1. Introduction
Arms, hands, and fingers are vital for numerous activities of daily living, and thus the loss of one or both upper limbs is a devastating disability and significantly impacts an individual’s quality of life. Globally, an estimated 180 million people live with limb loss due to various causes (e.g., vascular conditions, trauma, and congenital factors) [
1]. Of these, approximately 58 million people are affected by traumatic limb amputation, with around 39% experiencing unilateral or bilateral upper limb loss [
2]. In a recent publication, Rivera et al. [
3] estimated that approximately 2.3 million people in the U.S. are living with limb loss, with around 200,000 affected by upper limb amputation. Furthermore, this number is projected to double by 2050.
Commercially available prostheses can help to restore functionality in individuals with limb deficiencies and enable them to perform activities of daily living that they otherwise would not be able to accomplish. Advancements in prosthetic technologies, especially myoelectric control, have enabled modern prostheses to make significant improvements in the pursuit of mimicking the versatility of natural limbs. Electromyography (EMG) sensing from the residual limb stump using cutaneous surface electrodes is the most ubiquitous approach to controlling robotic prostheses. In this approach, dating back to the 1970s, cutaneous electrodes are placed within the inner surface of a prosthetic socket and sense EMG signals from the surface of the skin associated with muscle contractions in the residual limb [
4,
5]. In the most simplistic embodiment, which is still the most commonly used, two oppositely placed bipolar electrodes are used for prosthesis control; one electrode pair overlies the flexor muscles, and the second electrode pair overlies the extensor muscles. However, this first-generation embodiment only captures a small subset of EMG signals that are involved in hand and wrist movements and, hence, can only be used for very limited control of a prosthetic device (even though the prosthetic device itself is capable of far more complex movements). To execute a greater set of movements with this electrode-pair embodiment, the user must initiate specific muscle contractions (e.g., two rapid muscle contractions) to change the state of the prosthesis (e.g., change the mode, change the selected gesture, etc.). While this approach does technically allow the user to perform a greater number of gestures, it is unnatural, time consuming, and cognitively burdensome. This contributes heavily to a large abandonment rate of myoelectric prostheses, ranging from ~30–40%, observed to this day [
6,
7].
Thus, more advanced prosthesis control systems using a greater number of surface electrodes combined with machine learning algorithms, commonly referred to as myoelectric pattern recognition (MPR), have been proposed and shown to achieve high classification accuracy in the literature [
8,
9,
10,
11,
12,
13,
14]. However, with a few exceptions, the gesture set tested in these studies has been relatively small and often included limited involvement of individual fingers (e.g., one recent study only tested six gestures, and none involved individual fingers [
8]). While there is consistent evidence that classification performance improves with increasing electrode count [
9,
10], most of these systems use experimental electrode arrays suitable for acute lab settings. They remain at an early academic research stage, and only a few have progressed to commercial viability [
15,
16,
17]. These commercially available sensor arrays and accompanying MPR algorithms for decoding muscle signals can, in principle, enable more versatile and complex prosthesis movements. This is expected to translate into greater prosthesis control, and, indeed, various controlled studies have demonstrated that MPR provides superior control compared to conventional dual-electrode direct control in real-world settings [
18,
19].
However, both MPR and conventional prosthesis control systems suffer from the same challenges arising from sEMG. For instance, the fact that cutaneous electrodes sit farther from the source of the EMG signal (i.e., underlying muscle) reduces the signal-to-noise ratio (SNR), sometimes to unusable levels when abundant soft tissues or scar tissues are present. The sEMG interface is also highly susceptible to electromagnetic interference, reducing the SNR even further. In addition, the non-polarizable, high-impedance electrode–skin interface also makes it susceptible to motion artifacts that often result in unwanted activation of the prosthesis. Low electrode density, typically eight or fewer electrode pairs, and placement challenges make it difficult to isolate discrete EMG signals from specific muscle groups. Instead, crosstalk results in recorded sEMG being a summation of signals from multiple muscles. Moreover, relative movement of surface electrodes on the skin, especially with socket rotation, can reduce the reliability of MPR controllers. Finally, skin perspiration alters the electrical impedance of the source–sense interface, adding variability to the recordings [
19]. These challenges make current sEMG-controlled prostheses bulky due to the integration of sensors and wiring into the socket, unreliable due to inconsistent signal detection, and inconvenient, as they often create an unnatural user experience and require retraining. Therefore, current myoelectric prosthesis performance fails to meet users’ needs, leading to a high abandonment rate. Salminger et al. [
7] observed overall abandonment rates of upper limb prostheses of ~44% in a population of mainly myoelectric prosthesis users (~92%). This, combined with high rates of compensation injuries, depression, and loss of autonomy observed in populations with limb loss, demonstrates a clear need for better solutions that can provide more intuitive, accurate, and reliable prosthesis control to broadly improve patient outcomes and reduce prosthesis abandonment [
20,
21,
22].
The Phantom X prosthesis control system (Phantom Neuro Inc., Austin, TX, USA) aims to address several of the aforementioned limitations of the current sEMG-based prosthesis control systems, with a goal of enabling a clinically scalable solution. At the heart of the Phantom X system (
Figure 1) is a multi-electrode implantable sensor array that is circumferentially placed around a user’s forearm and senses EMG signals at a high spatial resolution. Considerable evidence exists that implantable electrodes result in more accurate prosthesis control. For example, the concept of invasive recording electrodes that directly record from the surface of the muscle (epimysial) or from within the muscle itself (intramuscular) is well known and has yielded the most advanced motor control of upper limb prostheses in humans to date, albeit in an experimental, non-scalable setting [
23,
24,
25]. However, the invasive nature of the Phantom X system warrants that the development of associated algorithms be conducted in phases to minimize risk to patients. In the first phase, largely constituted by this Assessment of Gesture Accuracy for a Multi-electrode EMG-Sensor-Array-Based Prosthesis Control System (ASCENT) study, we evaluated the performance of the Phantom X system using a non-invasive EMG sensor array. In a subsequent phase, longitudinal clinical studies will be undertaken with the implantable version containing the aforementioned electrode array(s). The objective of this study was to evaluate the performance of the Phantom X system, a novel platform with strong potential for rapid clinical translation. The Phantom X system employs a computationally efficient machine-learning-based control algorithm driven by input from a high-density array of 32 unipolar electrodes, designed to replicate the form factor of its implantable array, across a broader set of gestures intended to meaningfully enhance daily task performance for users. These results establish a critical foundation for future human studies with the implanted system and represent a meaningful step toward scalable, high-performance myoelectric control.
2. Materials and Methods
The ASCENT study was a single-arm, single-center, non-randomized, non-blinded study requiring one in-office visit by the participating subjects. This study was conducted in accordance with the principles of Good Clinical Practice (GCP) and the Declaration of Helsinki. Institutional Review Board (IRB) approval was obtained prior to study initiation, and all the participants provided written informed consent before any study-related procedures were performed. This study was registered at clinicaltrials.gov before first subject enrollment (NCTNCT06446037).
We enrolled able-bodied and amputee participants 21 years of age or older who signed the informed consent and were able to follow the study directions. The amputee subjects were required to have amputation at the transradial level either in one or both arms. Individuals meeting one or more of the following criteria were excluded from this study: (1) previously diagnosed muscle pathology, (2) cognitive impairment limiting their ability to follow study directions, (3) impaired muscle function and/or ability to perform normal phantom hand movements (applicable to amputees only), (4) allergies to skin adhesive materials necessary for cutaneous electrode placement, (5) excessive hair growth on arms and inability to shave off the hair for electrode placement, (6) arms or residual limbs with insufficient diameter to accommodate the wearing of two cutaneous sensor arrays, (7) injuries, bruises, or open wounds on the arm that need to be instrumented for the study, and (8) pregnant women.
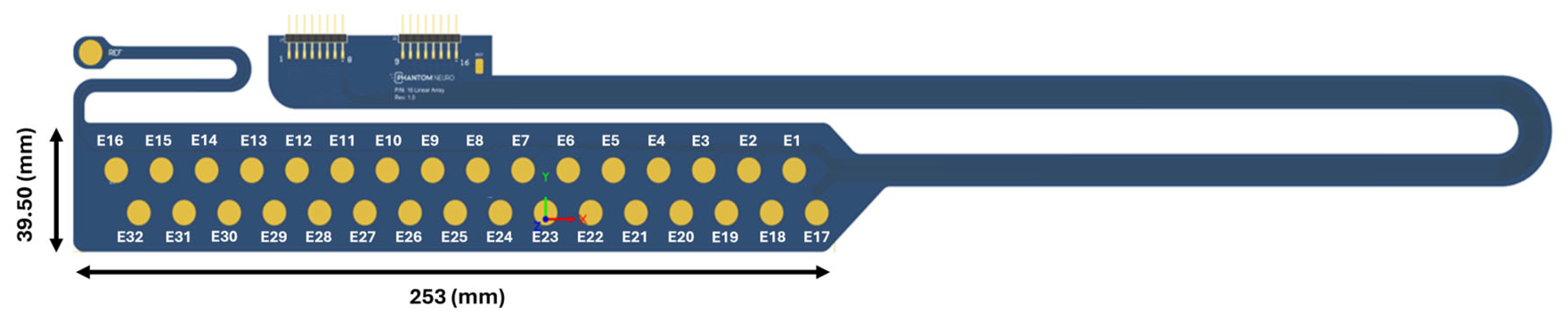
After obtaining informed consent, the subjects came in for a single in-office visit that lasted for up to 3 h. The subjects were instrumented with cutaneous electrode arrays with 32 electrodes (
Figure 2) circumferentially wrapped around the subject’s forearm. Each electrode of the array was gold-plated and 7.5 mm in diameter. The adjacent electrodes were spaced 7 mm apart and staggered by 12 mm. The array was adhered to the skin with a 1.1 mm thick foam pad (3M PE Foam, 3M, Minneapolis, MN, USA) and terminated into an EMG hardware board. The openings over the gold-plated contacts were cut into the foam, forming wells for electrode paste (Weaver and Company, Aurora, CO, USA). In addition, reference and ground electrodes (H124SG Kendall—Cardinal Health, Dublin, OH, USA) were attached near the array and then to the reference and ground terminal of the EMG board.
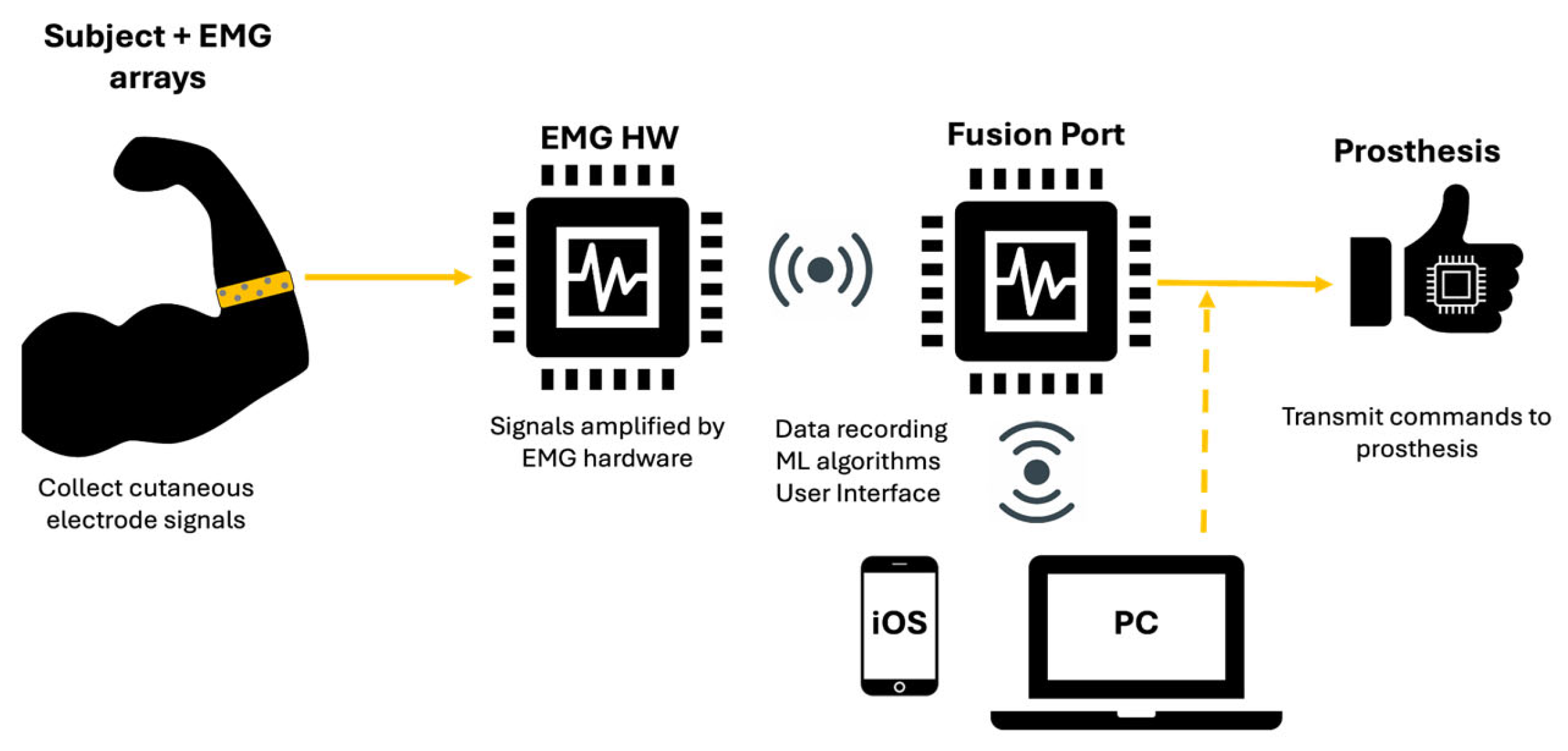
The EMG signals sensed by the electrodes were input into custom electronics hardware that amplified and filtered the signals. The amplifiers had a gain of 20×. The hardware used a bandpass filter of 5 Hz–4 kHz. The pre-processed EMG signals were wirelessly transferred to an edge computing component of the Phantom X system known as the Fusion Port. The Fusion Port was connected to a Windows desktop computer via a universal serial bus (USB) running machine learning (ML) algorithm that decoded the EMG signals into prosthesis control signals (
Figure 3). The computer running the ML algorithm was also used for data storage and as a user interface to provide gesture instructions. The control signals delivered by the Fusion Port controlled a desk-mounted prosthesis (Psyonic, San Diego, CA, USA) via a wired connection.
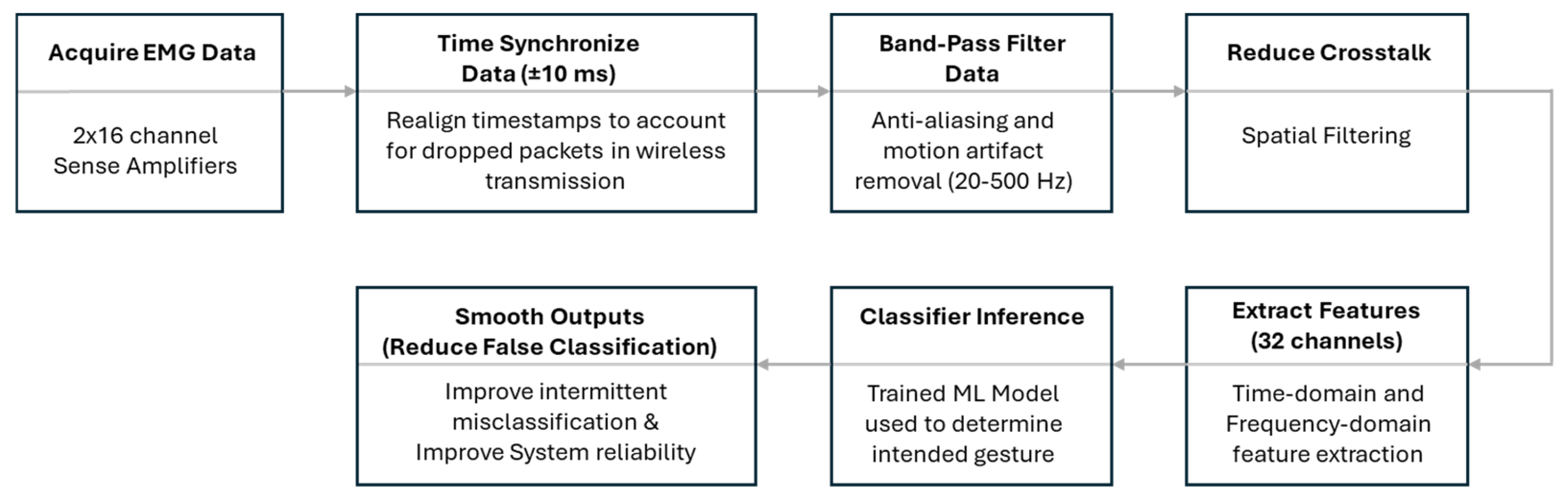
A block diagram of the ML algorithm is shown in
Figure 4. In brief, once the EMG signals were sensed, they were amplified and transmitted to the Fusion Port. The data were then passed from the Fusion Port to the PC, where time synchronization was performed to correct for any lost data packets during transmission. The signals were then band-pass filtered between 20 Hz and 500 Hz to remove motion artifacts, followed by spatial filtering to reduce crosstalk between channels. A proprietary algorithm was subsequently used to extract both time-domain and frequency-domain features from the EMG data. During the training phase, these features were mapped to specific gestures, enabling the development of a trained model that could recognize intended gestures based on incoming EMG features. In the final stage, post-processing was applied to reduce transient misclassifications and enhance overall system reliability.
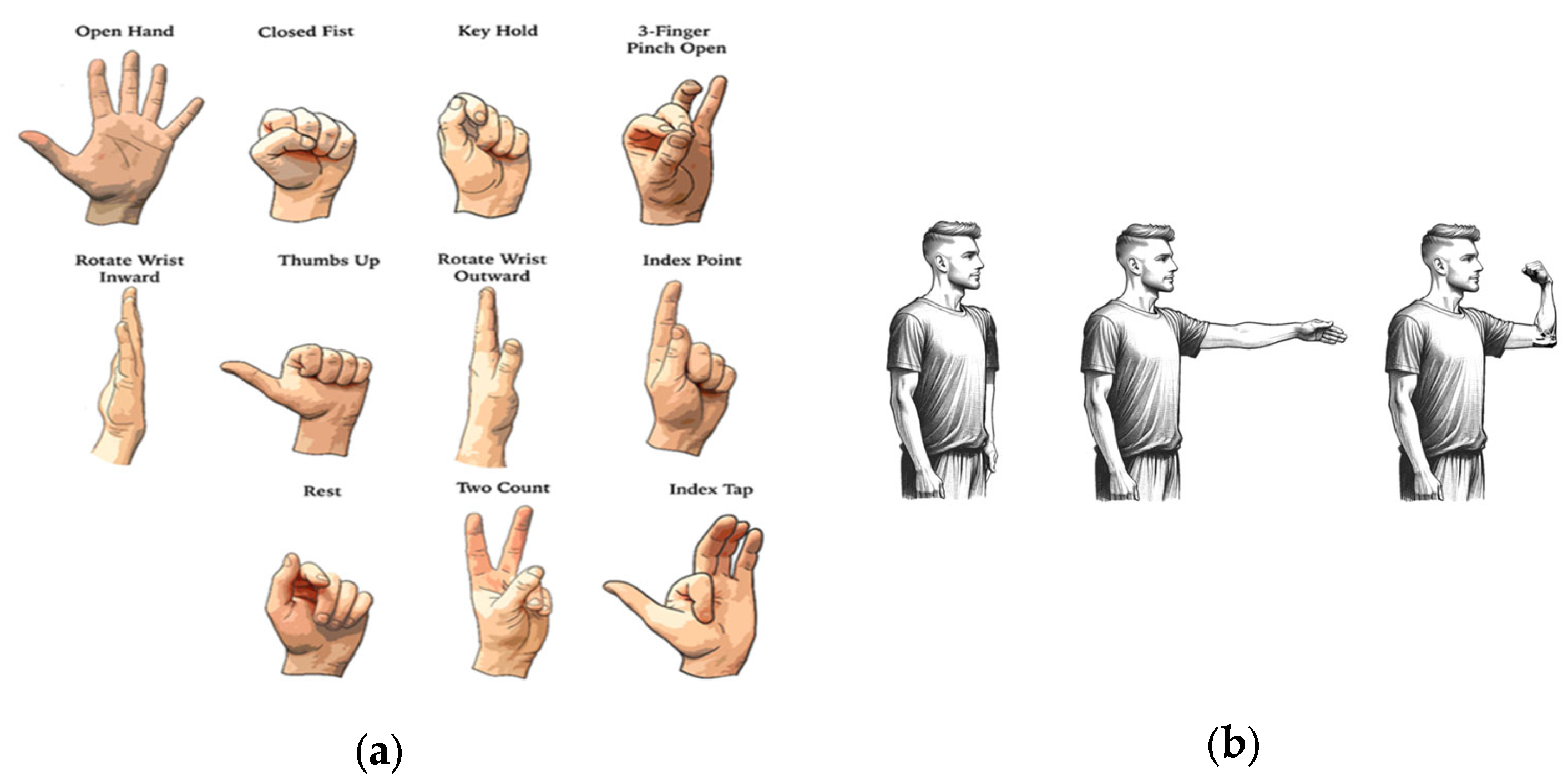
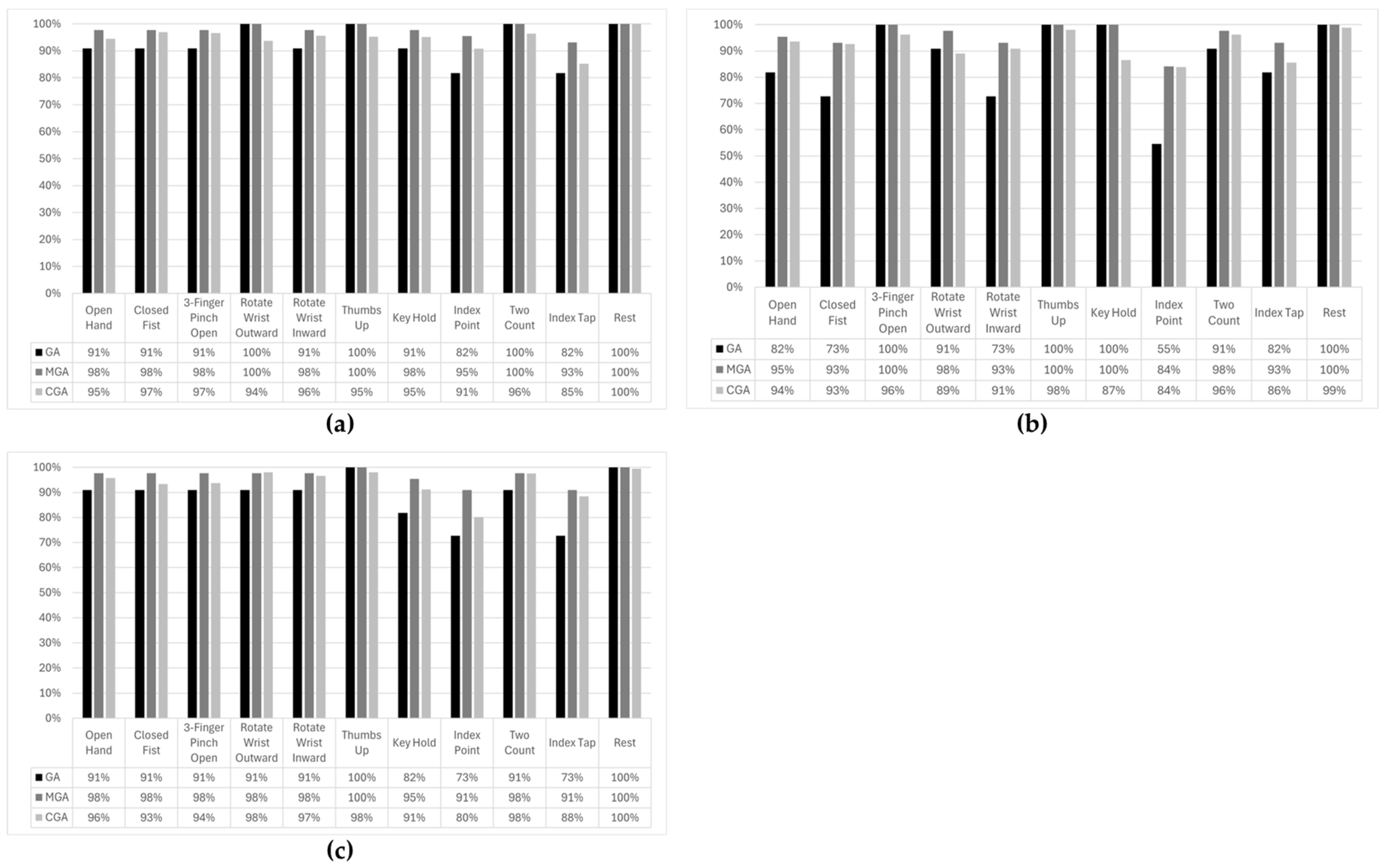
After the subjects were fully instrumented, they started a training session that lasted ~30 min. During this session, the subjects were first instructed to perform eleven distinct gestures in three distinct postures (
Figure 5) with an objective to train the machine learning (ML) algorithm. The three postures included (i) arm straight down, parallel to the body, referred to as ‘Arm Parallel’; (ii) arm perpendicular to the body, referred to as ‘Arm Perpendicular’; and (iii) arm bent at the elbow and hand pointing to the sky, referred to as ‘Arm Right-Angled’. The instructor-led training session used an interface to prompt the user to perform the gestures at the correct time. The gesture was initiated in the Arm Parallel posture and maintained as the subject transitioned into other two postures. During the training session, the desktop prosthesis was disconnected from the desktop computer.
Once the algorithm had been trained on all eleven gestures and in three postures, the desk-mounted prosthesis was connected to the control system, and the subjects were required to perform the various gestures in all three postures. The motion and gestures performed by the desk-mounted prosthesis were monitored. The reproduction of the gesture by the desk-mounted prosthesis was graded by assigning letter grades A, B, or C. The letters signified the varying fidelity of gesture execution as follows:
A: Gesture reproduced accurately;
B: Gesture reproduced with minor difficulty (e.g., digit mislocated initially but finally located in the right location);
C: Gesture reproduced with major difficulty (e.g., multiple attempts before correct gesture is attained).
In situations where clear grade assignment was ambiguous, agreement of two investigators was used to arrive at the final grade. The gesture accuracy was calculated in two different ways by assigning different weights to the gesture execution grades:
A = 1.0,
B = 0.75, and
C = 0.5. The following formulae were used for the accuracy computation:
The gesture accuracy was also computed by observing the output of the ML classifying algorithm and is referred to as Classifier Gesture Accuracy (CGA). The ML classifier produced decisions on 128 sample data windows from 32 channels with data collected at a sampling rate of 1250 Hz per channel. Proprietary features were computed on each window of data. The classifier produced 25 decisions per second. The participant performed each gesture in each posture for 5 s. This produced approximately 125 decisions per gesture. To calculate the classifier gesture accuracy (CGA) for a given posture, the number of correct classifier inferences was divided by the total number of decisions produced for a specific gesture in each posture:
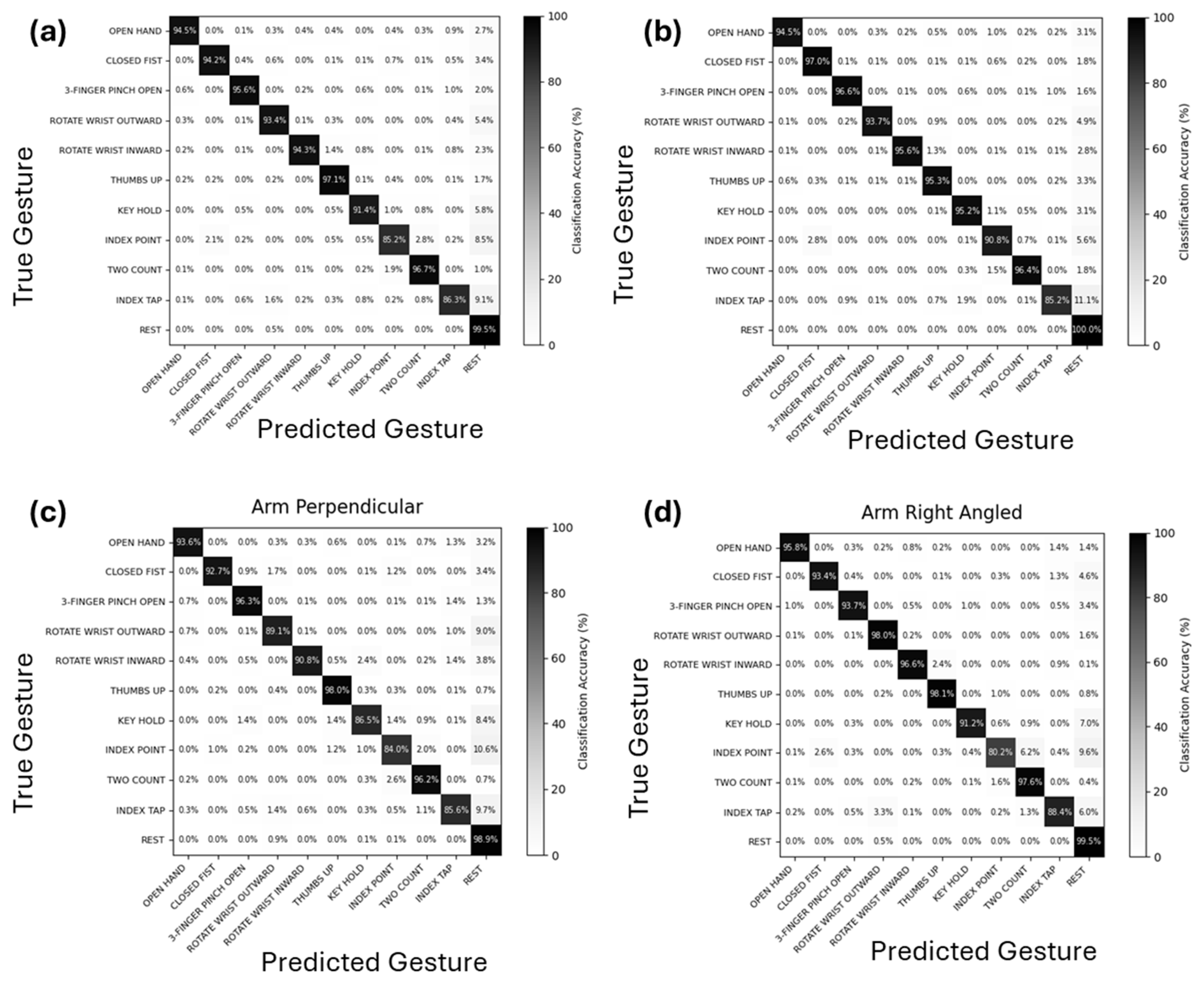
The performance of the algorithm was also assessed by constructing confusion matrices for all the postures combined and for each individual posture. These matrices were constructed using classifier gesture accuracies (CGAs) for each gesture. The gesture accuracy and misclassifications for each gesture were averaged across all the participants to produce the confusion matrices.
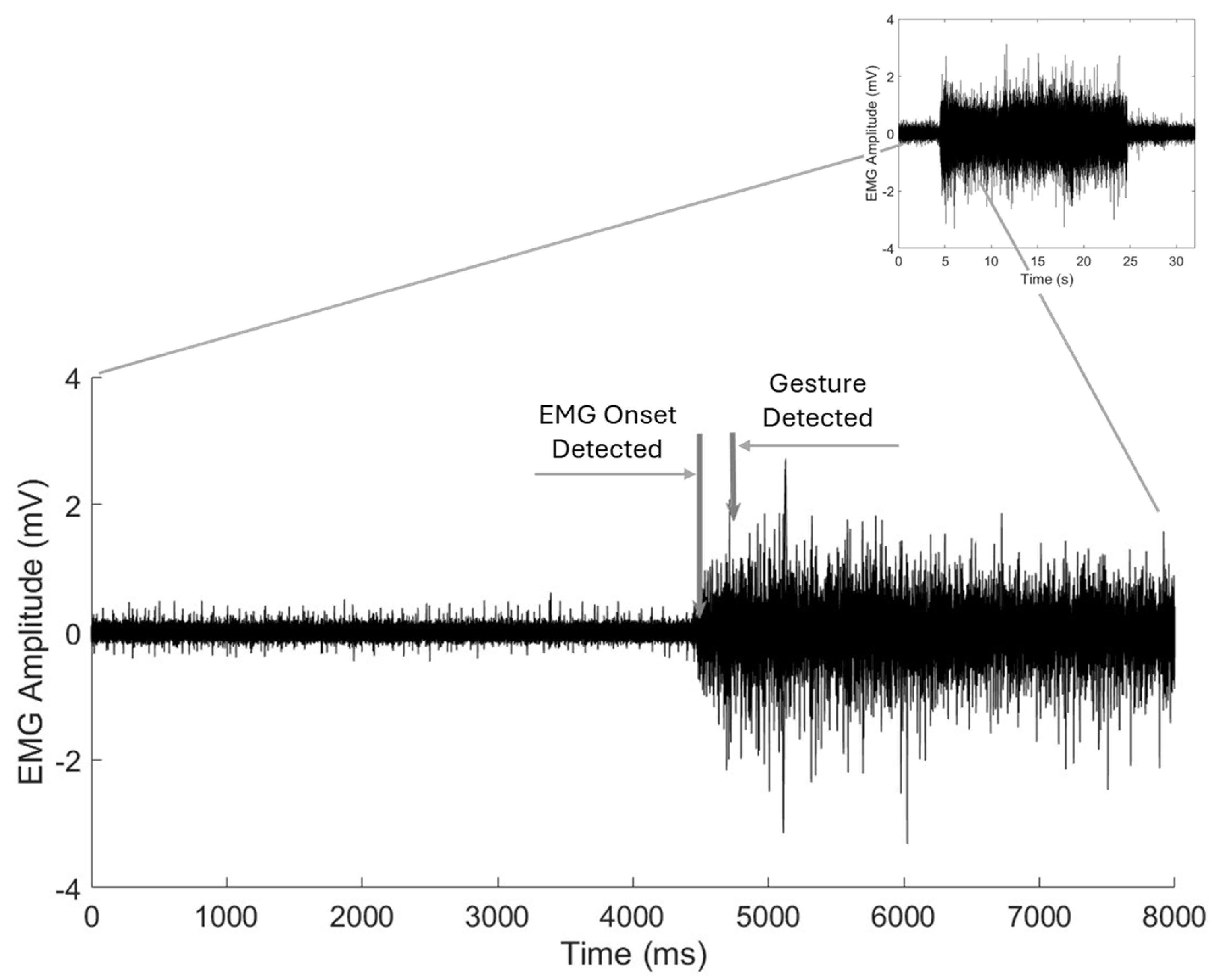
We also calculated the latency in gesture detection by the Phantom X algorithm. For this calculation, EMG signals from all the channels were superimposed, and latency was measured from the onset of EMG activity to the point of gesture detection by the algorithm. This latency calculation does not include the delay introduced by the prosthetic hand in physically executing the gesture.
At the end of the study session, the amputee subjects were required to complete a user satisfaction survey rating their satisfaction with the performance of Phantom X system relative to their current prosthesis, if they had one, on a scale from 1 to 5 (5 being most satisfied). They were also asked whether they would use the prosthesis for more, less, or the same amount of time if the prosthesis was equipped with the Phantom X control system. They also had an option to leave any additional descriptive comments.
Wherever applicable means were compared using one-way ANOVA. A significance level of α = 0.05 was set, and analyses were conducted in Microsoft Excel.
4. Discussion
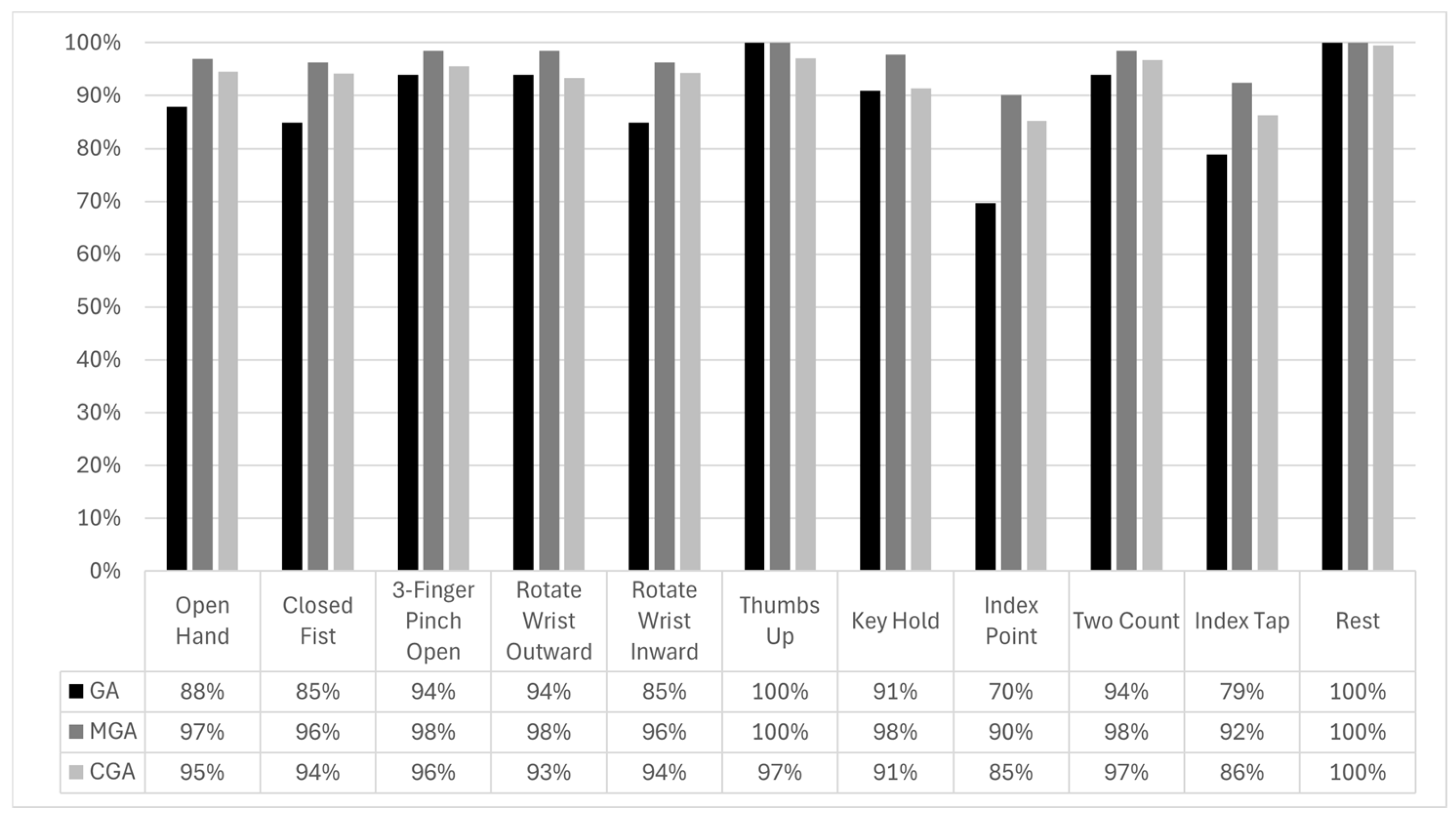
The results of our ASCENT study demonstrate the potential effectiveness of the Phantom X system in improving myoelectric prosthesis control through its multi-electrode EMG sensor array and ML algorithm. The system exhibited high overall accuracy in executing a broad range of gestures, with an average GA of 89.0%, MGA of 96.8%, and CGA of 93.6%. These findings indicate that the Phantom X control system can provide highly accurate gesture recognition, even in a non-invasive testing environment. The strong correlation between GA, MGA, and CGA suggests that the system’s machine learning algorithm reliably translates EMG signals into control commands for the prosthesis.
The Gesture Accuracy (GA) only included perfectly executed gestures. When partially executed gestures were included, the Modified Gesture Accuracy (MGA) increased from a GA of 89.0% to an MGA of 96.8%. These results, achieved with minimal training, likely represent a conservative estimate of the system’s potential performance. It is noteworthy to mention that the signal-to-noise ratio is expected to be significantly higher with the implantable electrodes because the amplitude of sensed EMG signals scales with the reciprocal of the square of the distance between the source (i.e., muscle) and electrodes [
26,
27]. Furthermore, an additional improvement in performance is anticipated as users gain experience with the Phantom X system. Thus, the performance observed in the ASCENT study is likely a lower limit of the performance the Phantom X system with implantable electrodes might demonstrate in a real-world at-home setting. However, the ASCENT study only included two amputee participants, with most subjects being able-bodied, which limits the generalizability of the findings to the broader amputee population.
A notable observation from this study was the variation in gesture accuracy across different arm postures. The Arm Perpendicular posture exhibited the lowest accuracy, although the difference was not statistically significant. This may be attributed to biomechanical factors, such as muscle recruitment variations and changes in skin–electrode contact due to arm positioning. Future iterations of the Phantom X system could incorporate adaptive algorithms that account for posture-related variations to further enhance performance. It is worth noting that the use of implanted electrodes, as would be the case for Phantom X system, has been shown to mitigate problems related to limb positioning without training for all possible positions [
28].
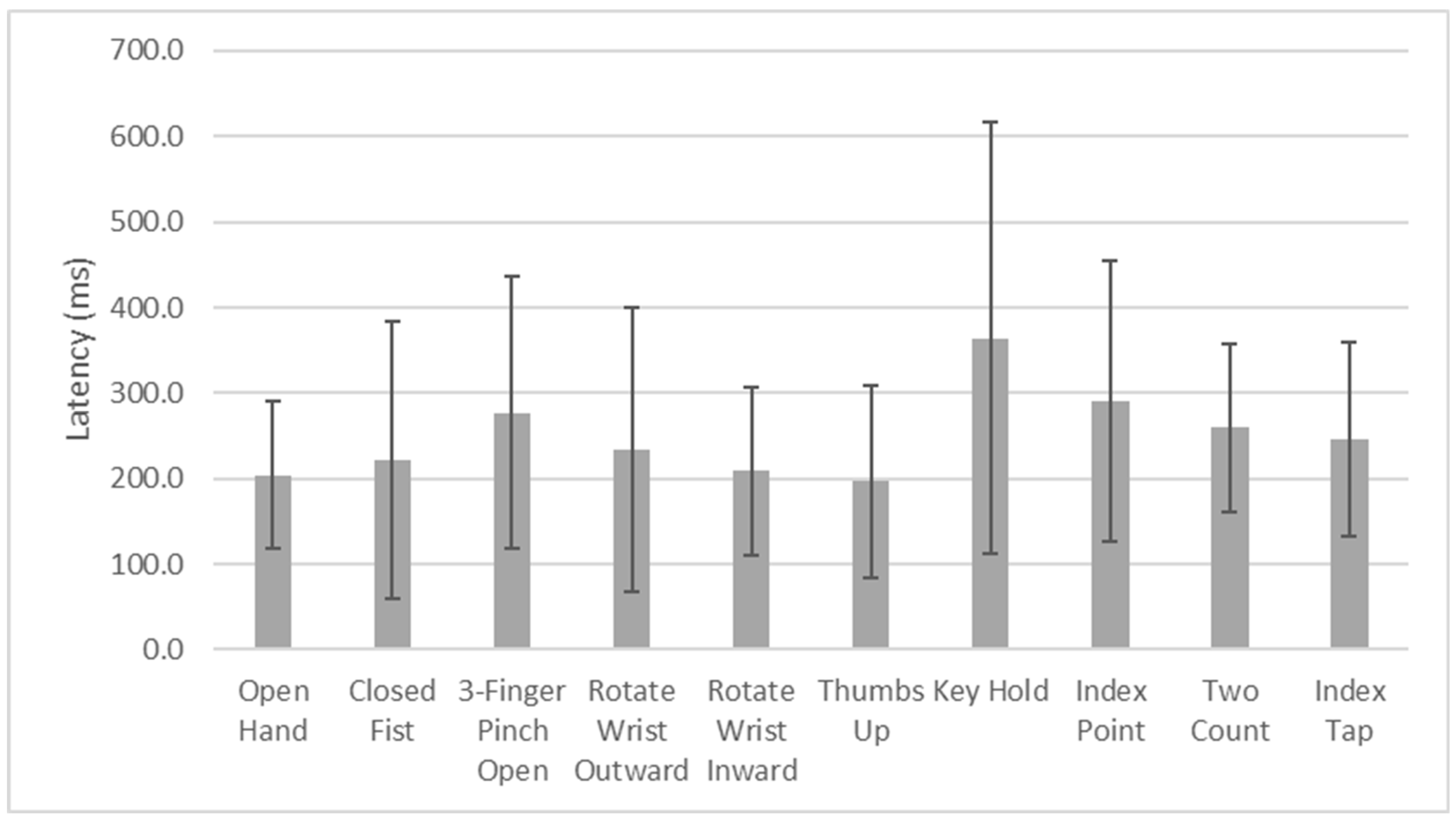
Gesture-specific analysis revealed that most gestures had high recognition rates, with Thumbs Up achieving the highest accuracy (GA = 100%). However, certain gestures, such as Index Point and Index Tap, exhibited lower accuracy (GA of 70% and 79% GA, respectively). These gestures were particularly challenging in the arm perpendicular posture, where Index Point accuracy dropped to 55%. The reasons for lower accuracy for these gestures and deterioration in the Arm Perpendicular posture may be several. First, these gestures involve isolated index finger movement and tend to produce more localized and lower-amplitude EMG signals, which may be harder to distinguish from baseline or other gestures. Second, the fact that surface electrodes are more susceptible to crosstalk may make the small differences associated with these gestures more difficult to discern [
29,
30]. Finally, the arm posture has been shown to significantly affect EMG pattern recognition performance due to changes in muscle length, orientation, and electrode–skin impedance [
31,
32]. Specifically, the Arm Perpendicular posture may introduce mechanical shifts in the muscle belly and residual limb geometry, leading to reduced signal fidelity or altered spatial activation patterns.
The confusion matrices provided deeper insight into gesture misclassification patterns, revealing that the majority of the misclassifications involved gestures being misclassified as Rest. Gestures that engaged minimal muscle mass, such as Index Point and Index Tap, which involve activation of only one or two fingers, tend to produce lower-amplitude EMG signals. As discussed above, this likely led to missed detections of finger activity, resulting in these gestures being more frequently classified as Rest. Misclassification was also more common between gestures that were visually and functionally similar. For instance, confusion between Index Point and Two Count occurred relatively often, with each being misidentified as the other at a higher rate compared to dissimilar gesture pairs.
The lower accuracy observed for certain gestures, such as Index Point and Index Tap, along with the frequent misclassification of gestures that are functionally and visually similar suggest a need for further refinement in signal decoding to improve robustness across postures. One potential strategy is to incorporate additional training data along with clearer and more standardized user instructions to enhance model performance. Alternatively, integrating advanced signal processing techniques may help better distinguish between overlapping muscle activations and improve gesture classification accuracy. Finally, EMG signals from smaller and deeper muscles are expected to be significantly stronger when recorded with implanted electrodes compared to surface electrodes, which could improve the classification accuracy of gestures that rely on these lower-mass muscle groups.
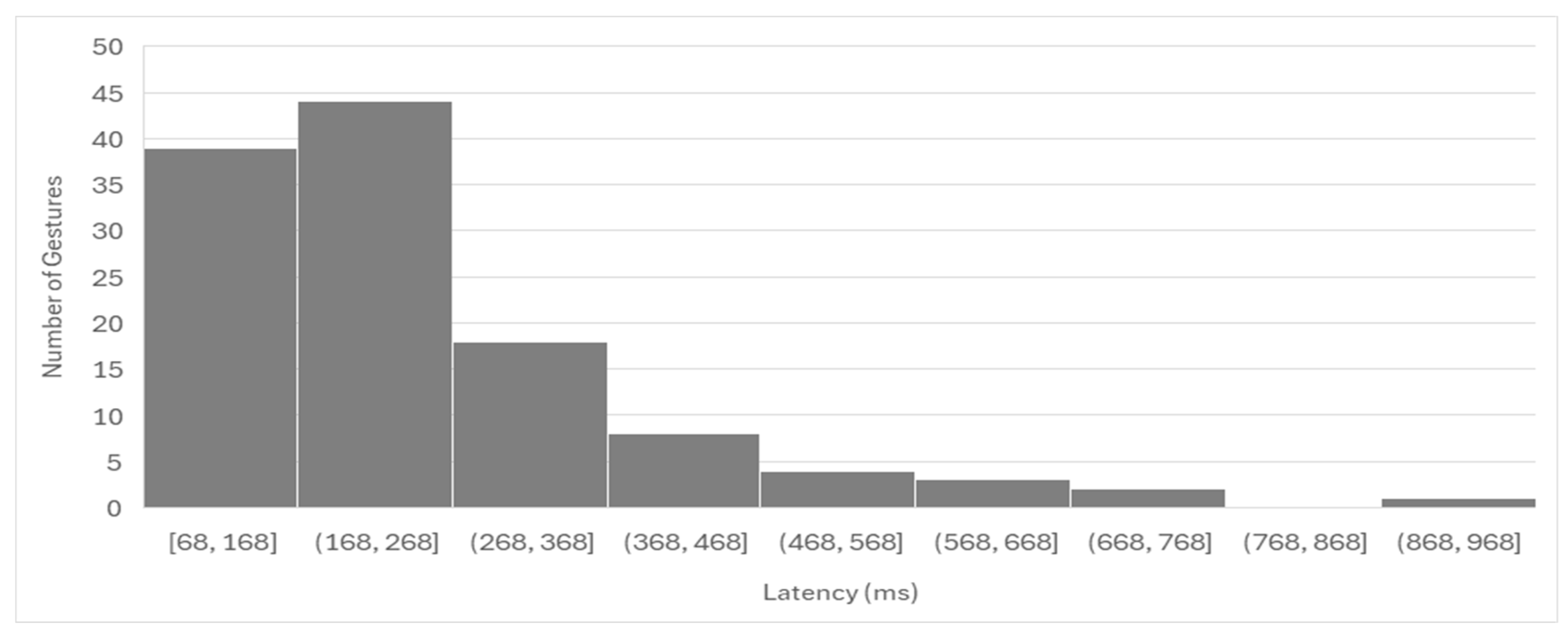
Latency analysis showed a mean response time of 250.5 ms, with the vast majority of gestures initiated within 500 ms. While this latency is within an acceptable range for real-time prosthesis control, minimizing delays could further enhance the user experience. The longest average latency was observed for the Key Hold gesture (364.4 ms), followed by Index Point (291.0 ms). These findings may be indicative of the complexity of muscle activation patterns required for certain gestures or similarities in muscle activation patterns relative to other gestures, necessitating further optimization of signal processing and classification algorithms for certain specific gestures. Moreover, the participants were not instructed to perform the gestures at a particular speed. Therefore, the rate at which a participant contracted their muscles to achieve a gesture could have greatly affected the calculated latency between EMG onset and the detection of the correct gesture and likely contributed to the observed spread in latency times. Finally, additional factors likely contributed to the variability in latency times, including the signal-to-noise ratio (SNR) of the recorded EMGs, which may have been affected by the quality of surface electrode contact with the skin, and the pattern and discriminability of the EMG signals, which influence the processing time required by the algorithm to classify gestures accurately. It is worth noting that our algorithm was designed using fully executed gestures, which involved longer EMG segments. An alternative approach would be to use partially executed gestures and shorter EMG segments, which could reduce latency. However, this would likely come at the cost of reduced algorithm performance and lower classification accuracy. From a practical, real-world task performance perspective, delays in the range of 300–400 ms appear to be acceptable and may not significantly impact functional performance [
33,
34,
35]. It is possible that improvement in the signal-to-noise ratio (SNR) with Phantom X’s implantable sensor array may reduce algorithm processing time for gesture detection and narrow the distribution of latency, thereby minimizing the occurrence of prolonged delays.
Comparing the performance of the Phantom X system in the ASCENT study to gesture control outcomes reported in the literature for transradial amputees with direct control (DC) and more advanced pattern recognition systems is essential and valuable. However, data specifically addressing gesture accuracy in two-electrode DC systems, which are currently the standard of care in myoelectric control, are limited. Studies that report such metrics highlight significant limitations of DC systems, including their constrained ability to support multiple gestures without mode switching and their cognitively demanding operation. For instance, Kuiken et al. [
36] evaluated gesture accuracy in three transradial amputees, reporting results ranging from 84% to 91%. Other studies have assessed functional outcomes using functional assessment instruments, such as the Assessment of Capacity of Myoelectric Control (ACMC) [
37,
38] and the Southampton Hand Assessment Procedure (SHAP) [
39] but did not include gesture accuracy metrics for direct comparison with the ASCENT study [
40]. Nonetheless, DC systems face challenges due to their non-intuitive, time-consuming, and cognitively taxing nature. Despite this, Phantom X achieves comparable accuracy to DC systems while offering a more extensive gesture set, greater ease of use, and reduced cognitive burden for the user.
When comparing Phantom X to newer pattern-recognition systems, which are not yet widely adopted as standard care, data remain sparse. Simon et al. [
19] reported an average gesture accuracy of 81.5% (range: 75% to 95%) for eight patients performing up to six gestures in an at-home setting using a control system called Complete Control System (Coapt LLC, Chicago, IL, USA). However, it is important to note that the ASCENT study was conducted in an in-office environment, making direct comparisons difficult. In another in-office study using a research-grade system (Cyberhand [
41] combined with a pattern-recognition algorithm), Cipriani et al. [
42] reported 79% accuracy for seven hand gestures in seven transradial amputees. Additional research with non-commercial systems and various classifier techniques has reported classification accuracy ranging from 60% to 96% [
43,
44,
45]. These findings underscore the need for standardized benchmarks while highlighting Phantom X’s potential to outperform traditional and MPR systems using sEMG in terms of accuracy and reliability.
As our study predominantly included able-bodied participants, generalizing the findings to a broader amputee population is limited. Muscle activation in transradial upper limb amputees differs from that of able-bodied individuals due to anatomical changes, altered load distribution, and compensatory strategies. Amputees often rely on residual forearm and proximal muscles, which may exhibit atypical activation patterns or co-contractions [
43,
44,
45]. Furthermore, EMG signal quality in amputees can be more variable due to changes in muscle mass, scarring, and altered limb geometry [
32]. However, there are also similarities; for instance, amputees retain voluntary control over their residual muscles and, with appropriate training, can generate activation patterns that closely approximate those of able-bodied individuals [
46].
Finally, the ASCENT study evaluated the performance of the Phantom X system in a controlled, in-office setting. Thus, its performance in real-world, ambulatory environments remains unknown. Future studies will evaluate the performance of the Phantom X system with an implantable array in a larger cohort of the amputee patient population using longitudinal study designs, where participants will use the system in an at-home setting over extended durations (e.g., several weeks to months).
5. Limitations
Despite the promising accuracy results, it is important to acknowledge the limitations of our study. First, this study utilized non-invasive surface EMG electrodes rather than the fully implanted Phantom X system. While the surface electrodes provided a useful approximation of expected performance, they are subject to signal degradation due to skin impedance, movement artifacts, and crosstalk from adjacent muscles. Transition to the fully implantable system is expected to mitigate these issues, likely improving signal fidelity and, consequently, control accuracy.
Second, this study was conducted in a relatively controlled in-office environment, which does not fully replicate real-world conditions, where users may encounter varying levels of physical activity, environmental noise, and prolonged usage challenges. Future studies incorporating long-term, at-home testing will be important in assessing the real-world usability and durability of the Phantom X system.
Third, the sample size was relatively small (n = 11), with two amputee participants. While the high accuracy observed in both the able-bodied and amputee participants is promising, as discussed above, larger studies with a greater representation of amputees are needed to validate the system’s effectiveness in its target user population.
Finally, while gesture accuracy for the two amputees of different genders was among the highest observed across all the subjects, variations in residual limb anatomy and muscle recruitment patterns among a broader amputee population may introduce unique challenges that warrant further investigation.