Clinical Outcomes of Zirconia Abutments for Implant Dentistry: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Questions
- How are the clinical performances and outcomes of zirconia abutments?
- What are the advantages of the application of zirconia abutments?
2.2. Eligibility Criteria
- Types of studies: Randomised clinical trials, case–control studies, cross-sectional studies, and cohort studies.
- Types of participants: Studies concerning patients with zirconia abutments for implant rehabilitations; only clinical studies were included in this review.
- Types of interventions: The comparison of the clinical performances of zirconia abutments. An assessment of these performances has been made through the performance of case–control, cross-sectional, cohort and clinical RCT studies.
- Outcome types: The identification of the advantages of using zirconia abutments for clinical use.
- Articles published in non-English languages;
- Studies where non-zirconia abutments were tested;
- Animal or in vitro studies;
- An absence of ethics committee approval.
2.3. Search Strategy
2.4. Research
2.5. Data Collection
| Number of Patients | Number of Implants | Main Outcome | Follow-Up | Study Type | Study Reference | Setting |
|---|---|---|---|---|---|---|
| 123 | 291 | One abutment fracture. Abutment survival rate of 99.66%. | 4 yy | Retrospective cohort study | Parpaiola et al., 2020 [8] | Multicentric: five in Italy and one in England |
| 20 | 40 | Canine, premolar and molar were replaced, half with zirconia abutments and half with Ti ones. No complications during duration of observation (too short), but zirconia, like all ceramics, aged faster than metal. | 3 yy | Randomised controlled trial | Zembic et al., 2009 [14] | University of Zurich (Switzerland) |
| 141 | 158 | Comparison between standard and switching platform for zirconia abutments. No difference between two systems in terms of fracture patterns; difference was found between internal and external hexagon. Survival and success rate of 93,8% and 81,2% (12 yy fu) for standard and 90% and 84% (5 yy fu) for platform switching. | 12 yy | Retrospective study | Passos et al., 2016 [16] | University of British Columbia (Canada) |
| 24 | 42 | No abutment fractures (4 veneering chips). | 1 y | Retrospective study | Nothdurft et al., 2010 [23] | Saarland University (Germany) |
| 56 | 89 | Internal hexagon, comparison of three different groups: titanium, titanium nitride and zirconia abutments. Total of 5 fractures in zirconia group at connection level (4 in posterior region and 1 during clinical phase), none in titanium group. In all failed restorations, fractures in abutment connection were evident at stem level. | 3 yy | Randomised controlled trial | Ferrari et al., 2016 [24] | Department of Prostho- dontics of the University of Siena (Italy) |
| 32 | 49 | 32 patients at T0, 24 patients and 39 implants at T1. Two-piece implant, cemented abutment. Two fracture lines were observed in two different patients, and 6 abutments (in six patients) fractured at connection level (2 after 4 yy, 1 after 5 yy and 3 after 6 yy). Six crown–abutment complexes lost retention. Cumulative rate of survival of 83% over 6 yy. | 6 yy | Cohort prospective study | Cionca et al., 2021 [25] | University Hospitals of Geneva, Geneva, Switzerland |
3. Results
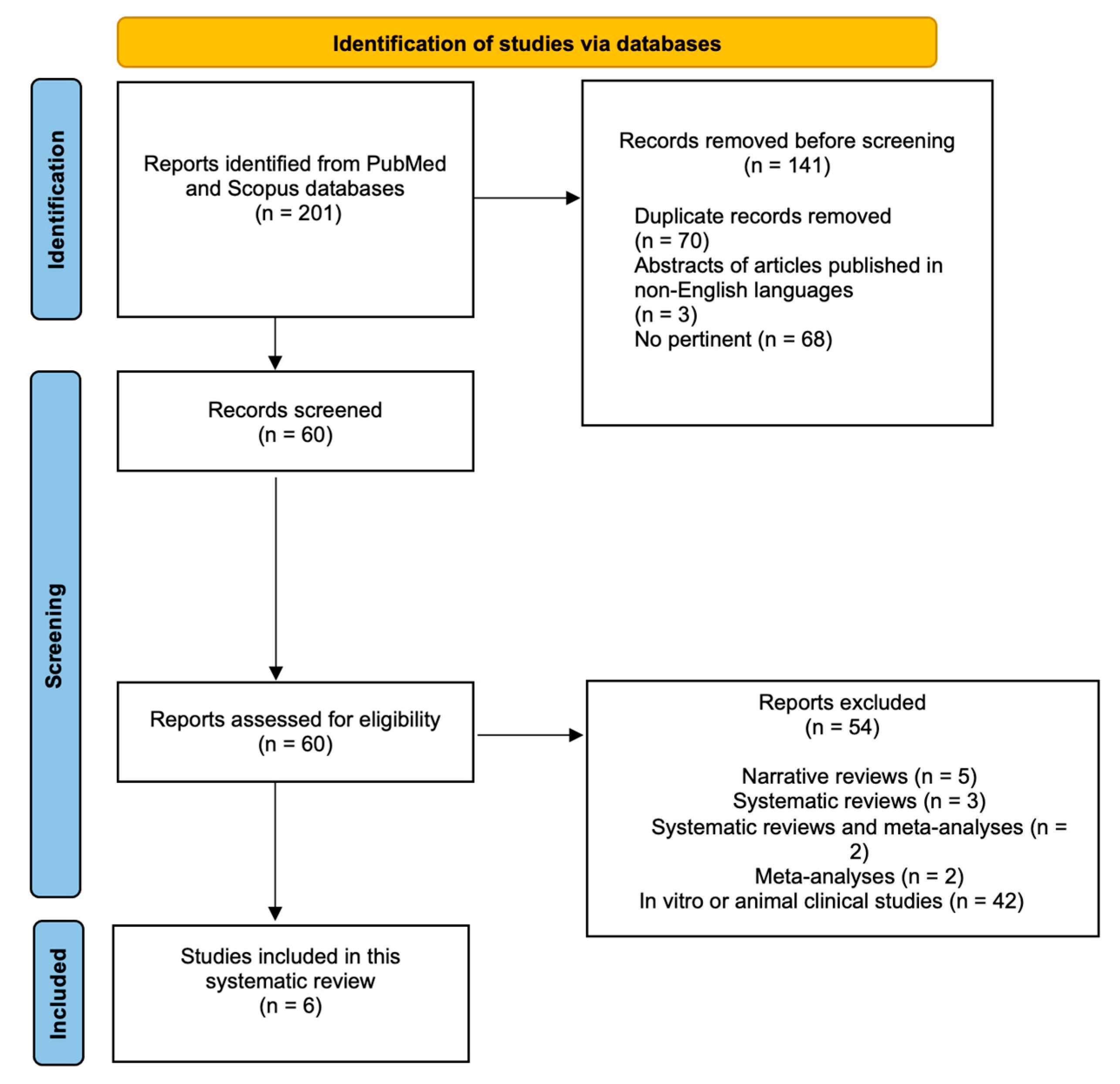
3.1. Search Outcome
3.2. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz-Martín, I.; Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of Modified Abutment Characteristics on Peri-implant Soft Tissue Health: A Systematic Review and Meta-analysis. Clin. Oral Implants Res. 2018, 29, 118–129. [Google Scholar] [CrossRef]
- Daneshvar, S.; Matthews, D.; Michuad, P.-L.; Ghiabi, E. Success and Survival Rates of Dental Implants Restored at an Undergraduate Dental Clinic: A 13-Year Retrospective Study with a Mean Follow-up of 5.8 Years. Int. J. Oral Maxillofac. Implants 2016, 31, 870–875. [Google Scholar] [CrossRef]
- Goto, T. Osseointegration and Dental Implants; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 24, ISBN 9780813813417. [Google Scholar]
- Lupi, S.M.; Todaro, C.; De Martis, D.; Blasi, P.; Rodriguez y Baena, R.; Storelli, S. The Conometric Connection for the Implant-Supported Fixed Prosthesis: A Narrative Review. Prosthesis 2022, 4, 458–467. [Google Scholar] [CrossRef]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term Implant Survival and Success: A 10–16-year Follow-up of Non-submerged Dental Implants. Clin. Oral Implants Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- de Almeida Basílio, M.; Cardoso, K.V.; Antonio, S.G.; Rizkalla, A.S.; Santos Junior, G.C.; Arioli Filho, J.N. Effects of Artificial Aging Conditions on Yttria-Stabilized Zirconia Implant Abutments. J. Prosthet. Dent. 2016, 116, 277–285. [Google Scholar] [CrossRef]
- Tischler, M. The Future of Implant Dentistry. Dent. Today 2016, 35, 84–85. [Google Scholar]
- Parpaiola, A.; Toia, M.; Norton, M.; Cecchinato, D.; Bressan, E.; Lops, D. CAD/CAM Implant Abutments: Peri-Implant Hard and Soft Tissue Response with Up to 4 Years of Follow-up—A Retrospective Cohort Study Evaluation. Int. J. Periodontics Restor. Dent. 2020, 40, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kerstein, R.B.; Radke, J. A Comparison of Fabrication Precision and Mechanical Reliability of 2 Zirconia Implant Abutments. Int. J. Oral Maxillofac. Implants 2008, 23, 1029–1036. [Google Scholar]
- Vozzo, L.M.; Azevedo, L.; Fernandes, J.C.H.; Fonseca, P.; Araújo, F.; Teixeira, W.; Fernandes, G.V.O.; Correia, A. The Success and Complications of Complete-Arch Implant-Supported Fixed Monolithic Zirconia Restorations: A Systematic Review. Prosthesis 2023, 5, 425–436. [Google Scholar] [CrossRef]
- Sordi, M.B.; Sarwer-Foner, S.N.D.; Schünemann, F.H.; Apaza-Bedoya, K.; Juanito, G.M.P.; Henriques, B.; Henriques, B.; Magini, R.S.; Benfatti, C.A.M. Biological Behavior of Titanium, Zirconia or PEEK Dental Implant-Abutments. In Biodental Engineering V; CRC Press: London, UK; Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 31–42. [Google Scholar]
- Foong, J.K.W.; Judge, R.B.; Palamara, J.E.; Swain, M.V. Fracture Resistance of Titanium and Zirconia Abutments: An in Vitro Study. J. Prosthet. Dent. 2013, 109, 304–312. [Google Scholar] [CrossRef]
- Halim, F.C.; Pesce, P.; De Angelis, N.; Benedicenti, S.; Menini, M. Comparison of the Clinical Outcomes of Titanium and Zirconia Implant Abutments: A Systematic Review of Systematic Reviews. J. Clin. Med. 2022, 11, 5052. [Google Scholar] [CrossRef]
- Zembic, A.; Sailer, I.; Jung, R.E.; Hämmerle, C.H.F. Randomized-Controlled Clinical Trial of Customized Zirconia and Titanium Implant Abutments for Single-Tooth Implants in Canine and Posterior Regions: 3-Year Results. Clin. Oral Implants Res. 2009, 20, 802–808. [Google Scholar] [CrossRef]
- Vazouras, K.; Margvelashvili-malament, M.; Kim, Y.J.; Weber, H.; Finkelman, M. An Esthetic Evaluation of Different Abutment Materials in the Anterior Maxilla: A Randomized Controlled Clinical Trial. J. Prosthodont. 2022, 31, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Passos, S.P.; Linke, B.; Larjava, H.; French, D. Performance of Zirconia Abutments for Implant-Supported Single-Tooth Crowns in Esthetic Areas: A Retrospective Study up to 12-Year Follow-Up. Clin. Oral Implants Res. 2016, 27, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Alkahtany, M.; Beatty, M.W.; Alsalleeh, F.; Petro, T.M.; Simetich, B.; Zhou, Y.; Feely, D.; Polyzois, G. Color Stability, Physical Properties and Antifungal Effects of ZrO2 Additions to Experimental Maxillofacial Silicones: Comparisons with TiO2. Prosthesis 2023, 5, 916–938. [Google Scholar] [CrossRef]
- Mavriqi, L.; Traini, T. Mechanical Properties of Translucent Zirconia: An In Vitro Study. Prosthesis 2023, 5, 48–59. [Google Scholar] [CrossRef]
- Sorrentino, R.; Ruggiero, G.; Toska, E.; Leone, R.; Zarone, F. Clinical Evaluation of Cement-Retained Implant-Supported CAD/CAM Monolithic Zirconia Single Crowns in Posterior Areas: Results of a 6-Year Prospective Clinical Study. Prosthesis 2022, 4, 383–393. [Google Scholar] [CrossRef]
- Barile, G.; Capodiferro, S.; De Rosa, G.; Muci, G.; Vanzanelli, A.; Corsalini, M. Screwed Monolithic Zirconia Crowns for Mono-Implant Posterior Rehabilitation: A Prospective Clinical Study on 41 Patients with a 7-Year Follow-Up. Prosthesis 2023, 5, 1037–1048. [Google Scholar] [CrossRef]
- Di Alberti, L.; Di Alberti, C.; Donini, F.; Lo Muzio, L.; Cadrobbi, F.; D’Agostino, A.; De Santis, D.; Bertossi, D. Clinical and Mechanical Evaluation of Screw-Retained Implant-Supported Zirconia Restorations. A 36 Months Prospective Clinical Study. Minerva Stomatol. 2018, 62, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Schnider, N.; Forrer, F.; Brägger, U.; Hicklin, S. Clinical Performance of One-Piece, Screw-Retained Implant Crowns Based on Hand-Veneered CAD/CAM Zirconia Abutments After a Mean Follow-up Period of 2.3 Years. Int. J. Oral Maxillofac. Implants 2018, 33, 188–196. [Google Scholar] [CrossRef]
- Nothdurft, F.; Pospiech, P. Prefabricated zirconium dioxide implant abutments for single-tooth replacement in the posterior region: Evaluation of peri-implant tissues and superstructures after 12 months of function. Clin. Oral Implant. Res. 2010, 21, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Tricarico, M.G.; Cagidiaco, M.C.; Vichi, A.; Gherlone, E.F.; Zarone, F.; Sorrentino, R. 3-Year Randomized Controlled Prospective Clinical Trial on Different CAD-CAM Implant Abutments. Clin. Implant Dent. Relat. Res. 2016, 18, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Two-piece Zirconia Implants Supporting All-Ceramic Crowns: Six-year Results of a Prospective Cohort Study. Clin. Oral Implants Res. 2021, 32, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.; Kloukos, D.; Petridis, H.; Pandis, N. An Assessment of the Risk of Bias in Randomized Controlled Trial Reports Published in Prosthodontic and Implant Dentistry Journals. Int. J. Prosthodont. 2015, 28, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and Validation of a Risk-of-Bias Tool for Assessing in Vitro Studies Conducted in Dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus Titanium in Dentistry: A Review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef]
- Sadowsky, S.J. Has Zirconia Made a Material Difference in Implant Prosthodontics? A Review. Dent. Mater. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Jung, R.E.; Holderegger, C.; Sailer, I.; Khraisat, A.; Suter, A.; Hämmerle, C.H.F. The Effect of All-Ceramic and Porcelain-Fused-to-Metal Restorations on Marginal Peri-Implant Soft Tissue Color: A Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2008, 28, 357–365. [Google Scholar]
- Jung, R.E.; Sailer, I.; Hämmerle, C.H.F.; Attin, T.; Schmidlin, P. In Vitro Color Changes of Soft Tissues Caused by Restorative Materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251. [Google Scholar]
- Denry, I.; Kelly, J.R. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Yüzügüllü, B.; Avci, M. The Implant-Abutment Interface of Alumina and Zirconia Abutments. Clin. Implant Dent. Relat. Res. 2008, 10, 113–121. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanno, T.; Milleding, P.; Ortengren, U. Zirconia as a Dental Implant Abutment Material: A Systematic Review. Int. J. Prosthodont. 2010, 23, 299–309. [Google Scholar]
- Zhang, Y.; Lawn, B.R. Evaluating Dental Zirconia. Dent. Mater. 2019, 35, 15–23. [Google Scholar] [CrossRef]
- Remísio, M.; Borges, T.; Castro, F.; Gehrke, S.; Fernandes, J.; Fernandes, G. Histologic Osseointegration Level Comparing Titanium and Zirconia Dental Implants: Meta-Analysis of Preclinical Studies. Int. J. Oral Maxillofac. Implants 2023, 38, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Peri-Implant Diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Tan, K.B.; Nicholls, J.I. Load Fatigue Performance of Implant-Ceramic Abutment Combinations. Int. J. Oral Maxillofac. Implants 2009, 24, 636–646. [Google Scholar]
- Freifrau von Maltzahn, N.; Bernard, S.; Kohorst, P. Two-part Implant Abutments with Titanium and Ceramic Components: Surface Modification Affects Retention Forces—An In-vitro Study. Clin. Oral Implants Res. 2019, 30, 903–909. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, C.; Bocchieri, S.; Sambataro, S.; Surace, G.; Stumpo, C.; Fiorillo, L. Occlusal Load Considerations in Implant-Supported Fixed Restorations. Prosthesis 2020, 2, 252–265. [Google Scholar] [CrossRef]
- Ban, S.; Yasuoka, Y.; Sugiyama, T.; Matsuura, Y. Translucent and Highly Toughened Zirconia Suitable for Dental Restorations. Prosthesis 2023, 5, 60–72. [Google Scholar] [CrossRef]
- Nam, R.-K.; Lee, S.; Park, E.-J.; Kwon, H.-B.; Yoon, H.-I. Three-Dimensional Deformation and Wear of Internal Implant-Abutment Connection: A Comparative Biomechanical Study Using Titanium and Zirconia. Int. J. Oral Maxillofac. Implants 2018, 33, 1279–1286. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y.; Park, J.-M.; Chung, S. Comparison of Fit and Stability Between 3D-Printed and Milled Implant Abutments with Titanium-6Al-4V and Co-Cr Metal Alloys. Int. J. Oral Maxillofac. Implants 2023, 38, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. CAD/CAM Abutments versus Stock Abutments: An Update Review. Prosthesis 2022, 4, 468–479. [Google Scholar] [CrossRef]
- Çin, V.; İzgi, A.D.; Kale, E.; Yilmaz, B. Marginal and Internal Fit of Monolithic Zirconia Crowns Fabricated by Using Two Different CAD-CAM Workflows: An In Vitro Study. Prosthesis 2023, 5, 35–47. [Google Scholar] [CrossRef]


| Pubmed |
| “implant” AND “abutment” AND “zirconia” |
| “zirconia abutment” AND “mechanical properties” |
| Total of 201 articles found |
| Scopus |
| “implant” AND “abutment” AND “zirconia” |
| “zirconia abutment” AND “mechanical properties” |
| Total of 183 articles found |
| Adequate Sequence Generated | Allocation Concealment | Blinding | Complete Outcome Data | Registration Outcome Data | |
|---|---|---|---|---|---|
| Parpaiola et al., 2020 [8] |  |  |  |  |  |
| Zembic et al., 2009 [14] |  |  |  |  |  |
| Passos et al., 2016 [16] |  |  |  |  |  |
| Nothdurft et al., 2010 [23] |  |  |  |  |  |
| Ferrari et al., 2016 [24] |  |  |  |  |  |
| Cionca et al., 2021 [25] |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scribante, A.; De Martis, D.; Vezzoni, F.; Mirando, M.; Sfondrini, D.; Zampetti, P. Clinical Outcomes of Zirconia Abutments for Implant Dentistry: Systematic Review. Prosthesis 2025, 7, 113. https://doi.org/10.3390/prosthesis7050113
Scribante A, De Martis D, Vezzoni F, Mirando M, Sfondrini D, Zampetti P. Clinical Outcomes of Zirconia Abutments for Implant Dentistry: Systematic Review. Prosthesis. 2025; 7(5):113. https://doi.org/10.3390/prosthesis7050113
Chicago/Turabian StyleScribante, Andrea, Dario De Martis, Filippo Vezzoni, Maria Mirando, Domenico Sfondrini, and Paolo Zampetti. 2025. "Clinical Outcomes of Zirconia Abutments for Implant Dentistry: Systematic Review" Prosthesis 7, no. 5: 113. https://doi.org/10.3390/prosthesis7050113
APA StyleScribante, A., De Martis, D., Vezzoni, F., Mirando, M., Sfondrini, D., & Zampetti, P. (2025). Clinical Outcomes of Zirconia Abutments for Implant Dentistry: Systematic Review. Prosthesis, 7(5), 113. https://doi.org/10.3390/prosthesis7050113








