | 12.2 | ||||
| Ophididae | Ophididae | Oph | D | 42.8 | 0.02 | 15.00 | |||
| Paralepididae | Paralepididae NI | Par | M | 498.0 | 2.73 | 30.8 | |||
| Paralepis coregonoides | Pco | M | 1086.7 | 0.44 | 38.78 | ||||
| Sudis spp | M | 21.1 | 0.12 | 40.2 | |||||
| Paralichthyidae | Cubiceps spp | Cub | D | 800.6 | 4.40 | 12.5 | |||
| Etropus spp | D | 25.0 | 0.14 | 18.5 | |||||
| Syacium papillosum | D | 69.1 | 0.38 | 25.0 | |||||
| Phosichthyidae | Vinciguerria attenuata | Vat | M | 2.9 | 0.02 | 45.0 | 2604.6 | 1.05 | 43.81 |
| Vinciguerria nimbaria | Vni | M | 259.3 | 1.42 | 28.8 | ||||
| Vinciguerria spp | Vis | M | 97.3 | 0.53 | 32.3 | ||||
| Pomacentridae | Chromis chromis | Cch | D | 99.2 | 0.04 | 12.67 | |||
| Scombridae | Auxis rochei | Aro | OP | 45.1 | 0.02 | 5.00 | |||
| Auxis spp | Aus | OP | 74.7 | 0.41 | 17.1 | ||||
| Euthynnus alletteratus | Eal | OP | 10.5 | 0.06 | 20.5 | 103.7 | 0.04 | 5.00 | |
| Katsuwonus pelamis | Kpe | OP | 343.8 | 1.89 | 19.6 | 32.1 | 0.01 | 5.00 | |
| Thunnus alalunga | Tal | OP | 354.8 | 0.14 | 9.56 | ||||
| Thunnus spp | Ths | OP | 411.7 | 2.26 | 12.3 | ||||
| Thunnus thynnus | Tth | OP | 417.5 | 2.29 | 7.2 | 58052.2 | 23.31 | 8.07 | |
| Scorpaenidae | Scorpaenidae NI | D | 20.0 | 0.11 | 26.5 | ||||
| Serranidae | Anthias spp | D | 30.5 | 0.17 | 17.9 | ||||
| Epinephelinae NI | D | 62.1 | 0.34 | 16.1 | |||||
| Hemanthias spp | D | 58.0 | 0.32 | 20.8 | |||||
| Serranus spp | Ser | D | 145.4 | 0.80 | 16.6 | ||||
| Stomiatidae | Stomias boa | Sbo | M | 200.8 | 0.08 | 33.31 | |||
| Stomiidae | Melanostomiinae NI | M | 53.1 | 0.29 | 21.5 | ||||
| Synodontidae | Synodontidae | D | 20.7 | 0.11 | 13.0 | ||||
| Tetraodontidae | Tetraodontidae | D | 24.2 | 0.13 | 26.1 | ||||
| Triglidae | Triglidae | Tri | D | 18.0 | 0.10 | 20.9 | 44.7 | 0.02 | 5.00 |
| Axis 1 | ||||
| Parametric coefficients | Estimate | Std. Error | ||
| a (Intercept) | 0.003 | 0.029 | ||
| Smooth terms | edf | Ref.df | F | p-value |
| s1 (Salinity) | 1.875 | 1.984 | 10.54 | 5.97 × 10−5 *** |
| s2 (Fluorescence) | 1.883 | 1.986 | 19.56 | 8.39 × 10−8 *** |
| R-sq.(adj) = 0.418 | Deviance explained = 44% | |||
| Axis 2 | ||||
| Parametric coefficients | Estimate | Std. Error | ||
| a (Intercept) | 0.012 | 0.034 | ||
| Smooth terms | edf | Ref.df | F | p-value |
| s1 (Salinity) | 1.884 | 1.986 | 4.1 | 0.027 * |
| s2 (Fluorescence) | 1 | 1 | 4.90 | 0.029 * |
| R-sq.(adj) = 0.07 | Deviance explained = 9.66% | |||
| Axis 1 | ||||
| Parametric coefficients | Estimate | Std. Error | ||
| a (Intercept) | −2.5 × 10−11 | 0.06 | ||
| Smooth terms | edf | Ref.df | F | p-value |
| s1 (Temperature) | 1 | 1 | 76.75 | <2 × 10−12 *** |
| R-sq.(adj) = 0.66 | Deviance explained = 69.2% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, I.; Rasmuson, L.K.; Gerard, T.; Laiz-Carrion, R.; Hidalgo, M.; Lamkin, J.T.; Malca, E.; Ferra, C.; Torres, A.P.; Alvarez-Berastegui, D.; et al. Influence of the Seasonal Thermocline on the Vertical Distribution of Larval Fish Assemblages Associated with Atlantic Bluefin Tuna Spawning Grounds. Oceans 2021, 2, 64-83. https://doi.org/10.3390/oceans2010004
Alvarez I, Rasmuson LK, Gerard T, Laiz-Carrion R, Hidalgo M, Lamkin JT, Malca E, Ferra C, Torres AP, Alvarez-Berastegui D, et al. Influence of the Seasonal Thermocline on the Vertical Distribution of Larval Fish Assemblages Associated with Atlantic Bluefin Tuna Spawning Grounds. Oceans. 2021; 2(1):64-83. https://doi.org/10.3390/oceans2010004
Chicago/Turabian StyleAlvarez, Itziar, Leif K. Rasmuson, Trika Gerard, Raul Laiz-Carrion, Manuel Hidalgo, John T. Lamkin, Estrella Malca, Carmen Ferra, Asvin P. Torres, Diego Alvarez-Berastegui, and et al. 2021. "Influence of the Seasonal Thermocline on the Vertical Distribution of Larval Fish Assemblages Associated with Atlantic Bluefin Tuna Spawning Grounds" Oceans 2, no. 1: 64-83. https://doi.org/10.3390/oceans2010004
APA StyleAlvarez, I., Rasmuson, L. K., Gerard, T., Laiz-Carrion, R., Hidalgo, M., Lamkin, J. T., Malca, E., Ferra, C., Torres, A. P., Alvarez-Berastegui, D., Alemany, F., Quintanilla, J. M., Martin, M., Rodriguez, J. M., & Reglero, P. (2021). Influence of the Seasonal Thermocline on the Vertical Distribution of Larval Fish Assemblages Associated with Atlantic Bluefin Tuna Spawning Grounds. Oceans, 2(1), 64-83. https://doi.org/10.3390/oceans2010004





