The Roles of Leaflet Geometry in the Structural Deterioration of Bioprosthetic Aortic Valves †
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pibarot, P.; Dumesnil, J.G. Prosthetic heart valves: Selection of the optimal prosthesis and long-term management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.H.; Takkenberg, J.J. Will heart valve tissue engineering change the world? Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.B.; Herbertson, L.H.; Fontaine, A.A.; Deutsch, S. A detailed fluid mechanics study of tilting disk mechanical heart valve closure and the implications to blood damage. J. Biomech. Eng. 2008, 130, 041001. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J. Heart valve tissue engineering: Quo vadis? Curr. Opin. Biotechnol. 2011, 22, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.F.; Abraham, J.R.; Butany, J. Bioprosthetic heart valves: Modes of failure. Histopathology 2009, 55, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.F.; Seco, M.; Wu, J.J.; Edelman, J.B.; Wilson, M.K.; Vallely, M.P.; Byrom, M.J.; Bannon, P.G. Mechanical Versus Bioprosthetic Aortic Valve Replacement in Middle-Aged Adults: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2016, 102, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Vongpatanasin, W.; Hillis, L.D.; Lange, R.A. Prosthetic heart valves. N. Engl. J. Med. 1996, 335, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–185. [Google Scholar] [CrossRef] [PubMed]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L.; Takkenberg, J.J.; David, T.E.; Butchart, E.G.; Adams, D.H.; et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J. Thorac. Cardiovasc. Surg. 2008, 135, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Schoen, F.J. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J. Biomed. Mater. Res. 2002, 62, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Thubrikar, M.J.; Deck, J.D.; Aouad, J.; Nolan, S.P. Role of mechanical stress in calcification of aortic bioprosthetic valves. J. Thorac. Cardiovasc. Surg. 1983, 86, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vesely, I.; Boughner, D.; Song, T. Tissue buckling as a mechanism of bioprosthetic valve failure. Ann. Thorac. Surg. 1988, 46, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Dasi, L.P.; Simon, H.A.; Sucosky, P.; Yoganathan, A.P. Fluid mechanics of artificial heart valves. Clin. Exp. Pharmacol. Physiol. 2009, 36, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, S.; Bortolotti, U.; Wasserman, F.; Tindel, N.; Factor, S.M.; Frater, R.W. Long-term follow-up of the Ionescu-Shiley mitral pericardial xenograft. J. Thorac. Cardiovasc. Surg. 1984, 88, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Reul, G.J., Jr.; Cooley, D.A.; Duncan, J.M.; Frazier, O.H.; Hallman, G.L.; Livesay, J.J.; Ott, D.A.; Walker, W.E. Valve failure with the Ionescu-Shiley bovine pericardial bioprosthesis: Analysis of 2680 patients. J. Vasc. Surg. 1985, 2, 192–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vesely, I. The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovasc. Pathol. 2003, 12, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Walley, V.; Keon, W. Patterns of failure in Ionescu-Shiley bovine pericardial bioprosthetic valves. J. Thorac. Cardiovasc. Surg. 1987, 93, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, U.; Milano, A.; Guerra, F.; Mazzucco, A.; Mossuto, E.; Thiene, G.; Gallucci, V. Failure of Hancock pericardial xenografts: Is prophylactic bioprosthetic replacement justified? Ann. Thorac. Surg. 1991, 5, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, U.; Milano, A.; Thiene, G.; Guerra, F.; Mazzucco, A.; Valente, M.; Talenti, E.; Gallucci, V. Early mechanical failures of the Hancock pericardial xenograft. J. Thorac. Cardiovasc. Surg. 1987, 94, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Bortolotti, U.; Valente, M.; Milano, A.; Calabrese, F.; Talenti, E.; Mazzucco, A.; Gallucci, V. Mode of failure of the Hancock pericardial valve xenograft. Am. J. Cardiol. 1989, 63, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.S.; Sabbah, H.N.; Stein, P.D. Influence of stent height upon stresses on the cusps of closed bioprosthetic valves. J. Biomech. 1986, 19, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Ius, P.; Thiene, G.; Minarini, M.; Valente, M.; Bortolotti, U.; Talenti, E.; Casarotto, D. Low-profile porcine bioprosthesis (Liotta): Pathologic findings and mode of failure in the long-term. J. Heart Valve Dis. 1996, 5, 323–327. [Google Scholar] [PubMed]
- Lim, K.H.; Candra, J.; Yeo, J.H.; Duran, C.M.G. Flat or curved pericardial aortic valve cusps: A finite element study. J. Heart Valve Dis. 2004, 13, 792–797. [Google Scholar] [PubMed]
- Xiong, F.L.; Goetz, W.A.; Chong, C.K.; Chua, Y.L.; Pfeifer, S.; Wintermantel, E.; Yeo, J.H. Finite element investigation of stentless pericardial aortic valves: Relevance of leaflet geometry. Ann. Biomed. Eng. 2010, 38, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Bayoumi, A.S.; Chen, P.; Hobson, C.M.; Wagner, W.R.; Mayer, J.E.; Sacks, M.S. Optimal elastomeric scaffold leaflet shape for pulmonary heart valve leaflet replacement. J. Biomech. 2013, 46, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Loerakker, S.; Argento, G.; Oomens, C.W.; Baaijens, F.P. Effects of valve geometry and tissue anisotropy on the radial stretch and coaptation area of tissue-engineered heart valves. J. Biomech. 2013, 46, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, Y.; Ronsky, J.; Ali, I.; Basha, A.; Bhanji, A.; Narine, K. Effects of Leaflet Design on Transvalvular Gradients of Bioprosthetic Heart Valves. Cardiovasc. Eng. Technol. 2016, 7, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sacks, M.S. Finite element implementation of a generalized Fung-elastic constitutive model for planar soft tissues. Biomech. Model. Mechanobiol. 2005, 4, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 1993. [Google Scholar]
- Dabiri, Y.; Paulson, K.; Tyberg, J.; Ronsky, J.; Ali, I.; Di Martino, E.; Narine, K. Design of Bioprosthetic Aortic Valves using biaxial test data. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 3319–3322. [Google Scholar]
- Humphrey, J.D.; Strumpf, R.K.; Yin, F.C.P. A constitutive theory for biomembranes: Application to epicardial mechanics. J. Biomech. Eng. 1992, 114, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Biomechanical characterization of aortic valve tissue in humans and common animal models. J. Biomed. Mater. Res. A. 2012, 100, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Chuong, C.J. Orthotropic mechanical properties of chemically treated bovine pericardium. Ann. Biomed. Eng. 1998, 26, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.G.; Lafaro, R.J.; Moggio, R.A. Immediate structural valve deterioration of 27-mm Carpentier-Edwards aortic pericardial bioprosthesis. Ann. Thorac. Surg. 2004, 77, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Simulation of long-term fatigue damage in bioprosthetic heart valves: Effects of leaflet and stent elastic properties. Biomech. Model. Mechanobiol. 2014, 13, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, E.; Crofts, C. Pericardial heterograft valves: An assessment of leaflet stresses and their implications for heart valve design. J. Biomed. Eng. 1987, 9, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zioupos, P.; Barbenel, J.C.; Fisher, J. Anisotropic Elasticity and Strength of Glutaraldehyde Fixed Bovine Pericardium for Use in Pericardial Bioprosthetic Valves. J. Biomed. Mater. Res. 1994, 28, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Ferrans, V.J.; Boyce, S.W.; Jones, M.; Roberts, W.C. Structure and classification of cuspal tears and perforations in porcine bioprosthetic cardiac valves implanted in patients. Am. J. Cardiol. 1981, 48, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Krucinski, S.; Vesely, I.; Dokainish, M.; Campbell, G. Numerical simulation of leaflet flexure in bioprosthetic valves mounted on rigid and expansile stents. J. Biomech. 1993, 26, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, N.; Weaver, J.D.; Ekrami, Y.; Retta, S.M.; Wu, C. A Parametric Computational Study of the Impact of Non-circular Configurations on Bioprosthetic Heart Valve Leaflet Deformations and Stresses: Possible Implications for Transcatheter Heart Valves. Cardiovasc. Eng. Technol. 2016, 7, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Scharfschwerdt, M.; Meyer-Saraei, R.; Schmidtke, C.; Sievers, H.-H. Hemodynamics of the Edwards Sapien XT transcatheter heart valve in noncircular aortic annuli. J. Thorac. Cardiovasc. Surg. 2014, 148, 126–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, E.; Chen, J.F.; Dong, O.; Gao, S.; Massiello, A.; Fukamachi, K. Transcatheter heart valve with variable geometric configuration: In vitro evaluation. Artif. Organs. 2011, 35, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Vesely, I. Transcatheter valves: A brave New World. J. Heart Valve Dis. 2010, 19, 543–558. [Google Scholar] [PubMed]
- Mercer, J.; Benedicty, M.; Bahnson, H. The geometry and construction of the aortic leaflet. J. Thorac. Cardiovasc. Surg. 1973, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Brewer, R.J.; Deck, J.D.; Capati, B.; Nolan, S.P. The dynamic aortic root. Its role in aortic valve function. J. Thorac. Cardiovasc. Surg. 1976, 72, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Yoganathan, A.P. Heart valve function: A biomechanical perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1369–1391. [Google Scholar] [CrossRef] [PubMed]
- Thubrikar, M.; Harry, R.; Nolan, S.P. Normal aortic valve function in dogs. Am. J. Cardiol. 1977, 40, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Billiar, K.L.; Sacks, M.S. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. J. Biomech. Eng. 2000, 122, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Smith, D.B.; Hiester, E.D. A small angle light scattering device for planar connective tissue microstructural analysis. Ann. Biomed. Eng. 1997, 25, 678–689. [Google Scholar] [CrossRef] [PubMed]

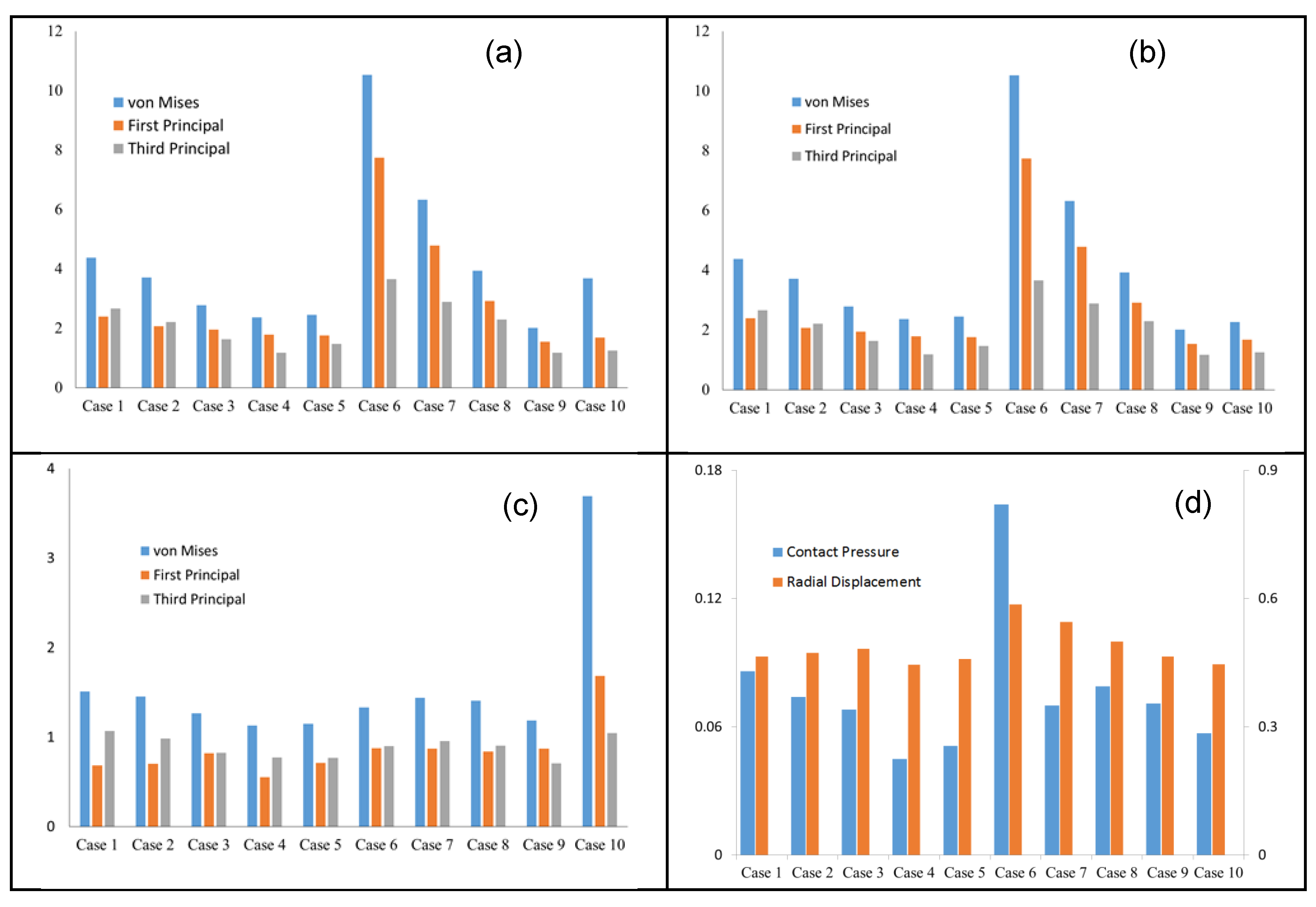
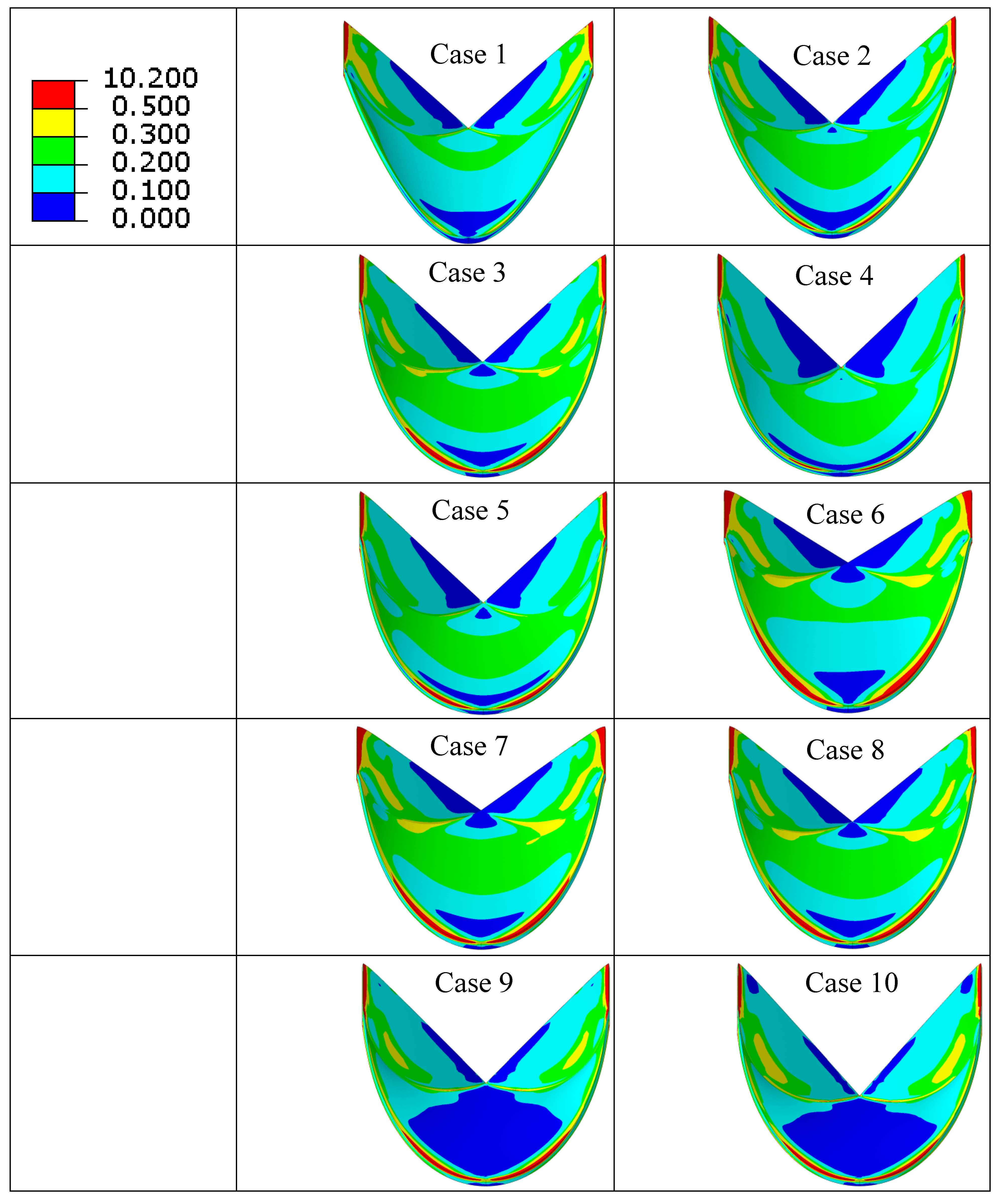
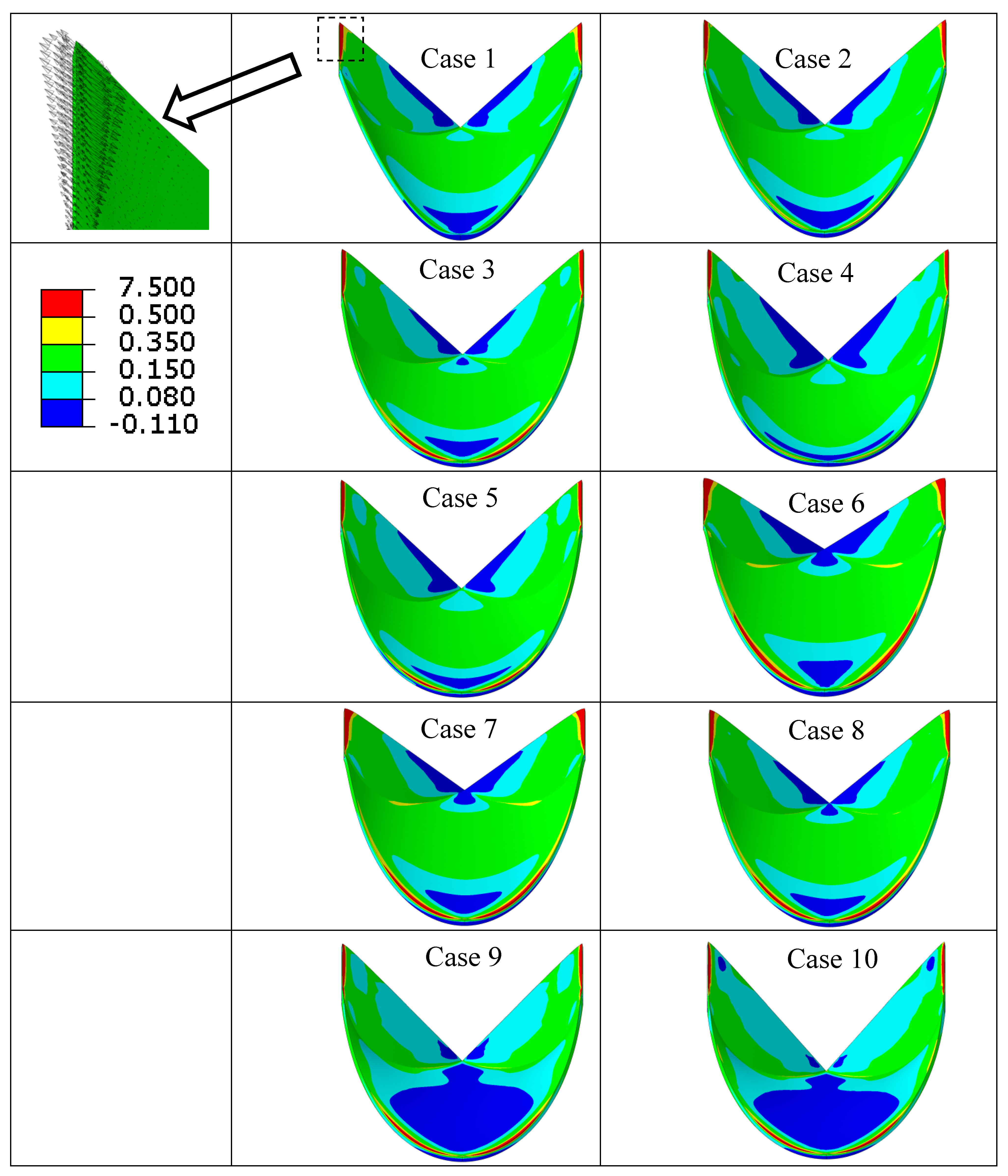
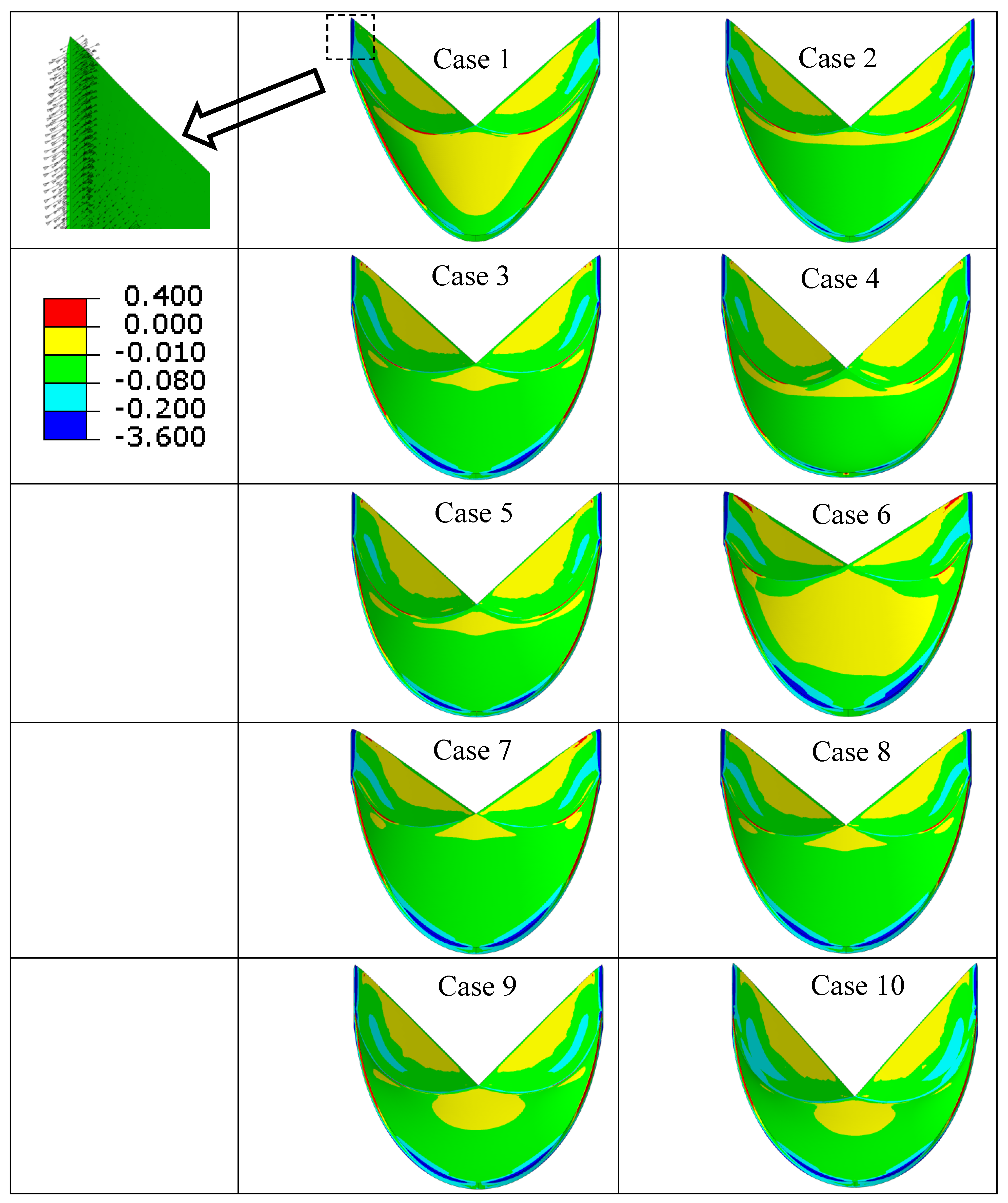

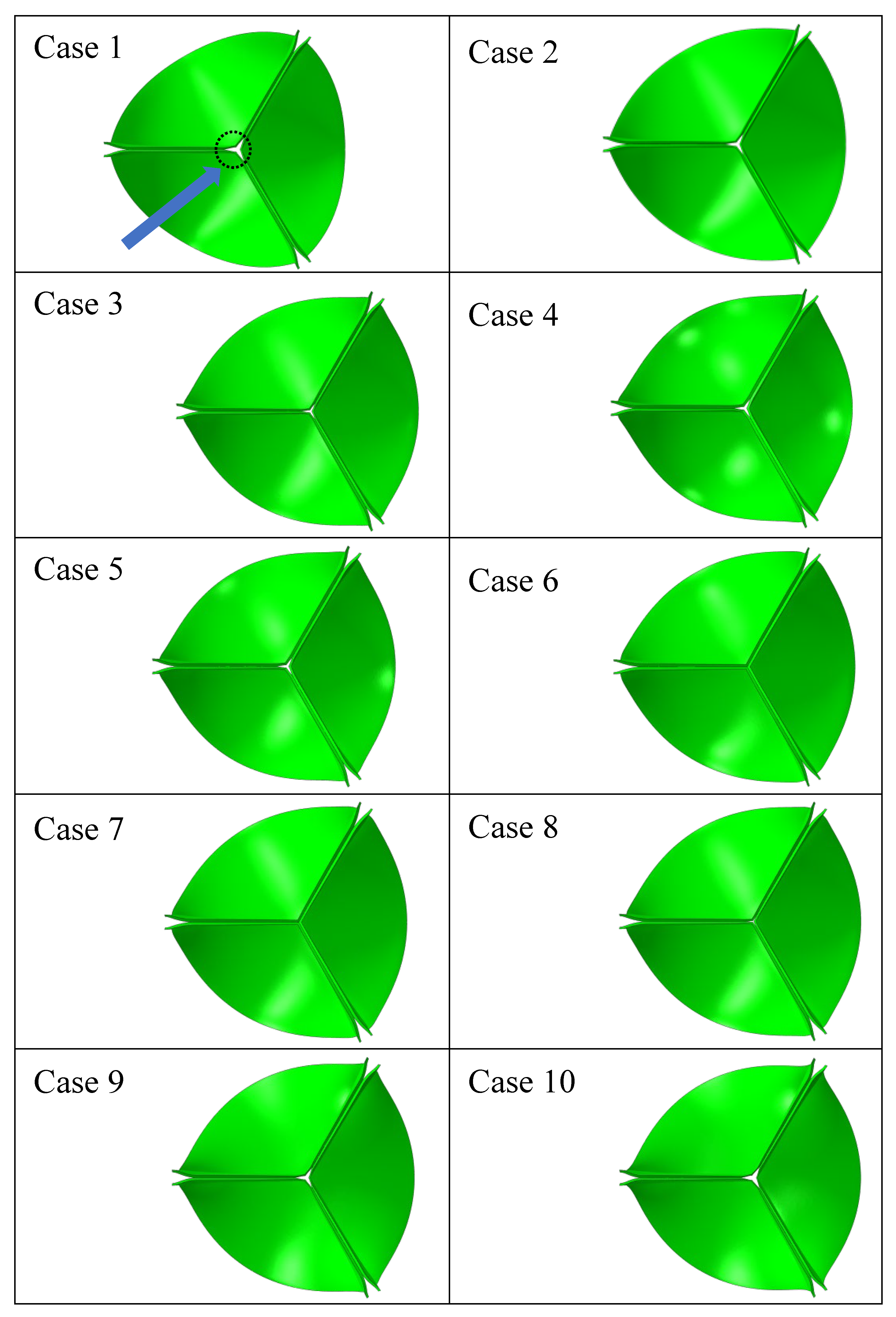
| Case Number | Leaflet Angle (Degree) | Radial Curvature | Circumferential Curvature |
|---|---|---|---|
| 1 | 54.4 | linear | Parabola |
| 2 | 54.4 | Linear | Spline |
| 3 | 54.4 | Linear | Circular |
| 4 | - | Parabola | Circular |
| 5 | - | Circular | Circular |
| 6 | 45.0 | Linear | Circular |
| 7 | 47.85 | Linear | Circular |
| 8 | 50.97 | Linear | Circular |
| 9 | 58.14 | Linear | Circular |
| 10 | 62.22 | Linear | Circular |
| (kPa). | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 8.297 | 151.375 | 0 | 56.994 | 0 | 0 | 50 | 200 | 60 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabiri, Y.; Narine, K. The Roles of Leaflet Geometry in the Structural Deterioration of Bioprosthetic Aortic Valves. Prosthesis 2025, 7, 86. https://doi.org/10.3390/prosthesis7040086
Dabiri Y, Narine K. The Roles of Leaflet Geometry in the Structural Deterioration of Bioprosthetic Aortic Valves. Prosthesis. 2025; 7(4):86. https://doi.org/10.3390/prosthesis7040086
Chicago/Turabian StyleDabiri, Yaghoub, and Kishan Narine. 2025. "The Roles of Leaflet Geometry in the Structural Deterioration of Bioprosthetic Aortic Valves" Prosthesis 7, no. 4: 86. https://doi.org/10.3390/prosthesis7040086
APA StyleDabiri, Y., & Narine, K. (2025). The Roles of Leaflet Geometry in the Structural Deterioration of Bioprosthetic Aortic Valves. Prosthesis, 7(4), 86. https://doi.org/10.3390/prosthesis7040086





