Fiber-Reinforced Composites in Fixed Prosthodontics: A Comprehensive Overview of Their Historical Development, Types, Techniques, and Longevity
Abstract
1. Introduction
2. Methodology
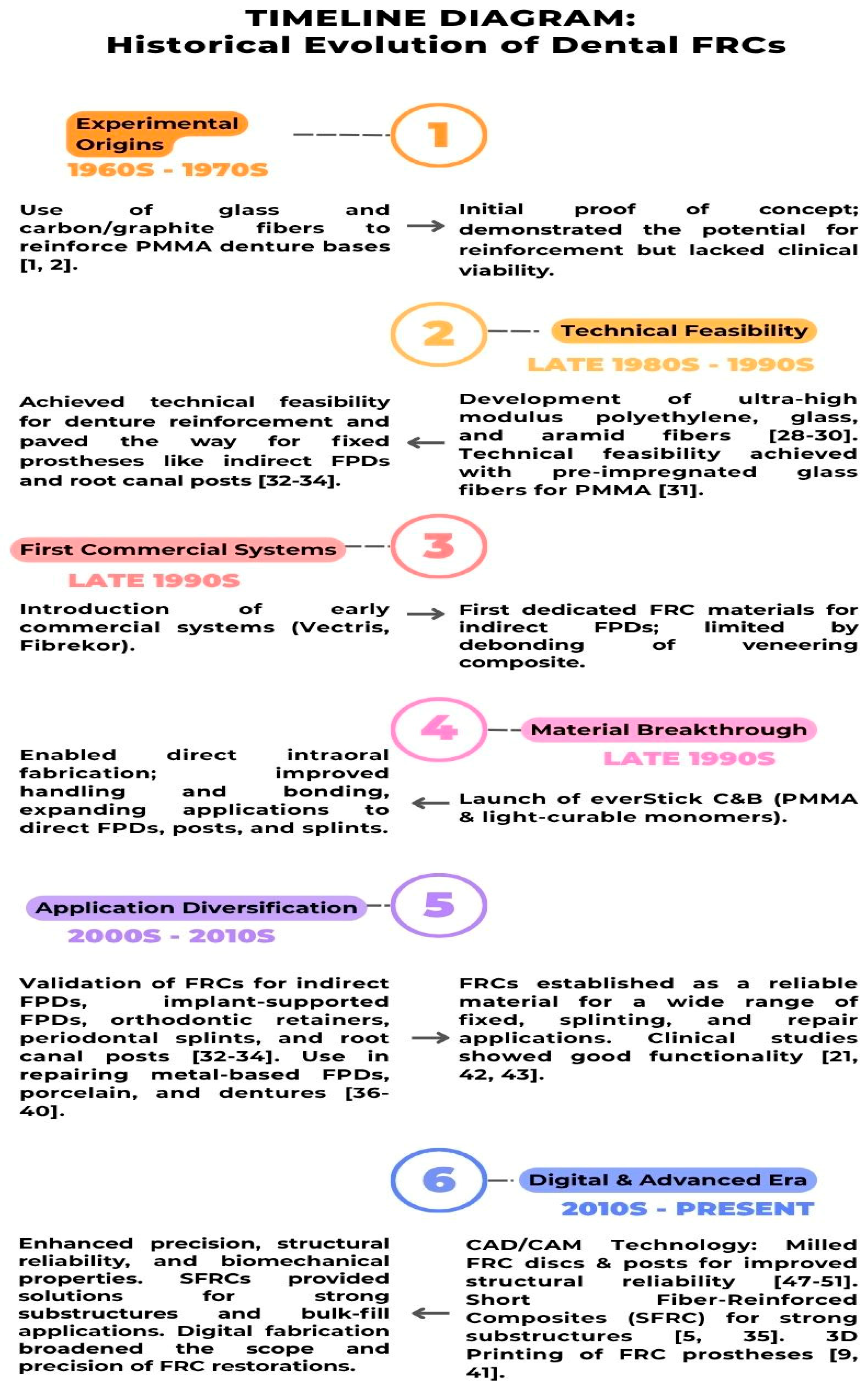
3. Historical Development and Evolution of FRC Materials
4. Types of Fiber-Reinforced Composites
4.1. Types of Fibers Used in Fiber-Reinforced Composites
| Fiber Type | Composition/Description | Advantages | Limitations | Applications in Dentistry |
|---|---|---|---|---|
| Carbon Fibers | Carbon content ~30–70%; higher content increases strength | High strength, fatigue resistance, toughens composites | Dark color reduces esthetics | Limited use; ongoing research on graphene-reinforced composites |
| Aramid Fibers | Aromatic polyamide (nylon-derived); high-crystallinity organic fiber | High impact and abrasion resistance; excellent fatigue resistance | Poor esthetics; limited bonding with resins due to surface inertness | Used when enhanced toughness is needed; bonding can be improved with surface treatments |
| Vectran Fibers | Aromatic polyester-based synthetic fibers | High abrasion and impact resistance | Expensive, difficult handling | Used in high-performance dental and medical materials |
| Polyethylene Fibers | High-strength organic fibers (e.g., UHMWPE) | Exhibit excellent toughness, impact resistance, and flexibility; enhance crack resistance in composites | Cost and handling limitations | Ideal for esthetic composite reinforcement |
| Other Organic Fibers | Includes polyester, acrylic, nylon, polypropylene, PBO, PBI, M5, and PI fibers | Lightweight, versatile, some variants highly esthetic | Some exhibit weak resin bonding | Alternative reinforcement options |
4.2. Types of Polymers Used in Fiber-Reinforced Composites
4.3. Resin Impregnation of Fibers
4.4. Glass Fiber Adhesion to the FRC Polymer Matrix
4.5. Dental Adhesives’ Adherence to FRC Construction
5. Characteristics of FRC- FPD’ Structure
6. Mechanical Properties and Load-Bearing Capacities of FRCs
7. Advantages and Challenges in FRC Prosthetic Application
8. Techniques of Fabricating FRC-FPDs
8.1. Direct Technique
8.2. Indirect Techniques
8.3. Semi-Direct Techniques
9. Discussion
9.1. Clinical Performance and Longevity of Direct and Indirect FRC-FPDs
9.2. Factors Affecting the Success and Survival of FRC-FPDs
9.3. Applications of FRCs in Dental Prosthetics
9.4. Fiber-Reinforced Composite FPD Supported by Implants
9.5. Factors Influencing FRC Material Selection
9.6. Clinical Considerations and Patient Selection
9.7. Comparison with Traditional Solutions
10. Advancements and Future Trends in FRC
Limitations and Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three dimensions |
| AC RBBs | All-ceramic-resin-bonded bridges |
| Bis-GMA | Bisphenol A-glycidyl methacrylate |
| CAD/CAM | Computer-Aided Design & Computer-Aided Manufacturing |
| EGDMA | ethylene glycol dimethacrylate |
| FPD | Fixed Partial Denture |
| FRCs | Fiber-Reinforced Composites |
| MPS | 3-trimethoxysilylpropyl methacrylate |
| PA | Polyamide |
| PC | Polycarbonate |
| PEEK | polyetheretherketone |
| PETG | poly(ethylene terephthalate glycol) |
| PMMA | Polymethyl methacrylate |
| PP | polypropylene |
| PU | polyurethane |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| sec-IPN | secondary interpenetrating polymer network |
| semi-IPN | Semi-interpenetrating polymer network |
| TEGDMA | Triethyleneglycol Dimethacrylate |
| UDMA | Urethane Dimethacrylate |
References
- Smith, D.C. Recent developments and prospects in dental polymers. J. Prosthet. Dent. 1962, 12, 1066–1078. [Google Scholar] [CrossRef]
- Schreiber, C.K. The clinical application of carbon fibre/polymer denture bases. Br. Dent. J. 1974, 137, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K. An overview of development and status of fiber-reinforced composites as dental and medical biomaterials. Acta Biomater. Odontol. Scand. 2018, 4, 44–55. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Matinlinna, J. Types of FRCs used in dentistry. In Clinical Guide to Principles of Fiber-Reinforced Composites in Dentistry; Woodhead Publishing: Cambridge, UK, 2017; pp. 11–34. [Google Scholar]
- McGarry, T.J.; Nimmo, A.; Skiba, J.F.; Ahlstrom, R.H.; Smith, C.R.; Koumjian, J.H.; Arbree, N.S. Classification system for partial edentulism. J. Prosthodont. 2002, 11, 181–193. [Google Scholar] [CrossRef]
- Edelhoff, D.; Stimmelmayr, M.; Schweiger, J.; Ahlers, M.O.; Güth, J.F. Advances in materials and concepts in fixed prosthodontics: A selection of possible treatment modalities. Br. Dent. J. 2019, 226, 739–748. [Google Scholar] [CrossRef]
- GC Europe. A World of Proof—Discover the Power of Fibres. Available online: https://www.gc.dental/europe/sites/europe.gc.dental/files/products/downloads/everstickcb/leaflet/LFL_everStickC%26B_en.pdf (accessed on 22 September 2024).
- Perea, L.; Matinlinna, J.P.; Tolvanen, M.; Lassila, L.V.; Vallittu, P.K. Fiber-reinforced composite fixed dental prostheses with various pontics. J. Adhes. Dent. 2014, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, D.; Shinya, A.; Lassila, L.V.; Gomi, H.; Nakasone, Y.; Vallittu, P.K.; Shinya, A. Framework design of an anterior fiber-reinforced hybrid composite fixed partial denture: A 3D finite element study. Int. J. Prosthodont. 2009, 22, 405–412. [Google Scholar]
- Alla, R.K.; Sanka, G.; Saridena, U.; Av, R.; Makv, R.; Mantena, S.R. Fiber-Reinforced Composites in Dentistry: Enhancing structural integrity and aesthetic appeal. Int. J. Dent. Mater. 2023, 5, 78–85. [Google Scholar] [CrossRef]
- Burgess, J.O.; Walker, R.; Davidson, J.M. Posterior resin-based composite: Review of the literature. Pediatr. Dent. 2002, 24, 465–479. [Google Scholar] [PubMed]
- Garoushi, S.; Vallittu, P. Fiber-reinforced composites in fixed partial dentures. Libyan J. Med. 2006, 1, 73–82. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Sevelius, C. Resin-bonded, glass fiber-reinforced composite fixed partial dentures: A clinical study. J. Prosthet. Dent. 2000, 84, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Lassila, L.V.; Vallittu, P.K. Comparison of load-bearing capacity of direct resin-bonded fiber-reinforced composite FPDs with four framework designs. J. Dent. 2007, 35, 578–582. [Google Scholar] [CrossRef]
- Garoushi, S.; Lassila, L.V.; Tezvergil, A.; Vallittu, P.K. Load bearing capacity of fibre-reinforced and particulate filler composite resin combination. J. Dent. 2006, 34, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.k.; Shinya, A. Structural properties of dental FRC structures. In Clinical Guide to Principles of Fiber-Reinforced Composites in Dentistry; Woodhead Publishing: Cambridge, UK, 2017; pp. 35–56. [Google Scholar]
- Jokstad, A.; Gokce, M.; Hjortsjo, C. A systematic review of the scientific documentation of fixed partial dentures made from fiber-reinforced polymer to replace missing teeth. J. Prosthet. Dent. 2006, 96, 321. [Google Scholar] [CrossRef]
- van Heumen, C.C.; Kreulen, C.M.; Creugers, N.H. Clinical studies of fiber-reinforced resin-bonded fixed partial dentures: A systematic review. Eur. J. Oral Sci. 2009, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.E.; Li, K.Y.; Murray, C.A. Longevity of fiber-reinforced composite fixed partial dentures (FRC FPD)-Systematic review. J. Dent. 2017, 61, 1–11. [Google Scholar] [CrossRef]
- Perrin, P.; Meyer-Lueckel, H.; Wierichs, R.J. Longevity of immediate rehabilitation with direct fiber reinforced composite fixed partial dentures after up to 9 years. J. Dent. 2020, 100, 103438. [Google Scholar] [CrossRef]
- Ang, Y.; Tan, C.G.; Yahaya, N. In-vitro performance of posterior fiber reinforced composite (FRC) bridge with different framework designs. Dent. Mater. J. 2021, 40, 584–591. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Bergamo, E.T.P.; Murcko, L.; Hirayama, M.; Perpetuini, P.; Speratti, D.; Bonfante, E.A. Fiber-reinforced composite partial fixed dental prostheses supported by short or extra-short implants: A 10 year retrospective study. Clin. Implant. Dent. Relat. Res. 2022, 24, 854–861. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Perpetuini, P.; Murcko, L.; Hirayama, M.; Morgan, K.; Marincola, M.; Bonfante, E.A.; Bergamo, E.T.P.; Ewers, R. Fiber-reinforced composite full-arch prosthetic reconstructions supported by three standard, short or extra-short implants: A two-center retrospective study. Clin. Oral Investig. 2023, 27, 4191–4203. [Google Scholar] [CrossRef]
- Brożek, R.; Koczorowski, R.; Dorocka-Bobkowska, B. Laboratory and clinical evaluation of polymer materials reinforced by fibers used in dentistry. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1855–1863. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Bonfante, E.A.; Bergamo, E.T.; Ewers, R. Partial fixed dental prostheses fabricated using fiber-reinforced composite resin supported by short and extra-short implants: A case series. J. Prosthodont. Res. 2024, 68, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Ladizesky, N.H. The integration of dental resins with highly drawn polyethylene fibres. Clin. Mater. 1990, 6, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ladizesky, N.H.; Ho, C.F.; Chow, T.W. Reinforcement of complete denture bases with continuous high performance polyethylene fibers. J. Prosthet. Dent. 1992, 68, 934–939. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Lassila, V.P. Reinforcement of acrylic resin denture base material with metal or fibre strengtheners. J. Oral Rehabil. 1992, 19, 225–230. [Google Scholar] [CrossRef]
- Vallittu, P.K. Oxygen inhibition of autopolymerization of polymethylmethacrylate-glass fibre composite. J. Mater. Sci. Mater. Med. 1997, 8, 489–492. [Google Scholar] [CrossRef]
- DeBoer, J.; Vermilyea, S.G.; Brady, R.E. The effect of carbon fiber orientation on the fatigue resistance and bending properties of two denture resins. J. Prosthet. Dent. 1984, 51, 119–121. [Google Scholar] [CrossRef]
- Grave, A.M.; Chandler, H.D.; Wolfaardt, J.F. Denture base acrylic reinforced with high modulus fibre. Dent. Mater. 1985, 1, 185–187. [Google Scholar] [CrossRef]
- Lassila, L.V.; Tanner, J.; Le Bell, A.M.; Narva, K.; Vallittu, P.K. Flexural properties of fiber reinforced root canal posts. Dent. Mater. 2004, 20, 29–36. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Vallittu, P.K. Use of woven glass fibres to reinforce a composite veneer. A fracture resistance and acoustic emission study. J. Oral Rehabil. 2002, 29, 423–429. [Google Scholar] [CrossRef]
- Ozcan, M.; van der Sleen, J.M.; Kurunmäki, H.; Vallittu, P.K. Comparison of repair methods for ceramic-fused-to-metal crowns. J. Prosthodont. 2006, 15, 283–288. [Google Scholar] [CrossRef]
- Gibreel, M.; Lassila, L.V.J.; Närhi, T.O.; Perea-Lowery, L.; Vallittu, P.K. Load-bearing capacity of simulated Locator-retained overdenture system. J. Prosthet. Dent. 2018, 120, 558–564. [Google Scholar] [CrossRef]
- Gibreel, M.; Lassila, L.V.J.; Närhi, T.O.; Perea-Lowery, L.; Vallittu, P.K. Fatigue resistance of a simulated single LOCATOR overdenture system. J. Prosthet. Dent. 2019, 122, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mohamed, B.A.; Al-Shamrani, S.S.; Ramakrishnaiah, R.; Perea-Lowery, L.; Säilynoja, E.; Vallittu, P.K. Influence of Monomer Systems on the Bond Strength Between Resin Composites and Polymerized Fiber-Reinforced Composite upon Aging. J. Adhes. Dent. 2019, 21, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Strassler, H.E.; Serio, C.L. Esthetic considerations when splinting with fiber-reinforced composites. Dent. Clin. N. Am. 2007, 51, 507–524. [Google Scholar] [CrossRef]
- Feinman, R.A.; Smidt, A. A combination porcelain/fiber-reinforced composite bridge: A case report. Pract. Periodontics Aesthet. Dent. 1997, 9, 925–929. [Google Scholar] [PubMed]
- Garoushi, S.; Vallittu, P. Chairside Fabricated Fiber-Reinforced Composite Fixed Partial Denture. Libyan J. Med. 2007, 2, 40–42. [Google Scholar] [CrossRef]
- Narva, K.K.; Vallittu, P.K.; Helenius, H.; Yli-Urpo, A. Clinical survey of acrylic resin removable denture repairs with glass-fiber reinforcement. Int. J. Prosthodont. 2001, 14, 219–224. [Google Scholar]
- Narva, K.K.; Lassila, L.V.; Vallittu, P.K. The static strength and modulus of fiber reinforced denture base polymer. Dent. Mater. 2005, 21, 421–428. [Google Scholar] [CrossRef]
- Khan, A.A.; Fareed, M.A.; Alshehri, A.H.; Aldegheishem, A.; Alharthi, R.; Saadaldin, S.A.; Zafar, M.S. Mechanical Properties of the Modified Denture Base Materials and Polymerization Methods: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 5737. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, E.T.P.; Yamaguchi, S.; Lopes, A.C.O.; Coelho, P.G.; de Araújo-Júnior, E.N.S.; Benalcázar Jalkh, E.B.; Zahoui, A.; Bonfante, E.A. Performance of crowns cemented on a fiber-reinforced composite framework 5-unit implant-supported prostheses: In silico and fatigue analyses. Dent. Mater. 2021, 37, 1783–1793. [Google Scholar] [CrossRef]
- Bonfante, E.A.; Suzuki, M.; Carvalho, R.M.; Hirata, R.; Lubelski, W.; Bonfante, G.; Pegoraro, T.A.; Coelho, P.G. Digitally produced fiber-reinforced composite substructures for three-unit implant-supported fixed dental prostheses. Int. J. Oral Maxillofac. Implant. 2015, 30, 321–329. [Google Scholar] [CrossRef]
- Suzaki, N.; Yamaguchi, S.; Nambu, E.; Tanaka, R.; Imazato, S.; Hayashi, M. Fabricated CAD/CAM Post-Core Using Glass Fiber-Reinforced Resin Shows Innovative Potential in Restoring Pulpless Teeth. Materials 2021, 14, 6199. [Google Scholar] [CrossRef]
- Mangoush, E.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Microstructure and Surface Characteristics of Short- Fiber Reinforced CAD/CAM Composite Blocks. Eur. J. Prosthodont. Restor. Dent. 2021, 29, 166–174. [Google Scholar] [CrossRef]
- Mangoush, E.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Shear-bond strength and optical properties of short fiber-reinforced CAD/CAM composite blocks. Eur. J. Oral Sci. 2021, 129, e12815. [Google Scholar] [CrossRef]
- Mangoush, E.; Säilynoja, E.; Prinssi, R.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Comparative evaluation between glass and polyethylene fiber reinforced composites: A review of the current literature. J. Clin. Exp. Dent. 2017, 9, e1408–e1417. [Google Scholar] [CrossRef] [PubMed]
- Barbucci, R. Integrated Biomaterials Science; Springer: New York, NY, USA, 2002. [Google Scholar]
- Dyer, S.R.; Lassila, L.V.J.; Jokinen, M.; Vallittu, P.K. Effect of fiber position and orientation on fracture load of fiber-reinforced composite. Dent. Mater. 2004, 20, 947–955. [Google Scholar] [CrossRef]
- Abdulamir, S.W.; Majeed, M.A. Fiber-Reinforced Composites in Operative Dentistry (A Literature Review). Tikrit J. Dent. Sci. 2024, 11, 271–284. [Google Scholar] [CrossRef]
- Cogswell, F.N. Thermoplastic Aromatic Polymer Composites; Woodhead Publishing: Cambridge, UK, 1992; 277p. [Google Scholar]
- Jancar, J.; Dibenedetto, A.T. Fibre reinforced thermoplastic composites for dentistry. J. Mater. Sci. Mater. Med. 1993, 4, 555–561. [Google Scholar] [CrossRef]
- Goldberg, A.J.; Burstone, C.J.; Hadjinikolaou, I.; Jancar, J. Screening of matrices and fibers for reinforced thermoplastics intended for dental applications. J. Biomed. Mater. Res. 1994, 28, 167–173. [Google Scholar] [CrossRef]
- Basavarajappa, S.; Perea-Lowery, L.; Alshehri, A.M.; Al-Kheraif, A.A.A.; Matinlinna, J.P.; Vallittu, P.K. Surface dissolution and transesterification of thermoset dimethacrylate polymer by dimethacrylate adhesive resin and organic catalyst-alcohol solution. Dent. Mater. 2020, 36, 698–709. [Google Scholar] [CrossRef]
- Karmaker, A.C.; Dibenedetto, A.T.; Goldberg, A.J. Extent of conversion and its effect on the mechanical performance of Bis-GMA/PEGDMA-based resins and their composites with continuous glass fibres. J. Mater. Sci. Mater. Med. 1997, 8, 369–374. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating Polymer Networks. In Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1994; pp. 3–38. [Google Scholar]
- Vallittu, P.K. Interpenetrating Polymer Networks (IPNs) in Dental Polymers and Composites. J. Adhes. Sci. Technol. 2009, 23, 961–972. [Google Scholar] [CrossRef]
- Pastila, P.; Lassila, L.V.; Jokinen, M.; Vuorinen, J.; Vallittu, P.K.; Mäntylä, T. Effect of short-term water storage on the elastic properties of some dental restorative materials—A resonant ultrasound spectroscopy study. Dent. Mater. 2007, 23, 878–884. [Google Scholar] [CrossRef]
- Mannocci, F.; Sherriff, M.; Watson, T.F.; Vallittu, P.K. Penetration of bonding resins into fibre-reinforced composite posts: A confocal microscopic study. Int. Endod. J. 2005, 38, 46–51. [Google Scholar] [CrossRef]
- Frese, C.; Decker, C.; Rebholz, J.; Stucke, K.; Staehle, H.J.; Wolff, D. Original and repair bond strength of fiber-reinforced composites in vitro. Dent. Mater. 2014, 30, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Geiger, S.; Ding, P.; Staehle, H.J.; Frese, C. Analysis of the interdiffusion of resin monomers into pre-polymerized fiber-reinforced composites. Dent. Mater. 2012, 28, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, I.E.; Ekstrand, K.; Bjork, N. Development of carbon/graphite fiber reinforced poly (methyl methacrylate) suitable for implant-fixed dental bridges. Dent. Mater. 1986, 2, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K. Some aspects of the tensile strength of undirectional glass fibre-polymethyl methacrylate composite used in dentures. J. Oral Rehabil. 1998, 25, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K. Ultra-high-modulus polyethylene ribbon as reinforcement for denture polymethyl methacrylate: A short communication. Dent. Mater. 1997, 13, 381–382. [Google Scholar] [CrossRef]
- Vallittu, P.K. Dimensional accuracy and stability of polymethyl methacrylate reinforced with metal wire or with continuous glass fiber. J. Prosthet. Dent. 1996, 75, 617–621. [Google Scholar] [CrossRef]
- Vallittu, P.K. Flexural properties of acrylic resin polymers reinforced with unidirectional and woven glass fibers. J. Prosthet. Dent. 1999, 81, 318–326. [Google Scholar] [CrossRef]
- Kolbeck, C.; Rosentritt, M.; Behr, M.; Lang, R.; Handel, G. In vitro examination of the fracture strength of 3 different fiber-reinforced composite and 1 all-ceramic posterior inlay fixed partial denture systems. J. Prosthodont. 2002, 11, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Vallittu, P.K. Bonding of resin composites to etchable ceramic surfaces—An insight review of the chemical aspects on surface conditioning. J. Oral Rehabil. 2007, 34, 622–630. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Ozcan, M.; Lassila, L.V.; Vallittu, P.K. Applications of trialkoxysilanes in dental biomaterials: A review. In Silanes and Other Coupling Agents; CRC Press: Boca Raton, FL, USA, 2007; pp. 199–215. [Google Scholar]
- Vallittu, P.K. Effect of 10 years of in vitro aging on the flexural properties of fiber-reinforced resin composites. Int. J. Prosthodont. 2007, 20, 43–45. [Google Scholar]
- Heikkinen, T.T.; Matinlinna, J.P.; Vallittu, P.K.; Lassila, L.V. Long term water storage deteriorates bonding of composite resin to alumina and zirconia short communication. Open Dent. J. 2013, 7, 123–125. [Google Scholar] [CrossRef]
- Norström, A.; Watson, H.; Engström, B.; Rosenholm, J. Treatment of E-glass fibres with acid, base and silanes. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 143–157. [Google Scholar] [CrossRef]
- Velo, M.M.A.C.; Nascimento, T.R.L.; Scotti, C.K.; Bombonatti, J.F.S.; Furuse, A.Y.; Silva, V.D.; Simões, T.A.; Medeiros, E.S.; Blaker, J.J.; Silikas, N.; et al. Improved mechanical performance of self-adhesive resin cement filled with hybrid nanofibers-embedded with niobium pentoxide. Dent. Mater. 2019, 35, e272–e285. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K.; Durgesh, B.H.; AlKheraif, A.A.; Hjerppe, J. From body-on-frame to unibody constructions and designs mimicking biological structures—An overview. Eur. J. Oral Sci. 2018, 126, 95–101. [Google Scholar] [CrossRef]
- Ruyter, I.E.; Svendsen, S.A. Flexural properties of denture base polymers. J. Prosthet. Dent. 1980, 43, 95–104. [Google Scholar] [CrossRef]
- Ruyter, I.E.; Øysaed, H. Conversion in denture base polymers. J. Biomed. Mater. Res. 1982, 16, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Lastumäki, T.M.; Lassila, L.V.; Vallittu, P.K. The semi-interpenetrating polymer network matrix of fiber-reinforced composite and its effect on the surface adhesive properties. J. Mater. Sci. Mater. Med. 2003, 14, 803–809. [Google Scholar] [CrossRef]
- Kim, J.K.; Mai, Y.W. Engineered Interfaces in Fiber Reinforced Composites; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Khan, A.A.; Perea-Lowery, L.; Al-Khureif, A.A.; AlMufareh, N.A.; Eldwakhly, E.; Säilynoja, E.; Vallittu, P.K. Interfacial Adhesion of a Semi-Interpenetrating Polymer Network-Based Fiber-Reinforced Composite with a High and Low-Gradient Poly(methyl methacrylate) Resin Surface. Polymers 2021, 13, 352. [Google Scholar] [CrossRef]
- Escobar, L.B.; Pereira da Silva, L.; Manarte-Monteiro, P. Fracture Resistance of Fiber-Reinforced Composite Restorations: A Systematic Review and Meta-Analysis. Polymers 2023, 15, 3802. [Google Scholar] [CrossRef]
- Khan, A.A.; Zafar, M.S.; Fareed, M.A.; AlMufareh, N.A.; Alshehri, F.; AlSunbul, H.; Lassila, L.; Garoushi, S.; Vallittu, P.K. Fiber-reinforced composites in dentistry—An insight into adhesion aspects of the material and the restored tooth construct. Dent. Mater. 2023, 39, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Bijelic-Donova, J.; Garoushi, S.; Lassila, L.V.; Vallittu, P.K. Oxygen inhibition layer of composite resins: Effects of layer thickness and surface layer treatment on the interlayer bond strength. Eur. J. Oral Sci. 2015, 123, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kallio, T.T.; Tezvergil-Mutluay, A.; Lassila, L.V.; Vallittu, P.K. The effect of surface roughness on repair bond strength of light-curing composite resin to polymer composite substrate. Open Dent. J. 2013, 7, 126–131. [Google Scholar] [CrossRef]
- Vallittu, P.K. High-aspect ratio fillers: Fiber-reinforced composites and their anisotropic properties. Dent. Mater. 2015, 31, 1–7. [Google Scholar] [CrossRef]
- Lassila, L.; Tuokko, J.; Suni, A.; Garoushi, S.; Vallittu, P.K. Effect of interfacial surface treatment on bond strength of particulate-filled composite to short fiber-reinforced composite. Biomater. Investig. Dent. 2022, 9, 33–40. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Kononen, M. Prosthodontic materials. Biomechanical aspects and materials properties. In A Textbook of Fixed Prosthodontics: The Scandinavian Approach; Publishing House Gothia: Stockholm, Sweden, 2000; pp. 116–130. [Google Scholar]
- Dyer, S.R.; Lassila, L.V.; Jokinen, M.; Vallittu, P.K. Effect of cross-sectional design on the modulus of elasticity and toughness of fiber-reinforced composite materials. J. Prosthet. Dent. 2005, 94, 219–226. [Google Scholar] [CrossRef]
- Lassila, L.V.; Nohrström, T.; Vallittu, P.K. The influence of short-term water storage on the flexural properties of unidirectional glass fiber-reinforced composites. Biomaterials 2002, 23, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Schütt, A.; Alander, P.; Schwaller, P.; Buerki, G.; Michler, J.; Cattani-Lorente, M.; Vallittu, P.K.; Krejci, I. Hydrothermal and mechanical stresses degrade fiber-matrix interfacial bond strength in dental fiber-reinforced composites. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 98–105. [Google Scholar] [CrossRef]
- Alander, P.; Lassila, L.V.J.; Tezvergil, A.; Vallittu, P.K. Acoustic emission analysis of fiber-reinforced composite in flexural testing. Dent. Mater. 2004, 20, 305–312. [Google Scholar] [CrossRef]
- Kardos, J.L. Short-fiber-reinforced polymeric composites, structure-property relations. In Handbook of Composite Reinforcements; Wiley-VCH: Weinheim, Germany, 1993; p. 593. [Google Scholar]
- Batdorf, S.B. Strength of composites. In Consice Encyclopedia of Composite Materials; Pergamon Press: Oxford, UK, 1994; p. 273. [Google Scholar]
- Garoushi, S.; Vallittu, P.; Lassila, L. Effect of Short Fiber Fillers on the Optical Properties of Composite Resins. J. Mater. Sci. Res. 2012, 1, 174. [Google Scholar] [CrossRef][Green Version]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Bonding performance and interfacial characteristics of short fiber-reinforced resin composite in comparison with other composite restoratives. Eur. J. Oral Sci. 2016, 124, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M. Longevity of fiber-reinforced resin composite (FRC) fixed dental prosthesis (FDP) and fabrication of direct FRC FDPs. In Clinical Guide to Principles of Fiber-Reinforced Composites in Dentistry; Woodhead Publishing: Cambridge, UK, 2017; pp. 203–210. [Google Scholar]
- Wolff, D.; Wohlrab, T.; Saure, D.; Krisam, J.; Frese, C. Fiber-reinforced composite fixed dental prostheses: A 4-year prospective clinical trial evaluating survival, quality, and effects on surrounding periodontal tissues. J. Prosthet. Dent. 2018, 119, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Burduroglu, D.; Guncu, M.B.; Keyf, F.; Özcan, M. Clinical Survival of Indirect, Anterior Surface-Retained Fiber-Reinforced Composite Fixed Dental Prosthesis: Up to 3-Year Follow-up. Eur. J. Prosthodont. Restor. Dent. 2019, 27, 90–94. [Google Scholar] [CrossRef]
- Kumbuloglu, O.; Özcan, M. Clinical survival of indirect, anterior 3-unit surface-retained fibre-reinforced composite fixed dental prosthesis: Up to 7.5-years follow-up. J. Dent. 2015, 43, 656–663. [Google Scholar] [CrossRef]
- Wolff, D.; Schach, C.; Kraus, T.; Ding, P.; Pritsch, M.; Mente, J.; Joerss, D.; Staehle, H.J. Fiber-reinforced composite fixed dental prostheses: A retrospective clinical examination. J. Adhes. Dent. 2011, 13, 187–194. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Piedra-Cascón, W.; Zandinejad, A.; Revilla-León, M. Fiber-reinforced composite fixed dental prosthesis using an additive manufactured silicone index. J. Esthet. Restor. Dent. 2020, 32, 626–633. [Google Scholar] [CrossRef]
- Revilla-León, M.; Fountain, J.; Piedra-Cascón, W.; Özcan, M.; Zandinejad, A. Workflow of a fiber-reinforced composite fixed dental prosthesis by using a 4-piece additive manufactured silicone index: A dental technique. J. Prosthet. Dent. 2021, 125, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Benitez Sellan, P.L.; Özcan, M.; Ferraz Caneppele, T.M.; Bresciani, E. A sectional precontoured metal matrix to improve the pontic contour and emergence profile for fiber-reinforced composite resin fixed dental prostheses. J. Prosthet. Dent. 2022, 128, 112–114. [Google Scholar] [CrossRef]
- Özcan, M.; Perea-Lowery, L.; Vallittu, P. 5—Fabrication of indirect fiber reinforced resin composite (FRC) dental devices. In A Clinical Guide to Fibre Reinforced Composites (FRCs) in Dentistry; Vallittu, P., Özcan, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 65–77. [Google Scholar]
- Elsaka, S.E. Repair bond strength of resin composite to a novel CAD/CAM hybrid ceramic using different repair systems. Dent. Mater. J. 2015, 34, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Goguta, L.M.; Candea, A.; Lungeanu, D.; Frandes, M.; Jivanescu, A. Direct Fiber-Reinforced Interim Fixed Partial Dentures: Six-Year Survival Study. J. Prosthodont. 2019, 28, e604–e608. [Google Scholar] [CrossRef] [PubMed]
- Collares, K.; Opdam, N.J.M.; Laske, M.; Bronkhorst, E.M.; Demarco, F.F.; Correa, M.B.; Huysmans, M. Longevity of Anterior Composite Restorations in a General Dental Practice-Based Network. J. Dent. Res. 2017, 96, 1092–1099. [Google Scholar] [CrossRef]
- Naumann, M.; Koelpin, M.; Beuer, F.; Meyer-Lueckel, H. 10-year survival evaluation for glass-fiber-supported postendodontic restoration: A prospective observational clinical study. J. Endod. 2012, 38, 432–435. [Google Scholar] [CrossRef]
- Malmstrom, H.; Dellanzo-Savu, A.; Xiao, J.; Feng, C.; Jabeen, A.; Romero, M.; Huang, J.; Ren, Y.; Yunker, M.A. Success, clinical performance and patient satisfaction of direct fibre-reinforced composite fixed partial dentures—A two-year clinical study. J. Oral Rehabil. 2015, 42, 906–913. [Google Scholar] [CrossRef]
- Aktas, G.; Basara, E.G.; Sahin, E.; Uctasli, S.; Vallittu, P.K.; Lassila, L.V. Effects of different cavity designs on fracture load of fiber-reinforced adhesive fixed dental prostheses in the anterior region. J. Adhes. Dent. 2013, 15, 131–135. [Google Scholar] [CrossRef]
- Ozcan, M.; Breuklander, M.H.; Vallittu, P.K. The effect of box preparation on the strength of glass fiber-reinforced composite inlay-retained fixed partial dentures. J. Prosthet. Dent. 2005, 93, 337–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behr, M.; Rosentritt, M.; Taubenhansl, P.; Kolbeck, C.; Handel, G. Fracture resistance of fiber-reinforced composite restorations with different framework design. Acta Odontol. Scand. 2005, 63, 153–157. [Google Scholar] [CrossRef]
- Tanoue, N.; Sawase, T.; Matsumura, H.; McCabe, J.F. Properties of indirect composites reinforced with monomer-impregnated glass fiber. Odontology 2012, 100, 192–198. [Google Scholar] [CrossRef]
- Kramer, E.J.; Meyer-Lueckel, H.; Wolf, T.G.; Schwendicke, F.; Naumann, M.; Wierichs, R.J. Success and survival of post-restorations: Six-year results of a prospective observational practice-based clinical study. Int. Endod. J. 2019, 52, 569–578. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Kramer, E.J.; Wolf, T.G.; Naumann, M.; Meyer-Lueckel, H. Longevity of composite build-ups without posts-10-year results of a practice-based study. Clin. Oral Investig. 2019, 23, 1435–1442. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Kramer, E.J.; Meyer-Lueckel, H. Risk Factors for Failure of Direct Restorations in General Dental Practices. J. Dent. Res. 2020, 99, 1039–1046. [Google Scholar] [CrossRef]
- Gresnigt, M.M.; Kalk, W.; Ozcan, M. Randomized clinical trial of indirect resin composite and ceramic veneers: Up to 3-year follow-up. J. Adhes. Dent. 2013, 15, 181–190. [Google Scholar] [CrossRef]
- Dyer, S.R.; Sorensen, J.A.; Lassila, L.V.; Vallittu, P.K. Damage mechanics and load failure of fiber-reinforced composite fixed partial dentures. Dent. Mater. 2005, 21, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.O.; Ozcan, M.; Michida, S.M.; de Melo, R.M.; Pavanelli, C.A.; Bottino, M.A.; Soares, L.E.; Martin, A.A. Conversion degree of indirect resin composites and effect of thermocycling on their physical properties. J. Prosthodont. 2010, 19, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A.; Asmussen, E. The effect of postcuring on quantity of remaining double bonds, mechanical properties, and in vitro wear of two resin composites. J. Dent. 2000, 28, 447–452. [Google Scholar] [CrossRef]
- Özcan, M.; Lamperti, S. Effect of mechanical and air-particle cleansing protocols of provisional cement on immediate dentin sealing layer and subsequent adhesion of resin composite cement. J. Adhes. Sci. Technol. 2015, 29, 2731–2743. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Watts, D.C.; Lassila, L.V.J. Polymerization shrinkage of experimental short glass fiber-reinforced composite with semi-inter penetrating polymer network matrix. Dent. Mater. 2008, 24, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Perea, L.; Matinlinna, J.P.; Tolvanen, M.; Mannocci, F.; Watson, T.F.; Vallittu, P.K. Penetration depth of monomer systems into acrylic resin denture teeth used as pontics. J. Prosthet. Dent. 2015, 113, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.; Liu, P.R. 3D-woven fiber-reinforced composite for CAD/CAM dental application. SAMPE journal. Soc. Adv. Mater. Process Eng. 2016, 2016, LB15-0138. [Google Scholar]
- Muhamad, A.; Ezzaldeen, A.; Nezar, W. Single Visit Replacement of Central Maxillary Using Fiber-Reinforced Composite Resin. IOSR J. Dent. Med. Sci. 2017, 16, 69–74. [Google Scholar] [CrossRef]
- Watted, N.; Abdulgani, A.; Muhamad, A. Aesthetic Replacement of Congenitally Missing Tooth Using Fiber-Reinforced Composite (FRC). Int. J. Dent. Health Sci. 2013, 1, 644–653. [Google Scholar]
- Yamuna, V.; Meshramkar, R. Anterior Fiber Reinforced Composite-fixed partial denture—A case report. Int. J. Curr. Res. 2019, 11, 650–653. [Google Scholar]
- Thirugnanam, S.; Cinthoori, C.S. Fibre Reinforced Fixed Partial Denture for Periodontally Compromised Anterior Teeth. J. Dent. Oral Sci. 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Muhamad, A.-H.; Abdulgani, A.; Mai, A. Ortho-Prostho Management of Hypodontia Using Fibre-Reinforced Composite Resin Bridge: An Interdisciplinary Approach. IOSR J. Dent. Med. Sci. (IOSR-JDMS) 2017, 16, 92–97. [Google Scholar]
- Nair, A.B.; Joseph, R. 9—Eco-friendly bio-composites using natural rubber (NR) matrices and natural fiber reinforcements. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 249–283. [Google Scholar]
- Derringer, G.C. Short Fiber-Elastomer Composites. J. Elastoplast. 1971, 3, 230–248. [Google Scholar] [CrossRef]
- Ozbakkaloglu, T.; Vincent, T. Axial Compressive Behavior of Circular High-Strength Concrete-Filled FRP Tubes. J. Compos. Constr. 2014, 18, 04013037. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Khandal, R.; Uppaluri, R.; Ghoshal, A. Bamboo Fiber Reinforced Polypropylene Composites and Their Mechanical, Thermal, and Morphological Properties. J. Appl. Polym. Sci. 2011, 119, 1619–1626. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhou, Y.; Huang, Z.M.; Batra, R.C. Experimental and micromechanical investigation of T300/7901 unidirectional composite strength. Polym. Compos. 2019, 40, 2639–2652. [Google Scholar] [CrossRef]
- Muhamad, A.-H.; Abdulgani, A. Aesthetic Replacement of Missing Tooth Using Fiber Splint. J. Dent. Med. Sci. 2021, 20, 18–23. [Google Scholar]
- Pjetursson, B.E.; Sailer, I.; Makarov, N.A.; Zwahlen, M.; Thoma, D.S. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part II: Multiple-unit FDPs. Dent. Mater. 2015, 31, 624–639. [Google Scholar] [CrossRef]
- Miettinen, M.; Millar, B.J. A review of the success and failure characteristics of resin-bonded bridges. Br. Dent. J. 2013, 215, E3. [Google Scholar] [CrossRef]
- Posti, J.P.; Piitulainen, J.M.; Hupa, L.; Fagerlund, S.; Frantzén, J.; Aitasalo, K.M.J.; Vuorinen, V.; Serlo, W.; Syrjänen, S.; Vallittu, P.K. A glass fiber-reinforced composite—Bioactive glass cranioplasty implant: A case study of an early development stage implant removed due to a late infection. J. Mech. Behav. Biomed. Mater. 2015, 55, 191–200. [Google Scholar] [CrossRef]
- Gogna, R.; Jagadis, S.; Shashikal, K. A comparative in vitro study of microleakage by a radioactive isotope and compressive strength of three nanofilled composite resin restorations. J. Conserv. Dent. 2011, 14, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Massironi, S.; Pieraccini, G.; Scribante, A.; Vallittu, P.K.; Lassila, L.V.; Gandini, P. Flexural strengths of conventional and nanofilled fiber-reinforced composites: A three-point bending test. Dent. Traumatol. 2014, 30, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Fraticelli, D.; Castellazzi, L.; Scribante, A.; Gandini, P. Clinical evaluation of bond failures and survival between mandibular canine-to-canine retainers made of flexible spiral wire and fiber-reinforced composite. J. Clin. Exp. Dent. 2014, 6, e145–e149. [Google Scholar] [CrossRef]
- Scribante, A.; Massironi, S.; Pieraccini, G.; Vallittu, P.; Lassila, L.; Sfondrini, M.F.; Gandini, P. Effects of Nanofillers on Mechanical Properties of Fiber-Reinforced Composites Polymerized with Light-Curing and Additional Postcuring. J. Appl. Biomater. Funct. Mater. 2015, 13, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Advancing Discontinuous Fiber-Reinforced Composites above Critical Length for Replacing Current Dental Composites and Amalgam. J. Nat. Sci. 2017, 3, e321. [Google Scholar]
- Perea, L.; Matinlinna, J.P.; Tolvanen, M.; Vallittu, P.K. Fracture behavior of pontics of fiber-reinforced composite fixed dental prostheses. Dent. Mater. J. 2015, 34, 746–753. [Google Scholar] [CrossRef][Green Version]
- Fráter, M.; Sáry, T.; Néma, V.; Braunitzer, G.; Vallittu, P.; Lassila, L.; Garoushi, S. Fatigue failure load of immature anterior teeth: Influence of different fiber post-core systems. Odontology 2021, 109, 222–230. [Google Scholar] [CrossRef]
- Fráter, M.; Sáry, T.; Molnár, J.; Braunitzer, G.; Lassila, L.; Vallittu, P.K.; Garoushi, S. Fatigue performance of endodontically treated premolars restored with direct and indirect cuspal coverage restorations utilizing fiber-reinforced cores. Clin. Oral Investig. 2022, 26, 3501–3513. [Google Scholar] [CrossRef]
- Otero, C.A.Y.; Bijelic-Donova, J.; Saratti, C.M.; Vallittu, P.K.; di Bella, E.; Krejci, I.; Rocca, G.T. The influence of FRC base and bonded CAD/CAM resin composite endocrowns on fatigue behavior of cracked endodontically-treated molars. J. Mech. Behav. Biomed. Mater. 2021, 121, 104647. [Google Scholar] [CrossRef]
- Mocquot, C.; Colon, P.; Fernando, D.; Jackson, P.; Pradelle-Plasse, N.; Grosgogeat, B.; Attik, N. The influence of experimental bioactive glasses on pulp cells behavior in vitro. Dent. Mater. 2020, 36, 1322–1331. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Posti, J.P.; Piitulainen, J.M.; Serlo, W.; Määttä, J.A.; Heino, T.J.; Pagliari, S.; Syrjänen, S.M.; Forte, G. Biomaterial and implant induced ossification: In vitro and in vivo findings. J. Tissue Eng. Regen. Med. 2020, 14, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, M.; Sameh, A.; Hegazy, S.; Närhi, T.O.; Vallittu, P.K.; Perea-Lowery, L. Effect of specific retention biomaterials for ball attachment on the biomechanical response of single implant-supported overdenture: A finite element analysis. J. Mech. Behav. Biomed. Mater. 2021, 122, 104653. [Google Scholar] [CrossRef] [PubMed]
| Sub-Classification | Composition/Properties | Advantages | Disadvantages/Limitations | Applications in Dentistry | Reference |
|---|---|---|---|---|---|
| Glass A | Alkali-based glass; low chemical resistance | Low cost, useful as filler in plastics | Poor strength and water resistance | Rarely used; mainly as filler | [49,50,51] |
| Glass C | High corrosion-resistant glass | Strong chemical protection | Lower mechanical strength compared to E-glass | Surface layers needing enhanced chemical durability | [49,50,51] |
| Glass E | Electric glass; superior electrical and mechanical properties | Most commonly used; high strength; good water resistance | Contains volatile substances like fluorine | Widely used in dental composites and reinforcement | [49,50,51] |
| Glass R | Calcium alumino-silicate reinforcement glass | High strength and acid corrosion resistance | Higher cost than E-glass | Applications requiring durability and strength | [49,50,51] |
| Glass S | High-strength, flexible glass produced via specialized manufacturing | Exceptional flexibility and tensile strength | Very expensive; limited dental applications | Rarely used in dentistry; mainly aerospace | [49,50,51] |
| FRC-FDP Type | Fiber Framework | Key Structural Features | Veneering & Aesthetic Considerations |
|---|---|---|---|
| Surface-Retained [14] | High volume of continuous fibers in the connector [15]. | Bonding wings placed incisally in wings to resist dislodging [17]. Requires large bonding area [17]. Fibers in bonding wings are protected with a polymerizable fluorinated resin (PFR) layer [17]. Connector thickness (palatal/lingual to buccal direction) is more critical than width for stiffness and durability [15]. Fibers should be positioned on the tension side of the prosthesis [15]. Minimal proximal preparations may be needed in anterior FPDs to optimize connector strength [15]. | Veneering composite is applied over the framework. The thin, translucent nature requires careful masking to prevent graying (dark oral background) [17]. |
| Inlay/Onlay-Retained [14] | Continuous unidirectional fibers run between cavities [16]. | Box preparation depth > 1.5 mm in sound tooth structure is required for vertical support [16]. An extra bonding wing is advised for canine abutments in canine-guided occlusion [16]. Capable of withstanding high forces (up to ~2600 N) [16]. Requires adhesive cementation with composite resin luting cements for a secondary IPN bond [16]. | Veneering layer on the occlusal surface should be at least 1.5 mm thick. Can be done with laboratory or direct restorative composites [16]. |
| Full-Coverage Retained [14] | Woven copings connected by continuous unidirectional fibers [17]. | The framework is built on full-coverage preparations, providing maximum retention and support. The design relies on the principles of a conventional fixed prosthesis [17]. | The entire framework is covered with veneering composite for aesthetics and polishability [17]. An opaque layer is often necessary to mask the dark oral background and prevent show-through in the connectors [17]. |
| Advantages | Disadvantages/Challenges |
|---|---|
| Superior Aesthetics: Metal-free framework provides excellent, natural aesthetics [13,16]. | Aesthetic Complications: Loss of surface shine and excessive translucency [13,16]. |
| Minimally Invasive: Requires little to no tooth preparation, preserving natural tooth structure [26]. | Fracture Risk: Potential for framework or veneer fracture if not designed correctly [13,16]. |
| Biocompatibility: Does not wear opposing tooth enamel [13,16]. | Technical Sensitivity: Success depends on correct framework design and the use of high-quality, pre-impregnated fibers to prevent issues [13,16]. |
| Strong Adhesion: Achieves high bond strength to tooth structure, exceeding that of traditional dental alloys [25]. | Reparability Required: While failures are often repairable, this requires careful analysis and skilled intraoral repair by the dentist [13,16]. |
| Cost-Effective: Offers a balance between functionality, aesthetics, and affordability. | Reparable: Most failures can be repaired directly in the patient’s mouth using composite resin technology [13,16]. |
| Technique | Description | Benefits | Drawbacks | Reference |
|---|---|---|---|---|
| Direct | No tooth preparation required; minimal enamel etching (micro-invasive). Composite is layered and completed in one workflow. | Minimal or no preparation. Single visit. Low cost. | Lower mechanical properties Rigid fibers and limited workspace. Challenging esthetics, finishing, and polishing. | [100,101] |
| Indirect | Teeth are prepared, impressions taken, and prosthesis fabricated in the laboratory. | Superior mechanical properties due to enhanced laboratory polymerization. Better esthetics and functional design. | Requires two visits. Preparation of cavities with parallel surfaces. Adhesion issues due to multiple polymerizations and potential dentin contamination. | [100,102,103] |
| Semi-direct | FRC-FPD fabricated chairside on a silicone cast and inserted in the same appointment. | Better esthetics and functional design than direct technique. Single visit. | Requires preparation of cavities with parallel surfaces. Longer chairside time. | [104] |
| Study | Reference | No. of FPDs | Follow-Up Duration | Survival Rate (%) | Success Rate (%) | Type of FRC FPD | Notes |
|---|---|---|---|---|---|---|---|
| Perrin et al. (2020) | [21] | 100 | Up to 9 years | 98.4% | 91.7% | Direct | everStickC&B, minimal invasive |
| Goguta et al. (2019) | [110] | 19 | 6 years | 94.7% | Not reported | Direct | everStickC&B; Inlay retainers |
| Goguta et al. (2019) | [110] | 4 | 6 years | 25% | Not reported | Direct | Hybrid retainers |
| Wolff et al. (2018) | [101] | 26 | 4 years | 73.5% | 46.2% | Direct & Semi-direct | 17/26 functioning; 12 success |
| Ahmed et al. (2017) | [20] | 592 | Mean 4.8 years | 94.5% | Not reported | Mixed | Systematic review |
| Van Heumen et al. (2009) | [19] | 435 | Mean 4.5 years | 73% | Not reported | Mixed | Early materials |
| Pooled Survival Rate | 86.2% | ||||||
| Pooled Success Rate | 82.3% (based on limited data) | ||||||
| Factor | Key Findings/Considerations | References |
|---|---|---|
| Study Location | Practice-based studies report higher failure rates compared to university-based randomized controlled trials, possibly due to stricter protocols and less routine in practice-based settings. | [118,119,120] |
| Dentist-Related Factors | Longevity of restorations may depend on operator skills and dentist profiles. | [111] |
| FRC-FPD survival may also be influenced when treatment is provided by a single dentist. | [21] | |
| Fabrication Method | Direct fabrication of FRC-FPDs is considered technique-sensitive, requiring higher clinical expertise. | [100] |
| Tooth Type | No significant survival difference was observed between anterior and posterior teeth; findings may be influenced by sample size and follow-up duration. | [20,21] |
| Cavity Design on Abutment | No significant difference found between no preparation (micro-invasive) and box preparation (minimally invasive). | [21,113] |
| Size & Method of Preparation | No significant difference between no preparation, shallow preparation, or deep preparation of abutment teeth. | [114,115] |
| No significant difference between cavities prepared with conventional inlay burs versus ultrasonic tips. | [115] | |
| Material Composition & Compatibility | Success depends on factors like adhesive protocols, chemical composition of FRC components, polymerization techniques/devices, and surface conditioning prior to adhesion. | [20,101,103,116,117,121] |
| Number of Pontics | Limited evidence is available; most studies focus on single-pontic restorations. | [20,21] |
| Study | Reference | No. of Prothesis | Follow-Up Duration | Prosthesis Survival Rate (%) | Prosthesis Success Rate (%) | Implant Survival Rate (%) | Type of FRC FPD | Notes |
|---|---|---|---|---|---|---|---|---|
| Cheng et al. (2022) | [23] | 121 (implants-261) | 10 years | 95.9% | 89.8% | Not reported | Partial FPDs | Short/extra-short implants; stable bone levels; longer spans linked to bone gain |
| Cheng et al. (2023) | [24] | Not explicitly stated (implants = 138) | 10 years | 97.8% | 90.8% | 96.5% | Complete FPDs | Extra-short implants viable; positive bone trends with some denture materials |
| Pooled Prosthesis Survival Rate | 86.2% Weighted across 121 (2022) and estimated 45 (2023) patients | |||||||
| Pooled Prosthesis Success Rate | 82.3% (Based on reported success from both studies) | |||||||
| Pooled Implant Survival Rate | 96.5% Only available from 2023 study | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fareed, M.A.; Masri, M.A.; Al-sammarraie, A.W.M.; Akil, B.M.E. Fiber-Reinforced Composites in Fixed Prosthodontics: A Comprehensive Overview of Their Historical Development, Types, Techniques, and Longevity. Prosthesis 2025, 7, 139. https://doi.org/10.3390/prosthesis7060139
Fareed MA, Masri MA, Al-sammarraie AWM, Akil BME. Fiber-Reinforced Composites in Fixed Prosthodontics: A Comprehensive Overview of Their Historical Development, Types, Techniques, and Longevity. Prosthesis. 2025; 7(6):139. https://doi.org/10.3390/prosthesis7060139
Chicago/Turabian StyleFareed, Muhammad Amber, Mazen Abdulmounem Masri, Almustafa Wisam Mustafa Al-sammarraie, and Buthena Mohamed Ehsan Akil. 2025. "Fiber-Reinforced Composites in Fixed Prosthodontics: A Comprehensive Overview of Their Historical Development, Types, Techniques, and Longevity" Prosthesis 7, no. 6: 139. https://doi.org/10.3390/prosthesis7060139
APA StyleFareed, M. A., Masri, M. A., Al-sammarraie, A. W. M., & Akil, B. M. E. (2025). Fiber-Reinforced Composites in Fixed Prosthodontics: A Comprehensive Overview of Their Historical Development, Types, Techniques, and Longevity. Prosthesis, 7(6), 139. https://doi.org/10.3390/prosthesis7060139







