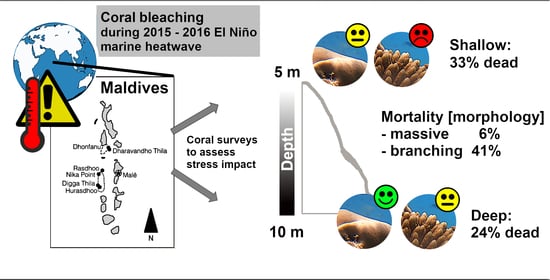
Severe Heat Stress Resulted in High Coral Mortality on Maldivian Reefs following the 2015–2016 El Niño Event
Abstract
1. Introduction
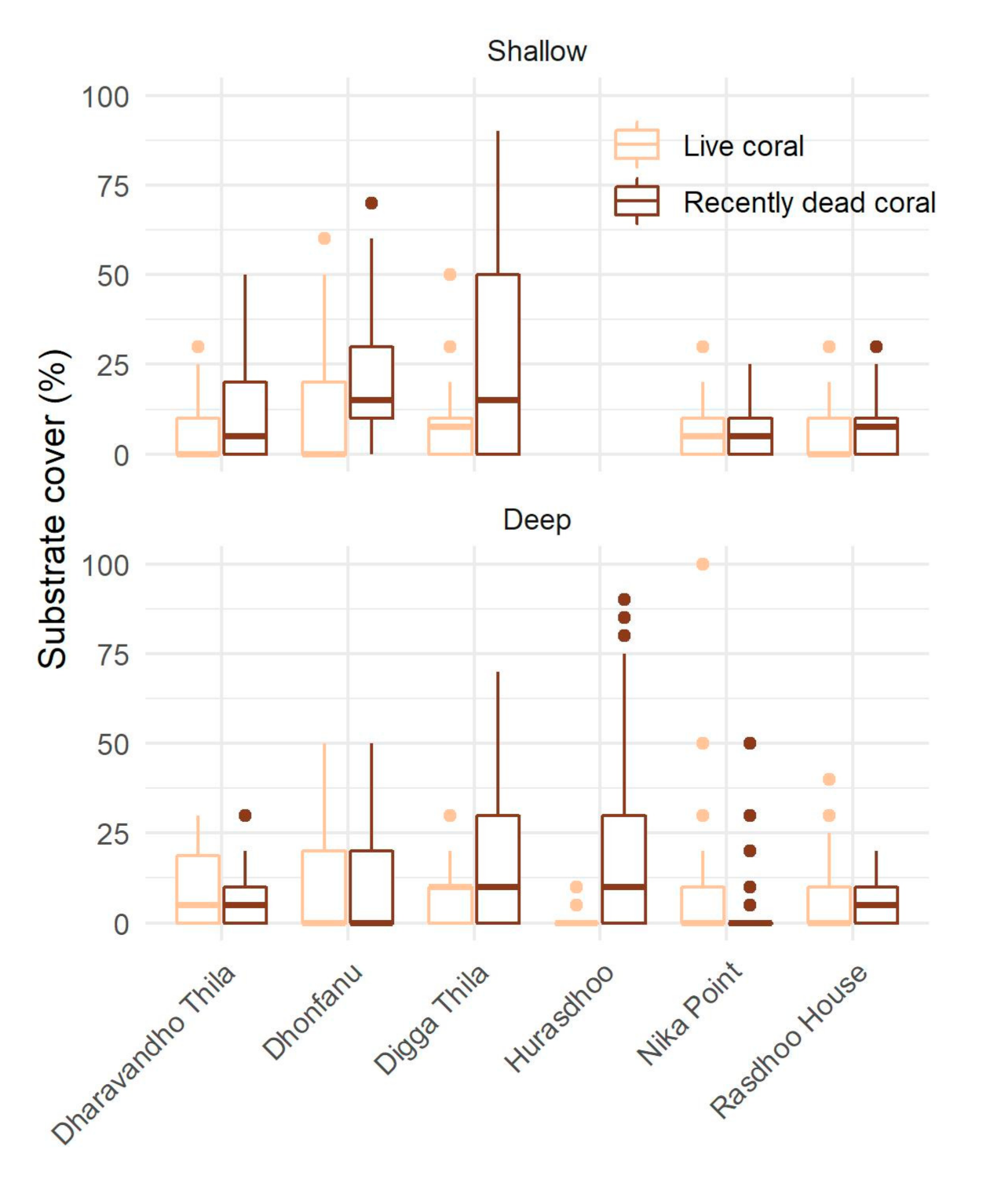
2. Results
3. Discussion
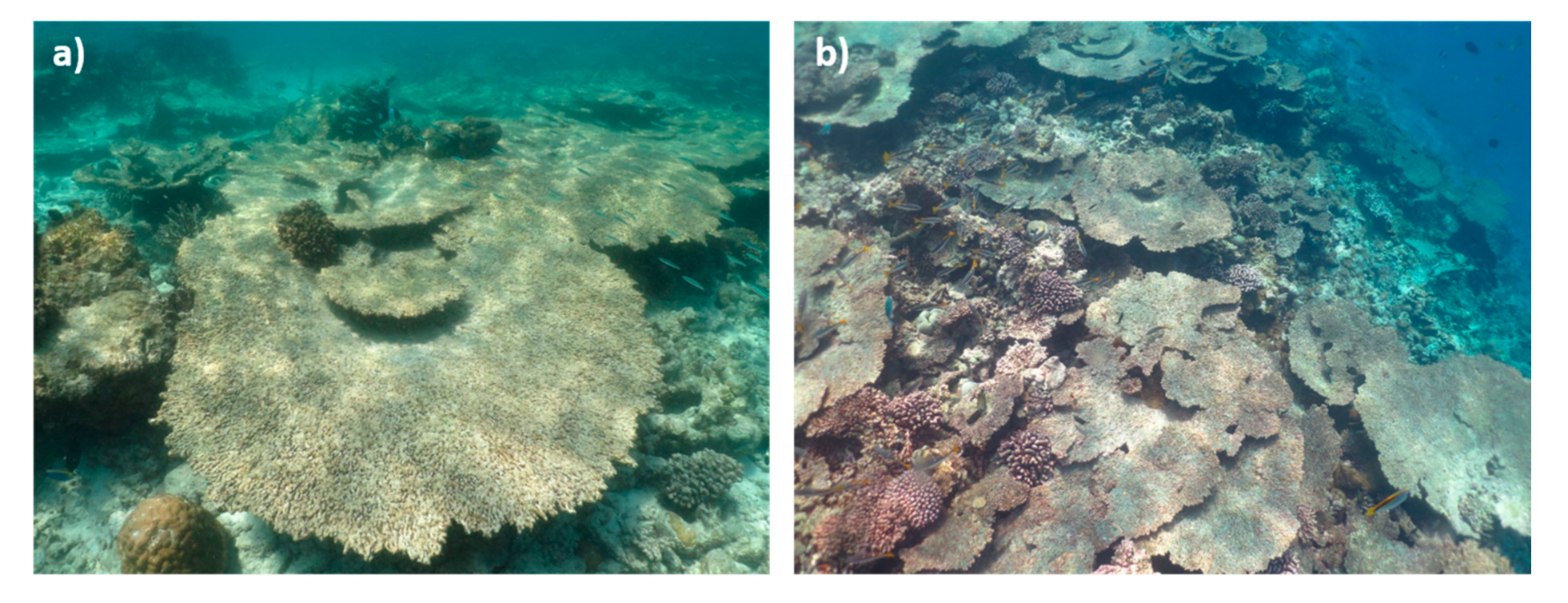
3.1. Bleaching and Mortality
3.2. Potential for Recovery of Maldivian Reefs
4. Materials and Methods
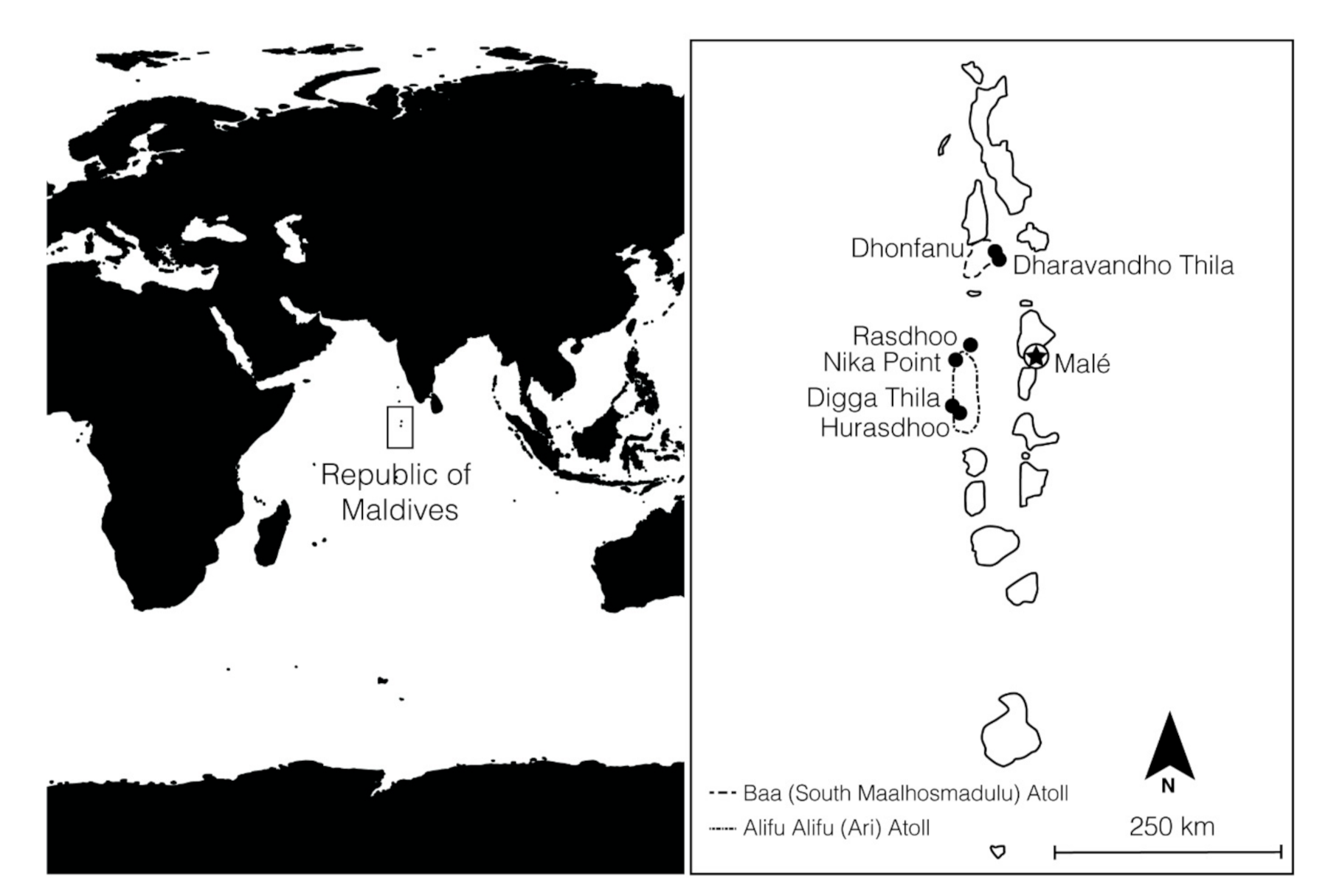
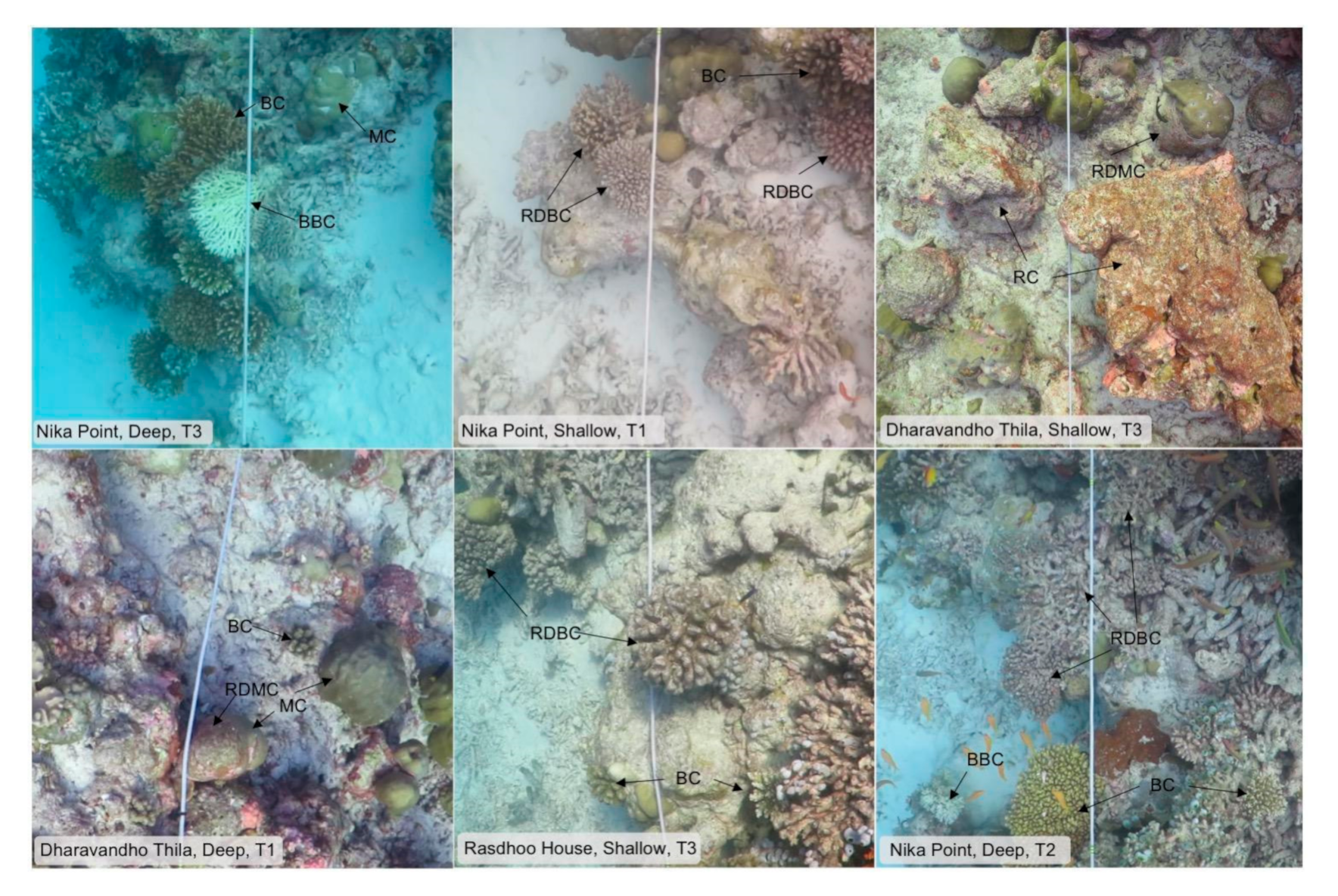
4.1. Field Surveys
4.2. Image Analyses
4.3. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and Temporal Patterns of Mass Bleaching of Corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; Van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. Nat. Publ. Group Lond. 2016, 6, 38402. [Google Scholar] [CrossRef] [PubMed]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 Global-Scale Coral Bleaching Event: Insights and Impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef]
- Barkley, H.C.; Cohen, A.L.; Mollica, N.R.; Brainard, R.E.; Rivera, H.E.; DeCarlo, T.M.; Lohmann, G.P.; Drenkard, E.J.; Alpert, A.E.; Young, C.W.; et al. Repeat Bleaching of a Central Pacific Coral Reef over the Past Six Decades (1960–2016). Commun. Biol. 2018, 1, 177. [Google Scholar] [CrossRef]
- Monroe, A.A.; Ziegler, M.; Roik, A.; Röthig, T.; Hardenstine, R.S.; Emms, M.A.; Jensen, T.; Voolstra, C.R.; Berumen, M.L. In Situ Observations of Coral Bleaching in the Central Saudi Arabian Red Sea during the 2015/2016 Global Coral Bleaching Event. PLoS ONE 2018, 13, e0195814. [Google Scholar] [CrossRef]
- Cowburn, B.; Moritz, C.; Grimsditch, G.; Solandt, J. Evidence of Coral Bleaching Avoidance, Resistance and Recovery in the Maldives during the 2016 Mass-Bleaching Event. Mar. Ecol. Prog. Ser. 2019, 626, 53–67. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Darling, E.S.; Maina, J.M.; Muthiga, N.A.; ’agata, S.D.; Jupiter, S.D.; Arthur, R.; Wilson, S.K.; Mangubhai, S.; Nand, Y.; et al. Temperature Patterns and Mechanisms Influencing Coral Bleaching during the 2016 El Niño. Nat. Clim. Change 2019, 9, 845–851. [Google Scholar] [CrossRef]
- Head, C.E.I.; Bayley, D.T.I.; Rowlands, G.; Roche, R.C.; Tickler, D.M.; Rogers, A.D.; Koldewey, H.; Turner, J.R.; Andradi-Brown, D.A. Coral Bleaching Impacts from Back-to-Back 2015–2016 Thermal Anomalies in the Remote Central Indian Ocean. Coral Reefs 2019, 38, 605–618. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Burdick, D.; Hoot, W.C.; Miller, R.M.; Brown, V.; Reynolds, T.; Gault, J.; Idechong, J.; Fifer, J.; Williams, A. Successive Bleaching Events Cause Mass Coral Mortality in Guam, Micronesia. Coral Reefs 2019, 38, 677–700. [Google Scholar] [CrossRef]
- Le Nohaïc, M.; Ross, C.L.; Cornwall, C.E.; Comeau, S.; Lowe, R.; McCulloch, M.T.; Schoepf, V. Marine Heatwave Causes Unprecedented Regional Mass Bleaching of Thermally Resistant Corals in Northwestern Australia. Sci. Rep. 2017, 7, 14999. [Google Scholar] [CrossRef]
- Ibrahim, N.; Mohamed, M.; Basheer, A.; Haleem, I.; Nistharan, F.; Schmidt, A.; Naeem, R.; Abdulla, A.; Grimsditch, G. Status of Coral Bleaching in the Maldives 2016; Maldives Marine Research Centre: Malé, Maldives, 2017; pp. 1–47. [Google Scholar]
- Cerutti, J.M.B.; Burt, A.J.; Haupt, P.; Bunbury, N.; Mumby, P.J.; Schaepman-Strub, G. Impacts of the 2014–2017 Global Bleaching Event on a Protected Remote Atoll in the Western Indian Ocean. Coral Reefs 2020, 39, 15–26. [Google Scholar] [CrossRef]
- Glynn, P.W. Widespread Coral Mortality and the 1982–83 El Niño Warming Event. Environ. Conserv. 1984, 11, 133–146. [Google Scholar] [CrossRef]
- Marcelino, L.A.; Westneat, M.W.; Stoyneva, V.; Henss, J.; Rogers, J.D.; Radosevich, A.; Turzhitsky, V.; Siple, M.; Fang, A.; Swain, T.D.; et al. Modulation of Light-Enhancement to Symbiotic Algae by Light-Scattering in Corals and Evolutionary Trends in Bleaching. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Epstein, H.; Torda, G. The Molecular Basis of Differential Morphology and Bleaching Thresholds in Two Morphs of the Coral Pocillopora Acuta. Sci. Rep. 2017, 7, 10066. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Warner, M.E.; Levas, S.J.; Aschaffenburg, M.D.; Schoepf, V.; McGinley, M.; Baumann, J.; Matsui, Y. The Cumulative Impact of Annual Coral Bleaching Can Turn Some Coral Species Winners into Losers. Glob. Chang. Biol. 2014, 20, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Safaie, A.; Silbiger, N.J.; McClanahan, T.R.; Pawlak, G.; Barshis, D.J.; Hench, J.L.; Rogers, J.S.; Williams, G.J.; Davis, K.A. High Frequency Temperature Variability Reduces the Risk of Coral Bleaching. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Berkelmans, R.; van Oppen, M.J.H. The Role of Zooxanthellae in the Thermal Tolerance of Corals: A ‘Nugget of Hope’ for Coral Reefs in an Era of Climate Change. Proc. R. Soc. B Biol. Sci. 2006, 273, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Boulotte, N.M.; Dalton, S.J.; Carroll, A.G.; Harrison, P.L.; Putnam, H.M.; Peplow, L.M.; van Oppen, M.J. Exploring the Symbiodinium Rare Biosphere Provides Evidence for Symbiont Switching in Reef-Building Corals. ISME J. 2016, 10, 2693–2701. [Google Scholar] [CrossRef]
- Carballo-Bolaños, R.; Denis, V.; Huang, Y.-Y.; Keshavmurthy, S.; Chen, C.A. Temporal Variation and Photochemical Efficiency of Species in Symbiodinaceae Associated with Coral Leptoria Phrygia (Scleractinia; Merulinidae) Exposed to Contrasting Temperature Regimes. PLoS ONE 2019, 14, e0218801. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Grupstra, C.G.B.; Barreto, M.M.; Eaton, M.; BaOmar, J.; Zubier, K.; Al-Sofyani, A.; Turki, A.J.; Ormond, R.; Voolstra, C.R. Coral Bacterial Community Structure Responds to Environmental Change in a Host-Specific Manner. Nat. Commun. Lond. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Cadotte, M.W. The New Diversity: Management Gains through Insights into the Functional Diversity of Communities. J. Appl. Ecol. 2011, 48, 1067–1069. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Norström, A.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative States on Coral Reefs: Beyond Coral–Macroalgal Phase Shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Chaves-Fonnegra, A.; Riegl, B.; Zea, S.; Lopez, J.V.; Smith, T.; Brandt, M.; Gilliam, D.S. Bleaching Events Regulate Shifts from Corals to Excavating Sponges in Algae-Dominated Reefs. Glob. Chang. Biol. 2018, 24, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.; Bellwood, D.R.; Cinner, J.E.; Hughes, T.P.; Norström, A.V.; Nyström, M. Managing Resilience to Reverse Phase Shifts in Coral Reefs. Front. Ecol. Environ. 2013, 11, 541–548. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef Degradation and the Loss of Critical Ecosystem Goods and Services Provided by Coral Reef Fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Fisheries Productivity under Progressive Coral Reef Degradation. J. Appl. Ecol. 2018, 55, 1041–1049. [Google Scholar] [CrossRef]
- Naseer, A.; Hatcher, B.G. Inventory of the Maldives? Coral Reefs Using Morphometrics Generated from Landsat ETM+ Imagery. Coral Reefs 2004, 23, 161–168. [Google Scholar] [CrossRef]
- Agardy, T.; Hicks, F.; Nistharan, F.; Fisam, A.; Schmidt, A.; Grimsditch, G. Ecosystem Services Assessment of North Ari Atoll Maldives; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Liu, G.; Rauenzahn, J.; Heron, S.; Eakin, C.M.; Skirving, W.; Christensen, T.; Strong, A.; Li, J. NOAA Coral Reef Watch 50 Km Satellite Sea Surface Temperature-Based Decision Support System for Coral Bleaching Management. NOAA Tech. Rep. NESDIS 2013, 143, 41. [Google Scholar]
- Perry, C.T.; Morgan, K.M. Post-Bleaching Coral Community Change on Southern Maldivian Reefs: Is There Potential for Rapid Recovery? Coral Reefs 2017, 36, 1189–1194. [Google Scholar] [CrossRef]
- Pisapia, C.; Burn, D.; Pratchett, M.S. Changes in the Population and Community Structure of Corals during Recent Disturbances (February 2016-October 2017) on Maldivian Coral Reefs. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Montefalcone, M.; Morri, C.; Bianchi, C.N. Influence of Local Pressures on Maldivian Coral Reef Resilience Following Repeated Bleaching Events, and Recovery Perspectives. Front. Mar. Sci. 2020, 7, 587. [Google Scholar] [CrossRef]
- Penin, L.; Adjeroud, M.; Schrimm, M.; Lenihan, H.S. High Spatial Variability in Coral Bleaching around Moorea (French Polynesia): Patterns across Locations and Water Depths. C. R. Biol. 2007, 330, 171–181. [Google Scholar] [CrossRef]
- Done, T.; Wooldridge, S. Learning to Predict Large-Scale Coral Bleaching from Past Events: A Bayesian Approach Using Remotely Sensed Data, in-Situ Data, and Environmental Proxies. Coral Reefs 2004, 23, 96–108. [Google Scholar] [CrossRef]
- Hoogenboom, M.O.; Frank, G.E.; Chase, T.J.; Jurriaans, S.; Álvarez-Noriega, M.; Peterson, K.; Critchell, K.; Berry, K.L.E.; Nicolet, K.J.; Ramsby, B.; et al. Environmental Drivers of Variation in Bleaching Severity of Acropora Species during an Extreme Thermal Anomaly. Front. Mar. Sci. 2017, 4, 376. [Google Scholar] [CrossRef]
- Kaniewska, P.; Anthony, K.R.N.; Hoegh-Guldberg, O. Variation in Colony Geometry Modulates Internal Light Levels in Branching Corals, Acropora Humilis and Stylophora Pistillata. Mar. Biol. 2008, 155, 649–660. [Google Scholar] [CrossRef]
- Swain, T.D.; DuBois, E.; Gomes, A.; Stoyneva, V.P.; Radosevich, A.J.; Henss, J.; Wagner, M.E.; Derbas, J.; Grooms, H.W.; Velazquez, E.M.; et al. Skeletal Light-Scattering Accelerates Bleaching Response in Reef-Building Corals. BMC Ecol. 2016, 16, 10. [Google Scholar] [CrossRef]
- Baird, A.H.; Marshall, P. Mortality, Growth and Reproduction in Scleractinian Corals Following Bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Vargas-Ángel, B.; Huntington, B.; Brainard, R.E.; Venegas, R.; Oliver, T.; Barkley, H.; Cohen, A. El Niño-Associated Catastrophic Coral Mortality at Jarvis Island, Central Equatorial Pacific. Coral Reefs 2019, 38, 731–741. [Google Scholar] [CrossRef]
- McClanahan, T.R. Bleaching Damage and Recovery Potential of Maldivian Coral Reefs. Mar. Pollut. Bull. 2000, 40, 587–597. [Google Scholar] [CrossRef]
- Edwards, A.J.; Clark, S.; Zahir, H.; Rajasuriya, A.; Naseer, A.; Rubens, J. Coral Bleaching and Mortality on Artificial and Natural Reefs in Maldives in 1998, Sea Surface Temperature Anomalies and Initial Recovery. Mar. Pollut. Bull. 2001, 42, 7–15. [Google Scholar] [CrossRef]
- Tkachenko, K.S. The Northernmost Coral Frontier of the Maldives: The Coral Reefs of Ihavandippolu Atoll under Long-Term Environmental Change. Mar. Environ. Res. 2012, 82, 40–48. [Google Scholar] [CrossRef]
- Morri, C.; Montefalcone, M.; Lasagna, R.; Gatti, G.; Rovere, A.; Parravicini, V.; Baldelli, G.; Colantoni, P.; Bianchi, C.N. Through Bleaching and Tsunami: Coral Reef Recovery in the Maldives. Mar. Pollut. Bull. 2015, 98, 188–200. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A. Community Change and Evidence for Variable Warm-Water Temperature Adaptation of Corals in Northern Male Atoll, Maldives. Mar. Pollut. Bull. 2014, 80, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, S.; Piccinetti, C.; Zaccanti, F. Tsunami Survey Expedition: Preliminary Investigation of Maldivian Coral Reefs Two Weeks after the Event. Environ. Monit. Assess. 2007, 131, 95–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pisapia, C.; Burn, D.; Yoosuf, R.; Najeeb, A.; Anderson, K.D.; Pratchett, M.S. Coral Recovery in the Central Maldives Archipelago since the Last Major Mass-Bleaching, in 1998. Sci. Rep. 2016, 6, 34720. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Pichon, M.; Benzoni, F.; Colantoni, P.; Baldelli, G.; Sandrini, M. Dynamics and Pattern of Coral Recolonization Following the 1998 Bleaching Event in the Reefs of the Maldives. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; Japanese Coral Reef Society: Tokyo, Japan, 2004; pp. 30–37. [Google Scholar]
- Schuhmacher, H.; Loch, K.; Loch, W.; See, W.R. The Aftermath of Coral Bleaching on a Maldivian Reef—a Quantitative Study. Facies 2005, 51, 80–92. [Google Scholar] [CrossRef]
- Goreau, T.; McClanahan, T.; Hayes, R.; Strong, A. Conservation of Coral Reefs after the 1998 Global Bleaching Event. Conserv. Biol. 2000, 14, 5–15. [Google Scholar] [CrossRef]
- Lasagna, R.; Albertelli, G.; Giovannetti, E.; Grondona, M.; Milani, A.; Morri, C.; Bianchi, C.N. Status of Maldivian Reefs Eight Years after the 1998 Coral Mass Mortality. Chem. Ecol. 2008, 24, 67–72. [Google Scholar] [CrossRef]
- Montefalcone, M.; Morri, C.; Bianchi, C.N. Long-Term Change in Bioconstruction Potential of Maldivian Coral Reefs Following Extreme Climate Anomalies. Glob. Chang. Biol. 2018, 24, 5629–5641. [Google Scholar] [CrossRef]
- Sheppard, C.R.C.; Harris, A.; Sheppard, A.L.S. Archipelago-Wide Coral Recovery Patterns since 1998 in the Chagos Archipelago, Central Indian Ocean. Mar. Ecol. Prog. Ser. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Polunin, N.V.C.; Jennings, S.; Chabanet, P.; Clark, S.; Spalding, M.D.; Letourneur, Y.; et al. Climate Warming, Marine Protected Areas and the Ocean-Scale Integrity of Coral Reef Ecosystems. PLoS ONE 2008, 3, e3039. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; et al. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Cowburn, B.; Samoilys, M.A.; Obura, D. The Current Status of Coral Reefs and Their Vulnerability to Climate Change and Multiple Human Stresses in the Comoros Archipelago, Western Indian Ocean. Mar. Pollut. Bull. 2018, 133, 956–969. [Google Scholar] [CrossRef]
- Jaleel, A. The Status of the Coral Reefs and the Management Approaches: The Case of the Maldives. Ocean Coast. Manag. 2013, 82, 104–118. [Google Scholar] [CrossRef]
- Nepote, E.; Bianchi, C.N.; Chiantore, M.; Morri, C.; Montefalcone, M. Pattern and Intensity of Human Impact on Coral Reefs Depend on Depth along the Reef Profile and on the Descriptor Adopted. Estuar. Coast. Shelf Sci. 2016, 178, 86–91. [Google Scholar] [CrossRef]
- Cowburn, B.; Moritz, C.; Birrell, C.; Grimsditch, G.; Abdulla, A. Can Luxury and Environmental Sustainability Co-Exist? Assessing the Environmental Impact of Resort Tourism on Coral Reefs in the Maldives. Ocean Coast. Manag. 2018, 158, 120–127. [Google Scholar] [CrossRef]
- Pancrazi, I.; Ahmed, H.; Cerrano, C.; Montefalcone, M. Synergic Effect of Global Thermal Anomalies and Local Dredging Activities on Coral Reefs of the Maldives. Mar. Pollut. Bull. 2020, 160, 111585. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Duckworth, A.; Jones, R. Impacts of Turbidity on Corals: The Relative Importance of Light Limitation and Suspended Sediments. Mar. Pollut. Bull. 2017, 117, 161–170. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Fisher, R.; Duckworth, A.; Jones, R. Mucous Sheet Production in Porites: An Effective Bioindicator of Sediment Related Pressures. Ecol. Indic. 2017, 77, 276–285. [Google Scholar] [CrossRef]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the Impacts of Sediments from Dredging on Corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Ricardo, G.F.; Jones, R.J.; Nordborg, M.; Negri, A.P. Settlement Patterns of the Coral Acropora Millepora on Sediment-Laden Surfaces. Sci. Total Environ. 2017, 609, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.L.E.; Hoogenboom, M.O.; Brinkman, D.L.; Burns, K.A.; Negri, A.P. Effects of Coal Contamination on Early Life History Processes of a Reef-Building Coral, Acropora Tenuis. Mar. Pollut. Bull. 2017, 114, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Jones, R. Cumulative Impacts: Thermally Bleached Corals Have Reduced Capacity to Clear Deposited Sediment. Sci. Rep. 2017, 7, 2716. [Google Scholar] [CrossRef]
- Fitt, W.K.; McFarland, F.K.; Warner, M.E.; Chilcoat, G.C. Seasonal Patterns of Tissue Biomass and Densities of Symbiotic Dinoflagellates in Reef Corals and Relation to Coral Bleaching. Limnol. Oceanogr. 2000, 45, 677–685. [Google Scholar] [CrossRef]
- Porter, J.W.; Fitt, W.K.; Spero, H.J.; Rogers, C.S.; White, M.W. Bleaching in Reef Corals: Physiological and Stable Isotopic Responses. Proc. Natl. Acad. Sci. USA 1989, 86, 9342–9346. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Bessell-Browne, P.; Jones, R. Synergistic and Antagonistic Impacts of Suspended Sediments and Thermal Stress on Corals. Nat. Commun. 2019, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Loder, J. Reef Check Australia Survey Methods; Reef Check Foundation Ltd.: Queensland, Australia, 2013; p. 18. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
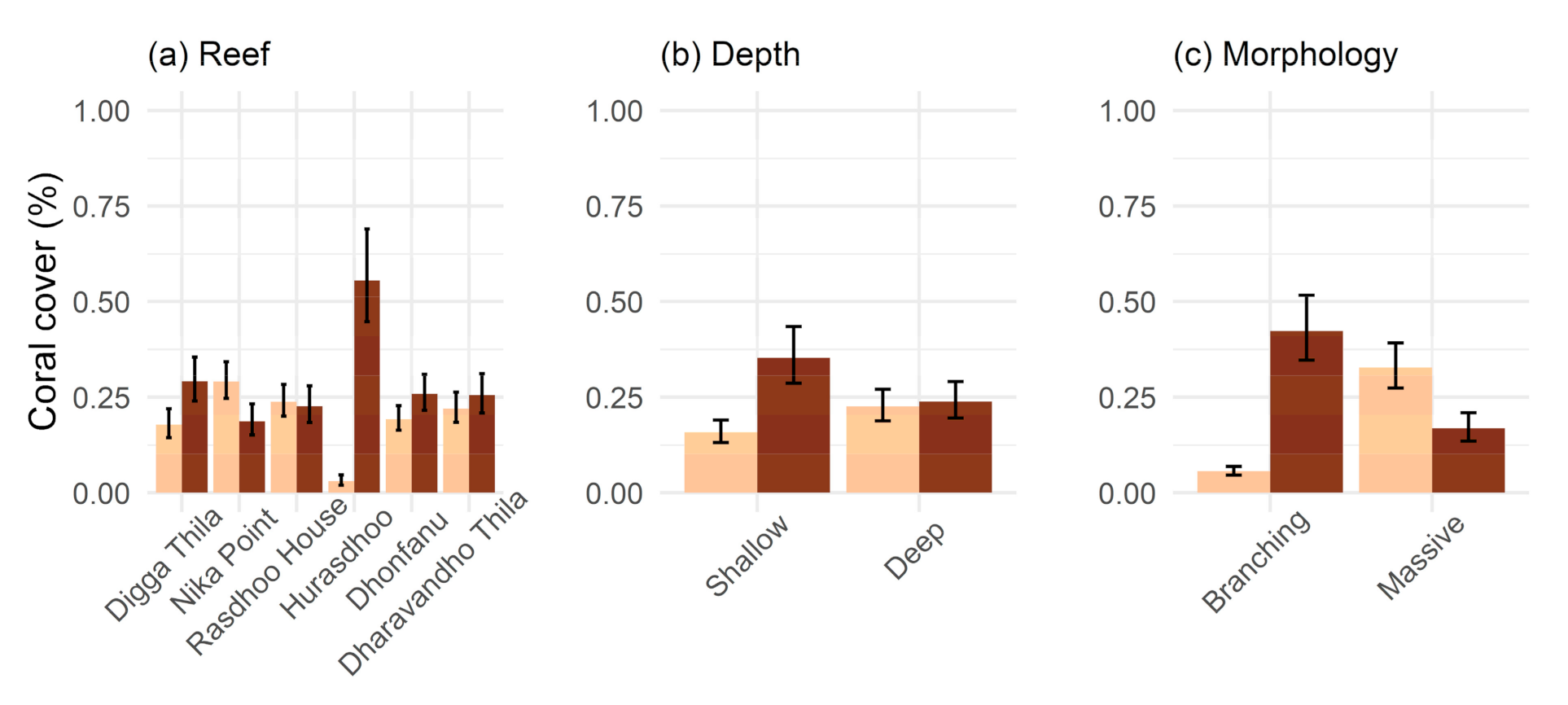

| Coral Cover | Model | n | AICc | δ AICc | ωi |
|---|---|---|---|---|---|
| (a) Live coral | Reef site + depth + morphology | 11 | 1013.3 | 0 | 1 |
| Reef site + morphology | 10 | 1036.7 | 23.31 | 0 | |
| Depth + morphology | 6 | 1153 | 139.64 | 0 | |
| Morphology | 5 | 1156.4 | 143.05 | 0 | |
| Reef site + depth | 10 | 1523.5 | 510.12 | 0 | |
| Reef site | 9 | 1537.7 | 524.31 | 0 | |
| Depth | 5 | 1635.1 | 621.79 | 0 | |
| Null | 4 | 1637 | 623.64 | 0 | |
| (b) Recently | Reef site + depth + morphology | 11 | 1034.7 | 0 | 1 |
| dead coral | Reef site + morphology | 10 | 1056.2 | 21.53 | 0 |
| Depth + morphology | 6 | 1093 | 58.25 | 0 | |
| Morphology | 5 | 1096.5 | 61.81 | 0 | |
| Reef site + depth | 10 | 1177.8 | 143.12 | 0 | |
| Reef site | 9 | 1193.5 | 158.75 | 0 | |
| Depth | 5 | 1236.9 | 202.18 | 0 | |
| Null | 4 | 1238.1 | 203.41 | 0 |
| Category | Description |
|---|---|
| Live hard coral | Live coral that is pigmented. Split into three morphology categories. |
| Bleached hard coral | Live coral that appears white or fluorescent in color and has lost normal pigment. Tissue has not been colonised by any algae and is therefore considered to still be alive. |
| Recently dead hard coral | Coral colonies that have recently died. Skeletons had a light covering of turf algae that distinguish them from translucent tissue in bleached coral and pigmented tissue in live coral. Algae cover was not well established and there was no breakdown of skeletal structure, suggesting coral mortality was recent. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessell-Browne, P.; Epstein, H.E.; Hall, N.; Buerger, P.; Berry, K. Severe Heat Stress Resulted in High Coral Mortality on Maldivian Reefs following the 2015–2016 El Niño Event. Oceans 2021, 2, 233-245. https://doi.org/10.3390/oceans2010014
Bessell-Browne P, Epstein HE, Hall N, Buerger P, Berry K. Severe Heat Stress Resulted in High Coral Mortality on Maldivian Reefs following the 2015–2016 El Niño Event. Oceans. 2021; 2(1):233-245. https://doi.org/10.3390/oceans2010014
Chicago/Turabian StyleBessell-Browne, Pia, Hannah E. Epstein, Nora Hall, Patrick Buerger, and Kathryn Berry. 2021. "Severe Heat Stress Resulted in High Coral Mortality on Maldivian Reefs following the 2015–2016 El Niño Event" Oceans 2, no. 1: 233-245. https://doi.org/10.3390/oceans2010014
APA StyleBessell-Browne, P., Epstein, H. E., Hall, N., Buerger, P., & Berry, K. (2021). Severe Heat Stress Resulted in High Coral Mortality on Maldivian Reefs following the 2015–2016 El Niño Event. Oceans, 2(1), 233-245. https://doi.org/10.3390/oceans2010014