Abstract
Background/Objectives: This study aimed to assess the long-term clinical performance of full-arch fixed restorations made of fiber-reinforced composite (FRC) supported by four ultra-short implants (4.0 × 5.0 mm) in patients with edentulous, atrophic mandibles. Methods: Ten patients were treated at Sapienza University of Rome and monitored over a 10-year period. Each case involved the placement of four plateau-design implants with a pure conometric connection and a calcium phosphate-treated surface. The final prostheses were fabricated using CAD/CAM-milled Trinia® fiber-reinforced composite frameworks. Clinical parameters included implant and prosthesis survival, marginal bone level (MBL), peri-implant probing depth (PPD), and patient-reported outcome measures (PROMs). Results: Implant and prosthesis survival reached 100% over the 10-year follow-up. MBL data showed a minor bone gain of approximately 0.11 mm per 5 years (p < 0.0001). PPD remained stable under 3 mm, with a minimal increase of 0.16 mm over the same period (p < 0.0001). PROMs reflected sustained high patient satisfaction. No technical complications, such as chipping or framework fracture, were observed. Conclusions: Rehabilitation of the edentulous mandible with ultra-short implants and metal-free FRC prostheses proved to be a minimally invasive and long-lasting treatment option. The 10-year follow-up confirmed excellent implant and prosthetic outcomes, favorable peri-implant tissue health, and strong patient satisfaction. Nonetheless, further studies with larger sample sizes are needed to confirm these encouraging results and strengthen the clinical evidence.
1. Introduction
The care of edentulous patients has been primarily addressed through the use of removable complete dentures, aiming to restore both functionality and aesthetic appearance. However, since 1965, the introduction of osseointegrated dental implants has provided a promising alternative as support for complete dentures [1]. Sivaramakrishnan and Sridharan in their meta-analysis confirmed that oral implantology can improve the quality of life of patients with total prosthesis through the realization of implant-supported rehabilitations [2]. Today, this approach has become a common practice with reliable long-term outcomes. Successfully placing dental implants requires an adequate amount of alveolar bone in terms of height and thickness. However, in daily clinical practice, it is common to encounter situations where the bone volume is significantly reduced. With a view to minimally invasive implant surgery, short (>6 mm to <10 mm) and extra-short or ultra-short (≤6 mm) implants [3,4] are a valid alternative to restore full-arches, resulting in a less traumatic approach, especially in severely atrophic mandibles. In a systematic review of RTCs, Amine et al. showed promising results in terms of bone loss with these types of implants that are increasingly used [5]. A recent pilot study reported encouraging preliminary results for full-arch, fiber-reinforced composite (FRC) prostheses fixed on four ultra-short 5.0 mm implants [6,7,8].
Similarly, in a recent pilot study, the current group has reported the same encouraging results [9].
The objective of the present research was to assess the long-term outcomes of full-arch FRC prostheses supported by ultra-short implants with sloping shoulder and pure conical connection concerning changes in marginal bone level and the survival of implants/prostheses. The researchers hypothesized that ultra-short implants would exhibit comparable clinical performance to that reported in the literature for standard-length implants.
In addition to the surgical aspect, the design of the prosthesis plays a significant role. In recent years, interest in prosthetic dentistry has shifted toward composite materials, with numerous studies evaluating the performance of fixed partial dentures made from these materials. Composite materials offer several benefits over traditional metal–ceramic systems, including enhanced aesthetics, superior biomechanical properties, and the ability to repair or modify the prosthesis chairside [10]. Recent research by Zaparolli et al. has examined various bar materials and manufacturing methods, demonstrating that fiber-reinforced composite (FRC) materials provide excellent functional, aesthetic, and biocompatibility results [11]. This case report aims to highlight the advantages of fixed implant–prosthetic rehabilitation using short implants and metal-free prosthetic materials.
Minimally invasive approaches are closely related to biomimetics. The term “biomimetics” derives from the Latin words bios, meaning life, and mimesis, meaning to copy or imitate. Coined by biophysicist and biomedical engineer Otto Schmitt in the 1950s, biomimetics refers to the study of natural mechanisms for designing innovative products that mimic nature [12].
The main goal of biomimetic dentistry is to achieve full functionality using materials that can mimic or entirely restore the natural biomechanics of the dento–periodontal complex. In this study, edentulous patients with maxillary atrophy are rehabilitated using biomimetic materials: four Ultra-Short implants measuring 4 × 5 mm with a pure conometric connection and plateau design, along with a “metal-free” fixed prosthesis characterized by a prosthetic substructure entirely formed from fiber-reinforced composite (FRC) materials (metal-free fixed restorations made from Trinia®, 501 Arborway, Boston, MA 02130, USA). The specific aims of the study were the following: (1) to ascertain survival rates of implants and prostheses; (2) to analyze bone level alterations over a ten-year follow-up period; and (3) to investigate the patients’ satisfaction.
2. Materials and Methods
The study sample for this prospective, single-cohort study comprised ten volunteers receiving care at the Oral Surgery Department of Dental Sciences, Dental Clinic at “Sapienza” University of Rome (Italy). The study was conducted in accordance with the Helsinki Declaration and received approval from the local ethics committee. Patients were given an anamnestic questionnaire to identify any diseases that might potentially impact the study’s outcome, such as cardiovascular, endocrine, pulmonary, renal, or hepatic diseases. Patients who had undergone organ transplant or had a history of pathological tumor were excluded from participation.
Inclusion criteria for this study were as follows: patients in good overall health as determined by routine laboratory screening tests; edentulous mandible with adequate height and thickness (minimum 6 mm height and 5 mm thickness) for the placement of short implants. Assessment of mandibular anatomy and bone volume was conducted through clinical examination and radiographic assessments including orthopantomography and CT scans.
Exclusion criteria comprised the following: lack of informed consent or inability to provide informed consent, age under 18 or over 80 years, uncontrolled diabetes (target HbA1c < 6.5%), heavy smoking (more than ten cigarettes per day), tobacco chewing, alcohol abuse, untreated periodontal disease affecting the opposing arch, heightened risk of bacterial endocarditis, rheumatic diseases, prior bisphosphonate or interferon therapy, chronic glucocorticoid use, pregnancy, absence of compliance, physical disability that interferes with oral hygiene, and patients who took part in drug trials in the last thirty days.
Forty implants were surgically placed in ten patients, consisting of four males and six females. Among them, eight patients had a complete denture in the opposing dentition, while two retained their natural teeth. The patients received four short implants (Bicon LLC, Boston, MA, USA), measuring 4 mm in diameter and 5 mm in height, to support the fixed prosthesis. These implants feature a plateau design, a crestal module with a pure locking taper connection, sloping shoulder, abutment hemispheric profile, and a calcium phosphate surface treatment. Both the implants and abutments of the system are composed of the titanium alloy Ti6Al4V.
The prosthesis substructure is crafted from Trinia® (Bicon LLC, Boston, MA, USA), consisting of interlaced multidirectional, multilayered fiberglass embedded in an epoxy resin (FRC) matrix. It is supplied in pre-cured milling blocks for the CAD/CAM (computer-aided design/computer-aided manufacturing) technique. The substructure was further enhanced with denture teeth made of composite material.
The surgical procedure involved the placement of four short implants for each patient. Site preparation was conducted using a surgical guide. The initial pilot drill was utilized under irrigation, followed by atraumatic drills rotating at a speed of 50 rpm without irrigation. The final reamer size matched the implant size, and the implant was placed under pressure, flush with or up to 3 mm below the bone level (sub-crestal) (Figure 1). Bone harvested during osteotomy was positioned between the cortical bone of the surgical alveolus and the implant, above the implant shoulder.
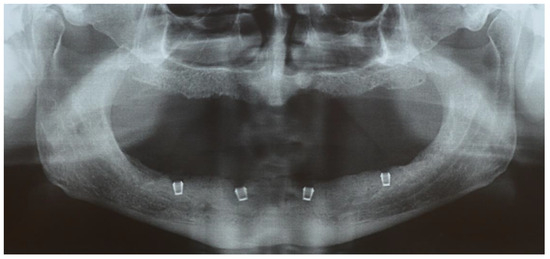
Figure 1.
The orthopantomogram shows the placement of the four implants 3 mm below the bone level.
All surgical procedures were performed by the same surgeon (A.C.). After a three-month healing period, implants were surgically exposed, and healing abutments were placed. Two weeks later, a full-arch impression of the implants and opposing teeth was taken using an A-type silicone material (Elite HD, Zhermack, Badia Polesine, Italy). Once ready, milled abutments were connected to the implants using a template to ensure correct orientation (Figure 2). The prosthesis was then cemented using a temporary luting agent (Temp Bond NE, Kerr) (Figure 3). Subsequently, occlusion was checked using a balanced occlusal scheme. The prosthetic teeth featured anatomical cusped morphology to promote efficient masticatory function. Occlusal contacts were verified using articulating paper during both static and dynamic mandibular movements. No signs of bruxism or other parafunctional habits were observed during the clinical evaluations or follow-up visits.
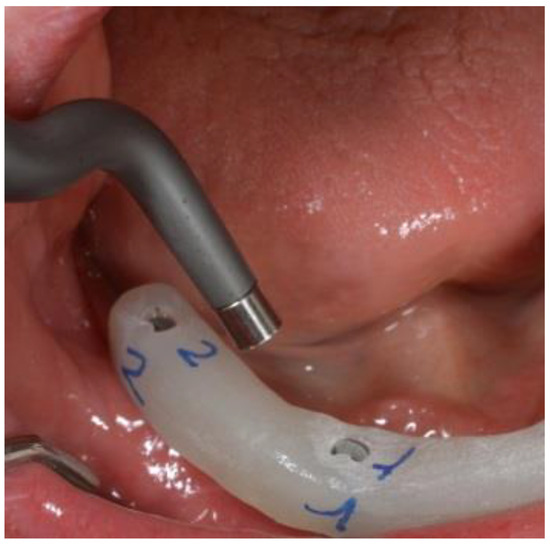
Figure 2.
The abutments were connected to the implants with the aid of a surgical template to ensure precise orientation.
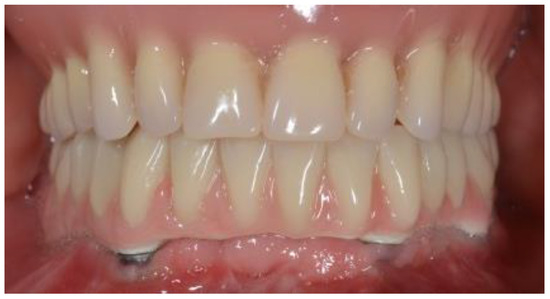
Figure 3.
Final prosthetic restoration.
All prostheses were fabricated by the same laboratory technician. A control panoramic radiograph was taken (Figure 4). Follow-up visits were scheduled at 12 and 24 months after prosthetic loading, and every 12 months thereafter, at the UOC Oral Surgery Department of Dental Sciences, Dental Clinic “Sapienza” at the University of Rome (Italy).
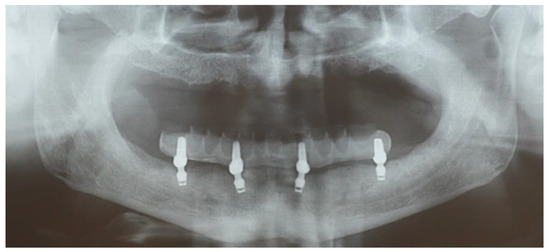
Figure 4.
The image shows the four implants and the prosthetic finalization.
Marginal bone level was assessed following the implant–abutment connection. It was defined as the maximum distance from the implant–abutment junction (IAJ) on the implant side to the marginal bone. Two mesial and distal sites were measured for each implant (IAJ and marginal bone). Mesial and distal bone levels were directly measured on X-ray films (analog orthopantomography) using a millimeter ruler, with measurements calibrated to the known size of the implant. All measurements were performed at each follow-up interval (baseline, 12 and 24 months after prosthetic loading, and annually thereafter up to 10 years) to monitor longitudinal changes in bone levels. A positive value indicated bone above the IAJ, while a negative value indicated bone below the IAJ. A score of zero was assigned when the bone level coincided with the IAJ. Therefore, negative values indicated bone loss, while positive values indicated bone gain. Horizontal marginal bone level was not considered in the measurements.
A single calibrated examiner recorded the mesial and distal aspects of each implant. The intra-class correlation coefficient was 0.83 (95% CI: 0.79–0.87), indicating a good reproducibility in radiographic measurements
These measurements were subsequently averaged to determine the mean implant bone level variation. Variation represented bone loss or gain compared to the baseline (prosthesis cementation) reference value, which was set as zero.
Patient perception was evaluated using a questionnaire where subjects rated various aspects on a scale from zero to ten. The categories included level of satisfaction, assessment of the prosthetic procedure, comfort, aesthetics, bite force, hygiene, phonetics, and implant stability. This questionnaire was administered at each follow-up visit, 12 and 24 months after prosthetic loading, and annually thereafter up to 10 years.
Descriptive statistics using mean and standard deviation for quantitative variables, and frequency and percentage for qualitative variables were used. The primary outcome variable was the implant survival rate. Considering that no implant was lost at the final follow-up, it was impossible to determine the risk factors for implant loss.
Linear regression models were performed in order to investigate the changes in the different secondary outcomes (MBL, PPD, PROMS) during the follow-up. The Shapiro–Wilk test was used to determine the normal distribution of the quantitative variables.
Differences in mean values at the follow-up visits were assessed by means of ANOVA for repeated measures with post hoc Bonferroni’s correction considering the different follow-up visits for the intra-group comparisons. The level of significance was set at p < 0.05. All statistical analyses were performed using a computer program (SPSS v 21.0, Chicago, IL, USA).
3. Results
The final sample included eight patients, aged 61.1 years (range 42–80), for a total of 32 implants. Two patients dropped out of the study. The implant survival rate was 100%. Also, the cumulative survival rate of the prostheses was 100%. Mean MBL is reported in Table 1. ANOVA for repeated measures showed significant changes over time on bone level at the 10-year follow-up (p < 0.0001). Surprisingly, this difference was due to a coronal growth of the hard tissues when compared to the baseline examination. This bone gain amounted to 0.11 mm every five years. Figure 5 reports the decrease of MBL over time. Figure 6 shows the clinical outcome at the 10-year follow-up.

Table 1.
Changes in MBL and PPD over time (ANOVA for repeated measures).
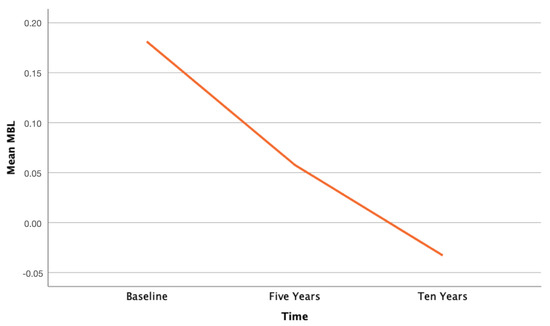
Figure 5.
Descriptive graph illustrating the decrease of MBL between baseline and last follow-up.
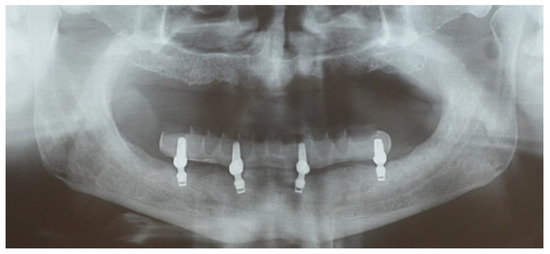
Figure 6.
The 10-year clinical outcome.
Table 1 also reports the mean difference in PPD. ANOVA for repeated measures showed significant increase over time on PPD at the 10-year follow-up (p < 0.0001). Although this difference was statistically significant, the PPD remained lower than 3 mm, thus assessing the healthy periodontal conditions of the included implants. Table 2 shows the changes of PPD over time, with a 0.16 mm increase every 5 years.

Table 2.
Differences over time in PROMS (ANOVA for repeated measures).
Figure 7 shows the slight increase of PPD over time.
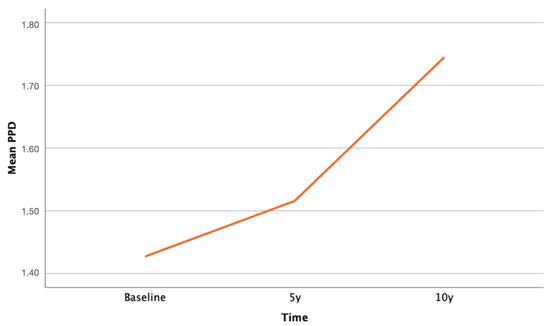
Figure 7.
Descriptive graph illustrating the increase of mean PPD between baseline and last follow-up.
Data about the PROMS are reported in Table 2. No statistically significant difference was shown in any of the outcomes of interest, which shows a high appreciation of the patients which remained unchanged or showed a slight increase during time.
Concerning the durability of the prosthesis and prosthetic complications, no fractures or chipping of the manufact were observed.
4. Discussion
The primary aim of this study was to evaluate the long-term clinical outcomes—over a period of up to 10 years—of fixed full-arch prostheses made of fiber-reinforced composite (FRC), supported by four ultra-short implants (4.0 × 5.0 mm) placed in severely atrophic mandibular ridges. The main clinical parameters assessed included implant and prosthetic survival rates, as well as changes in marginal bone levels (MBL) over time.
The findings demonstrated excellent clinical performance of this approach, with a 100% survival rate for both implants and prostheses, and stable MBL throughout the follow-up period. Probing pocket depths (PPD) consistently remained below 3 mm throughout the follow-up period, indicating stable peri-implant health and the absence of pathological inflammation. These results support the existing literature which suggests that short implants, even in compromised sites, can offer outcomes comparable to standard-length implants used in conjunction with bone augmentation procedures [13,14,15]. In particular, the implant-based survival rate observed in this study aligns with recent evidence highlighting the effectiveness of ultra-short implants in full-arch rehabilitations [16], and compares favorably with survival rates reported for standard-length implant-supported prostheses [17,18]. A recent systematic review has provided strong support for the use of ultra-short implants (≤6 mm) in full-arch and atrophic jaw rehabilitations [19].
Patient-reported outcomes also reflected consistently high levels of satisfaction, further supporting the clinical validity of this treatment protocol.
Several factors may account for the favorable results observed. One is the unique macrogeometry of the implant system used, which features healing chambers designed to facilitate bone formation and remodeling. Histological analyses of retrieved human samples have shown that bone initially forms as woven tissue within these chambers and subsequently reorganizes into a more structured, haversian-like architecture. This maturation process is known to enhance the mechanical integrity of peri-implant bone over time [20,21,22,23].
Moreover, the implants incorporate a dual platform-switch design aimed at directing occlusal loads coronally to the implant–abutment junction. The inward and upward tapering of the implant shoulder not only creates space for crestal bone preservation but also promotes compressive loading on both existing and regenerating bone at the crestal level. This design principle contributes to the long-term stability of peri-implant bone [24].
The present data showed that mean MBL values remained stable in the majority of patients, suggesting minimal bone remodeling after the initial healing phase. These results are further supported by findings from Ewers et al. [25], who reported that the vertical positioning of implants significantly affects MBL changes over time. Their study showed superior bone preservation when implants were placed slightly subcrestally, as opposed to epicrestal placement, which was associated with greater marginal bone loss. These observations suggest that apical positioning of ultra-short implants may be a key factor in maintaining peri-implant bone in the long term.
Several clinical and biomechanical studies have explored how different framework materials influence the outcomes of implant-supported fixed prostheses.
A recent systematic review highlighted that full-arch implant-supported prostheses have traditionally relied on cast noble (gold or silver–palladium) or non-noble metal alloys (cobalt–chromium) due to their excellent mechanical strength and long-standing clinical reliability. These materials have consistently demonstrated high survival rates for both implants and prostheses over the long term. In recent years, alternative materials such as titanium, zirconia, and various polymers such as carbon-fiber-reinforced composites have gained attention. These newer materials offer favorable mechanical properties, biocompatibility, and resistance to corrosion, along with promising clinical results. However, despite their advantages, prostheses fabricated with these innovative materials are still susceptible to biological and technical complications [26].
In a prospective study conducted in 2017, Pera et al. evaluated the clinical performance of carbon fiber frameworks in comparison to conventional metal frameworks for full-arch immediate loading implant rehabilitations. The findings indicated that carbon fiber structures represented a promising alternative to traditional metal frameworks, as they were associated with reduced marginal bone resorption and improved implant survival rates throughout the follow-up period [27].
Another material commonly used in full-arch implant-supported fixed dental prostheses due to its favorable mechanical and esthetic properties is zirconia. Comparative analyses have indicated that, while zirconia-based rehabilitations may involve higher initial costs than conventional metal–acrylic solutions, they are associated with a reduction in prosthetic complications and demonstrate higher long-term survival rates [28].
Recent retrospective studies support the clinical reliability of zirconia frameworks across various design configurations—such as zirconia-on-titanium, progressive monolithic zirconia, and zirconia-on-zirconia—with all approaches yielding satisfactory prosthetic and implant success rates, along with high levels of patient satisfaction. Nevertheless, further long-term prospective research involving larger patient populations is necessary to better define the indications and long-term benefits of these prosthetic options [29].
In our study, the use of FRC as the material for the fixed prosthesis may also have contributed to the favorable outcomes. FRC is characterized by a relatively low flexural modulus (~18 GPa), which is comparable to that of cortical bone [30].
Despite its flexibility, FRC offers mechanical performance comparable to that of conventional prosthetic materials.
Panoramic finite element analyses have shown that frameworks made of fiber-reinforced composite (FRC) distribute stress more evenly across the bone–implant interface compared to rigid metal frameworks like Cr–Co, reducing peak stress concentrations in the surrounding bone [31]. Several studies have assessed the clinical outcomes of implants and prostheses in fiber-reinforced composite (FRC) [32,33,34,35]. Their findings consistently indicate high survival rates for both implants and prostheses, even when ultra-short implants were used, along with stable marginal bone levels over time.
In addition to its biomechanical benefits, FRC has demonstrated excellent biocompatibility. It has been proposed as an effective alternative in the reconstruction of craniofacial bone defects [36], and it avoids the risk of corrosion and metal ion release associated with traditional metal alloys, such as cobalt and nickel [10]. Further advantages include improved esthetics, reduced cost, and compatibility with digital workflows, which together contribute to a simplified and efficient treatment process.
A notable limitation of the present study is the absence of reporting on the bone quality at implant sites, as classified by Lekholm and Zarb (D1–D4). Bone quality is widely recognized as a pivotal determinant of primary implant stability and long-term osseointegration success. The lack of this critical parameter constrains the interpretability of the findings, given that variations in bone density may substantially influence implant survival rates and marginal bone remodeling. Future research would benefit from incorporating standardized bone quality evaluations to enhance the robustness and generalizability of results. Additional potential limitations include the small sample size, the absence of a control group, and the use of panoramic radiographs to assess marginal bone levels. Two patients dropped out during the follow-up period, resulting in a final sample of eight participants and 32 implants. Therefore, the results should be interpreted with caution and considered preliminary. Further prospective, multicenter studies with larger sample sizes and the inclusion of control groups are necessary to validate the long-term efficacy and reproducibility of this treatment approach. Although panoramic imaging is commonly employed in long-term follow-up studies due to its broad coverage and reduced radiation dose, it is subject to inherent limitations such as image distortion and magnification variability, which can affect measurement accuracy. The decision to use panoramic radiographs was based on clinical feasibility and patient compliance over the extended 10-year follow-up. However, we acknowledge that periapical radiographs or cone-beam computed tomography (CBCT) could have offered more precise measurements. Future studies might benefit from incorporating these imaging modalities to enhance the accuracy of bone level assessments.
5. Conclusions
The findings of this 10-year clinical study suggest that full-arch rehabilitations supported by four ultra-short implants and restored with fiber-reinforced composite (FRC) prostheses represent a reliable and effective treatment option for patients with edentulous mandibular ridges. The high implant and prosthesis survival rates, combined with stable marginal bone levels and high patient satisfaction, support the long-term viability of this minimally invasive approach. The biomechanical advantages of both the implant design and the FRC material (namely stress modulation, bone preservation, and biocompatibility) appear to play a critical role in the success of the therapy. Although the results are promising, further multicenter studies with larger cohorts and longer follow-up periods are needed to validate these outcomes and establish definitive clinical guidelines.
Author Contributions
Conceptualization, A.C. and G.P.; methodology, A.C.; software, C.R.; validation, A.C.; formal analysis, C.R.; investigation, G.P.; resources, F.Z.; data curation, G.P.; writing—original draft preparation, G.P.; writing—review and editing, F.Z.; visualization, F.Z.; supervision, A.C.; project administration, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Sapienza” University of Rome (protocol code 628/13, Rome 13/06/2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Sivaramakrishnan, G.; Sridharan, K. Comparison of patient satisfaction with mini-implant versus standard diameter implant overdentures: A systematic review and meta-analysis of randomized controlled trials. Int. J. Implant Dent. 2017, 3, 29. [Google Scholar] [CrossRef]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental implant length and diameter: A proposed classification scheme. J. Prosthodont. 2017, 26, 252–260. [Google Scholar] [CrossRef]
- Neugebauer, J.; Vizethum, F.; Berger, C.; Bolz, W.; Bowen, A.; Deporter, D.; Ewers, R.; Fairbairn, P.; Felino, A.; Fortin, T.; et al. Update: Kurze, angulierte und durchmesserreduzierte Implantate-praxisleitfa. Den: 11. Europäische Konsensuskonferenz (EuCC). BDIZ/EDI Konkret 2016, 20, 88–90. [Google Scholar]
- Amine, M.; Guelzim, Y.; Benfaida, S.; Bennani, A.; Andoh, A. Short implants (5–8 mm) vs. long implants in augmented bone and their impact on peri-implant bone in maxilla and/or mandible: Systematic review. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 133–142. [Google Scholar] [CrossRef]
- Seemann, R.; Wagner, F.; Marincola, M.; Ewers, R. Fixed, fiber-rein-forced resin bridges on 5.0-mm implants in severely atrophic mandibles: Up to 5 years’ follow-up of a prospective cohort study. J. Oral Maxillofac. Surg. 2018, 76, 956. [Google Scholar] [CrossRef] [PubMed]
- Ewers, R.; Marincola, M.; Perpetuini, P.; Seemann, R.; Morgan, V.; Wu, R. Leichtgewicht im Praxistest—Restaurationen bei schwierigen Situationen und atrophen Kiefern. Z. Oral Implant. 2017, 13, 28. [Google Scholar]
- Ewers, R.; Perpetuini, P.; Morgan, V.; Marincola, M.; Wu, R.; Seemann, R. TRINIA TM—Metal-free restorations. Implants 2017, 1, 2–7. [Google Scholar]
- Petroni, G.; Passaretti, A.; Marincola, M.; Pompa, G.; Cicconetti, A. Alternative solution for mandible rehabilitation: Fixed full arch prosthesis on short implant, a randomized cohort study. J. Osseointegr. 2019, 11, 477–484. [Google Scholar]
- Passaretti, A.; Petroni, G.; Miracolo, G.; Savoia, V.; Perpetuini, A.; Cicconetti, A. Metal free, full arch, fixed prosthesis for edentulous mandible rehabilitation on four implants. J. Prosthodont. Res. 2018, 62, 264–267. [Google Scholar] [CrossRef]
- Zaparolli, D.; Peixoto, R.F.; Pupim, D.; Macedo, A.P.; Toniollo, M.B.; de Mattos, M.d.G.C. Photoelastic analysis of mandibular full-arch implant-supported fixed dentures made with different bar materials and manufacturing techniques. Mater. Sci. Eng. C 2017, 81, 144–147. [Google Scholar] [CrossRef]
- Harkness, J.M. An idea man (the life of Otto Herbert Schmitt). IEEE Eng. Med. Biol. Mag. 2004, 23, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Cristalli, M.P.; Dell’Aquila, D.; Bignozzi, I.; La Monaca, G.; Pilloni, A. Short dental implants: A systematic review. J. Dent. Res. 2012, 91, 25. [Google Scholar] [CrossRef]
- Atieh, M.; Zadeh, H.; Stanford, C.M.; Cooper, L.F. Survival of short dental implants for treatment of posterior partial edentulism: A systematic review. Int. J. Oral Maxillofac. Implant. 2012, 27, 1323–1331. [Google Scholar]
- Palacios, J.A.V.; Garcia, J.J.; Carames, J.M.M.; Quirynen, M.; da Silva Marques, D.N. Short implants versus bone grafting and standard-length implants placement: A systematic review. Clin. Oral Investig. 2018, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Anglada-Bosqued, A.; Salomó-Coll, O.; Hernández-Alfaro, F.; Wang, H.L.; Gargallo-Albiol, J. Short implants (<8mm) versus longer implants (≥8mm) with lateral sinus floor augmentation in posterior atrophic maxilla: A meta-analysis of RCT’s in humans. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e168–e179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afrashtehfar, K.I. The all-on-four concept may be a viable treatment option for edentulous rehabilitation. Evid.-Based Dent. 2016, 17, 56–57. [Google Scholar] [CrossRef]
- Patzelt, S.B.; Bahat, O.; Reynolds, M.A.; Strub, J.R. The All-on-Four Treatment Concept: A Systematic Review. Clin. Implant Dent. Relat. Res. 2014, 16, 836–855. [Google Scholar] [CrossRef]
- Rosa, A.; Pujia, A.M.; Arcuri, C. Complete Full Arch Supported by Short Implant (<8 mm) in Edentulous Jaw: A Systematic Review. Appl. Sci. 2023, 13, 7162. [Google Scholar] [CrossRef]
- Gil, L.F.; Suzuki, M.; Janal, M.N.; Tovar, N.; Marin, C.; Granato, R.; Bonfante, E.A.; Jimbo, R.; Gil, J.N.; Coelho, P.G. Progressive plateau root form dental implant osseointegration: A human retrieval study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1328–1332. [Google Scholar] [CrossRef]
- Baldassarri, M.; Bonfante, E.; Suzuki, M.; Marin, C.; Granato, R.; Tovar, N.; Coelho, P.G. Mechanical properties of human bone surrounding plateau root form implants retrieved after 0.3–24 years of function. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Bonfante, E.A.; Marin, C.; Granato, R.; Giro, G.; Suzuki, M. A human retrieval study of plasma-sprayed hydroxyapatite-coated plateau root form implants after 2 months to 13 years in function. J. Long-Term Eff. Med. Implant. 2010, 20, 335–342. [Google Scholar] [CrossRef]
- Coelho, P.G.; Marin, C.; Granato, R.; Suzuki, M. Histomorphologic analysis of 30 plateau root form implants retrieved after 8 to 13 years in function. A human retrieval study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 975–979. [Google Scholar] [CrossRef]
- Abdel-Latif, H.H.; A Hobkirk, J.; Kelleway, J.P. Functional mandibular deformation in edentulous subjects treated with dental implants. Int. J. Prosthodont. 2000, 13, 513–519. [Google Scholar]
- Ewers, R.; Marincola, M.; Perpetuini, P.; Morina, A.; Bergamo, E.T.P.; Cheng, Y.-C.; Bonfante, E.A. Severely atrophic mandibles restored with fiber-reinforced composite prostheses supported by 5.0-mm ultra-short implants present high survival rates up to eight years. J. Oral Maxillofac. Surg. 2022, 80, 81–92. [Google Scholar] [CrossRef]
- Delucchi, F.; De Giovanni, E.; Pesce, P.; Bagnasco, F.; Pera, F.; Baldi, D.; Menini, M. Framework Materials for Full-Arch Implant-Supported Rehabilitations: A Systematic Review of Clinical Studies. Materials 2021, 14, 3251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pera, F.; Pesce, P.; Solimano, F.; Tealdo, T.; Pera, P.; Menini, M. Carbon fibre versus metal framework in full-arch immediate loading rehabilitations of the maxilla—A cohort clinical study. J. Oral Rehabil. 2017, 44, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Askar, H.; Ravidà, A.; Gargallo-Albiol, J.; Travan, S.; Wang, H.-L. Long-term Clinical Outcomes and Cost-Effectiveness of Full-Arch Implant-Supported Zirconia-Based and Metal-Acrylic Fixed Dental Prostheses: A Retrospective Analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Marchio, V.; Cinquini, C.; Alfonsi, F.; Romeggio, S.; Stoppaccioli, M.; Zingari, F.; Priami, M.; Barone, A. Retrospective Analysis of Full-Arch Zirconia Rehabilitations on Dental Implants: Clinical Outcomes and Patient Satisfaction. Appl. Sci. 2025, 15, 416. [Google Scholar] [CrossRef]
- Cevik, P.; Schimmel, M.; Yilmaz, B. New generation CAD-CAM materials for implant-supported definitive frameworks fabricated by using subtractive technologies. BioMed Res. Int. 2022, 2022, 3074182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erkmen, E.; Meriç, G.; Kurt, A.; Tunç, Y.; Eser, A. Biomechanical comparison of implant retained fixed partial dentures with fiber reinforced composite versus conventional metal frameworks: A 3D FEA study. J. Mech. Behav. Biomed. Mater. 2011, 4, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Perpetuini, P.; Murcko, L.; Hirayama, M.; Morgan, K.; Marincola, M.; Bonfante, E.A.; Bergamo, E.T.P.; Ewers, R. Fiber-reinforced composite full-arch prosthetic reconstructions supported by three standard, short or extra-short implants: A two-center retrospective study. Clin. Oral Investig. 2023, 27, 4191–4203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Bergamo, E.T.P.; Murcko, L.; Hirayama, M.; Perpetuini, P.; Speratti, D.; Bonfante, E.A. Fiber-reinforced composite partial fixed dental prostheses supported by short or extra-short implants: A 10 year retrospective study. Clin. Implant Dent. Relat. Res. 2022, 24, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, D.; Marincola, M.; Díaz-Caballero, A.; Passaretti, A.; Cicconetti, A. Survival Rate, Biomechanical Complications, and Patient Satisfaction of Implant-Supported FRC Full-Arch Prostheses: A Retrospective Study with Follow up of 5 Years. J. Dent. 2024, 25, 268–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Y.-C.; Bonfante, E.A.; Bergamo, E.T.; Ewers, R. Partial fixed dental prostheses fabricated using fiber-reinforced composite resin supported by short and extra-short implants: A case series. J. Prosthodont. Res. 2024, 68, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.-A.; Rotaru, H.; Bâldea, I.; Boşca, A.B.; Berce, C.P.; Prejmerean, C.; Prodan, D.; Câmpian, R.S. Evaluation of the biocompatibility of new fiber-reinforced composite materials for craniofacial bone reconstruction. J. Craniofacial Surg. 2016, 27, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).