Masticatory Efficacy Following Implant Rehabilitation: Objective Assessment and Patient Perception Through Two-Color Mixing Test and Viewgum® Software
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Study Type
2.2. Participant Selection
- Surgical/prosthetic protocols (according to manufacturer’s instructions);
- Operator expertise (all implants placed by A.S-P.);
- Assessment methods (single calibrated examiner A.S-O.).
- Individuals aged 18 years or older.
- Patients who provided informed consent to participate in the study.
- Patients scheduled for dental implant placement.
- Patients without periodontal disease.
- Patients from whom pre- and postimplant placement samples could be collected, including after crown placement, during the data collection period.
- Patients with ≤4 missing teeth to be replaced with implants.
- Patients with multiple dental absences.
- Fully edentulous patients.
- Patients who did not provide written informed consent.
- Patients unable to understand the instructions for the tests.
- Patients who did not complete their treatment or attend follow-up visits.
- Patients whose definitive implant crown placement was scheduled outside the data collection period.
- Specific medical conditions that could affect masticatory function (uncontrolled metabolic diseases (HbA1c > 7%), neuromuscular disorders, medications affecting mastication).
2.3. Data Collection: Clinical Samples
- Patient medical history forms.
- Information sheet for adult participants.
- Informed consent form.
- Disposable blue nitrile gloves (Luna brand, OMARE SL, Lorquí, Spain).
- Surgical masks, blue, 3-layer, type IIR (OMARE SL, Lorquí, Spain).
- Hue-Check Gum® chewing gum, unflavored and lightly sweetened (Muri bei Bern, Switzerland).
- Transparent rectangular plastic bags, Pakico Zip (Coplasem, Derio, Bizkaia, Spain).
- Tongue depressors, approximately 1 mm thick.
- Glass slabs.
- iPhone 11 Pro (i11Pro): triple 12 MP camera, 1/2.55″ sensor, 1.4 µm pixel size, f/1.8 aperture (Apple Inc., Los Altos, CA, USA).
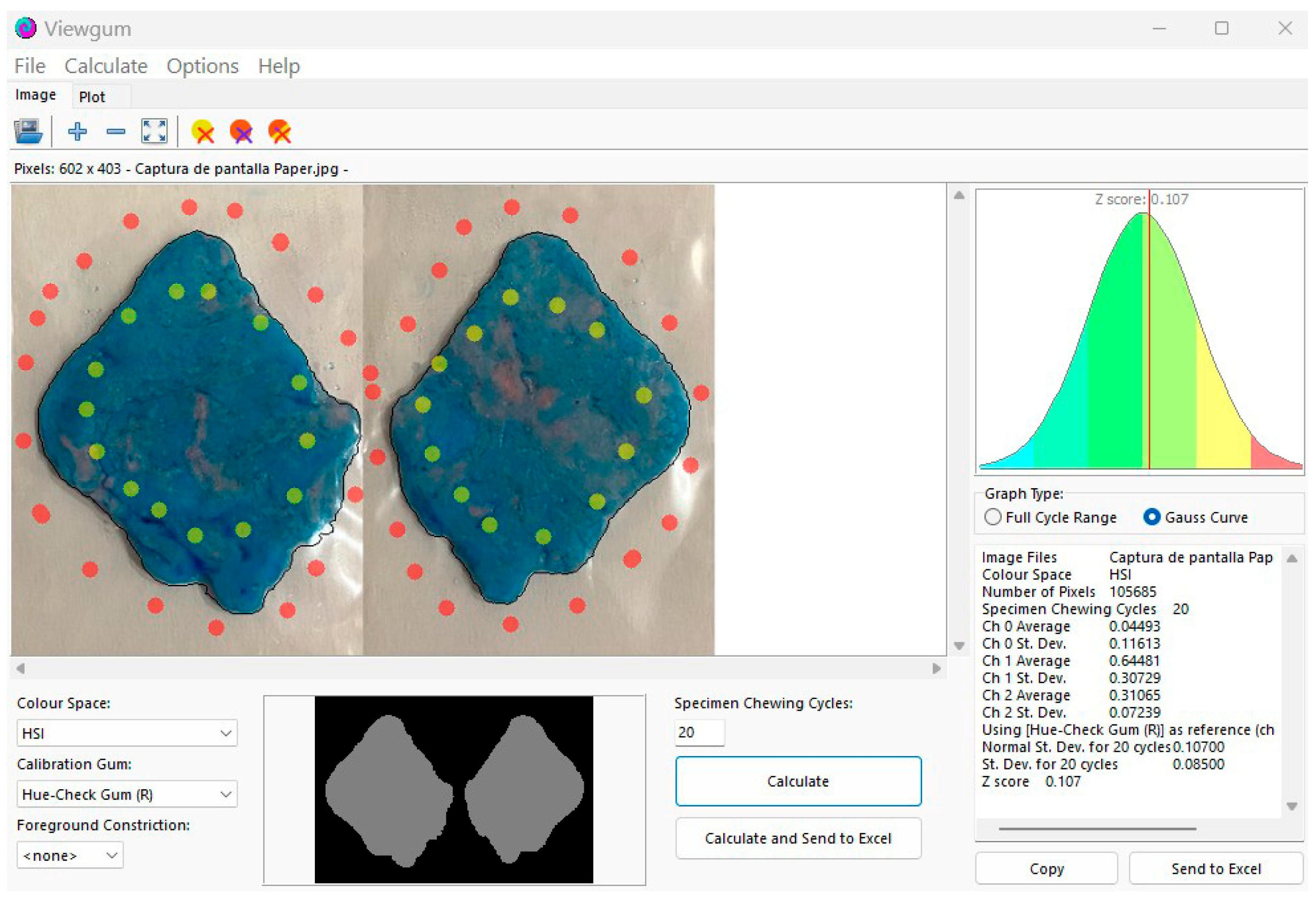
- Viewgum® software (dHAL Software, Kifissia, Greece).
- A 10 cm visual analog scale (VAS) sheet was used to quantify patient satisfaction with masticatory efficiency. One end, labeled “Could not be worse”, corresponds to a subjective masticatory capacity score of 0 (0 cm), whereas the other end, labeled “Could not be better”, corresponds to a score of 10 (10 cm). In this study, the VAS sheet was administered both before and after implant placement (Figure 1).
- A 20 cm transparent plastic ruler (FAIBO, Sucesores de Vda. E. Fajeda, S.L., Girona, Spain).
- This study was conducted using internal funding from the Periodontology Unit, with no financial or material support from product manufacturers. The authors declare no competing interests related to Hue-Check Gum® (Hue-Check GmbH) or ViewGum® (dHAL Software). Products were purchased at market price without vendor involvement in study design, execution, or analysis.
2.4. Procedure for Data Collection
2.5. Data Analysis
2.6. Statistical Analysis
- Descriptive Analysis: This analysis determined the maximum and minimum values, means, and standard deviations for the numerical variables. The frequencies of qualitative and scale variables were also analyzed, and box plots were generated and grouped by implant class.
- Inferential Analysis: Normality was assessed using the Shapiro–Wilk test. When a variable did not follow a normal distribution according to the Shapiro–Wilk test, the Wilcoxon signed-rank test was applied to assess statistically significant differences in the variables (e.g., VOH values) before and after prosthesis placement. A correlation coefficient and a linear regression analysis were performed to evaluate the relationship between the objective assessment (VOH values) and the subjective assessment using the VAS (cm). A value of p < 0.05 was considered to indicate statistical significance.
2.7. Ethical Considerations
3. Results
- Canine guidance (n = 18, 60%);
- Group function (n = 12, 40%).
3.1. Descriptive Statistics of Quantitative Variables
3.2. Inferential Statistics
3.2.1. Shapiro–Wilk Normality Tests
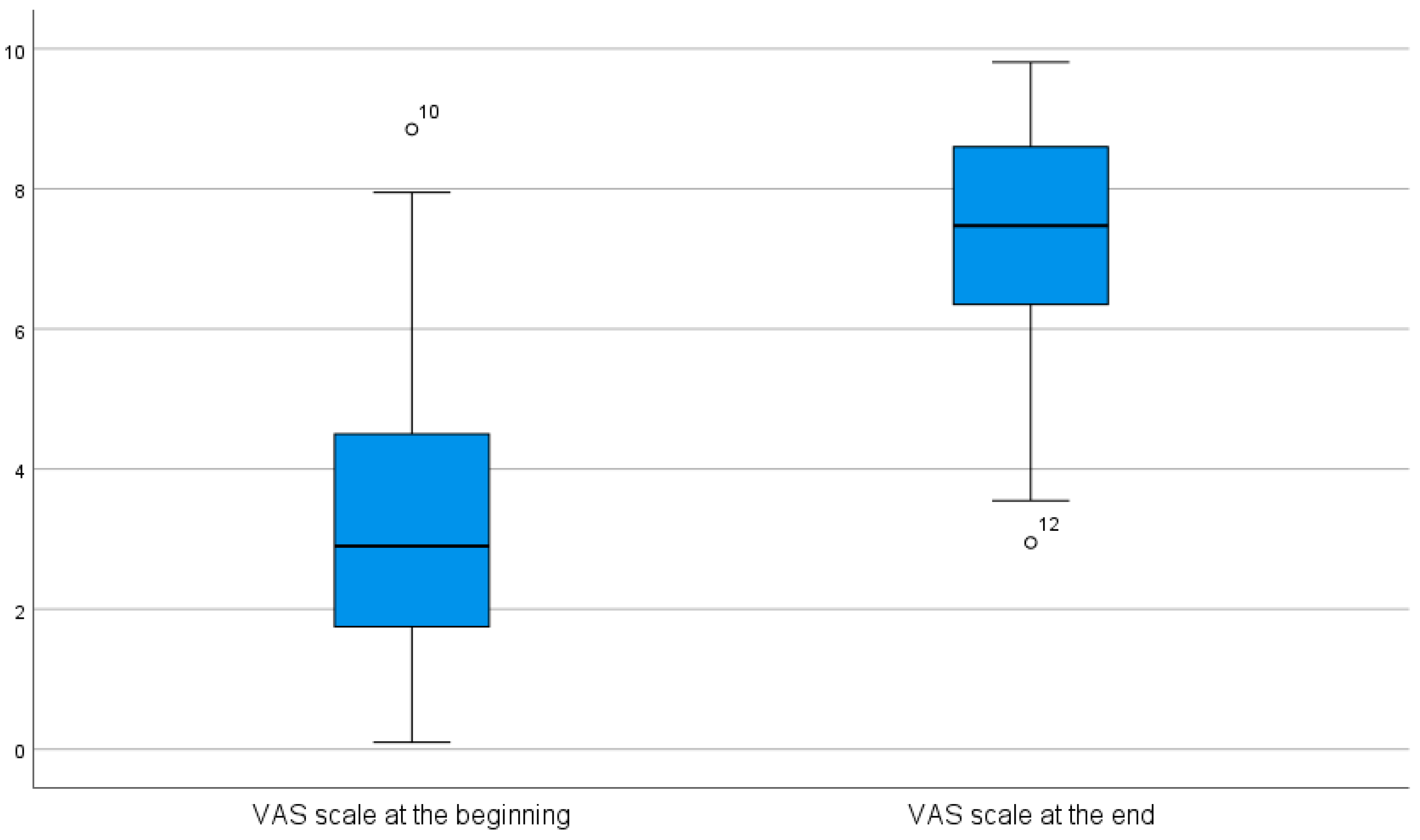
3.2.2. Wilcoxon Signed-Rank Test for Paired Samples of VAS Values
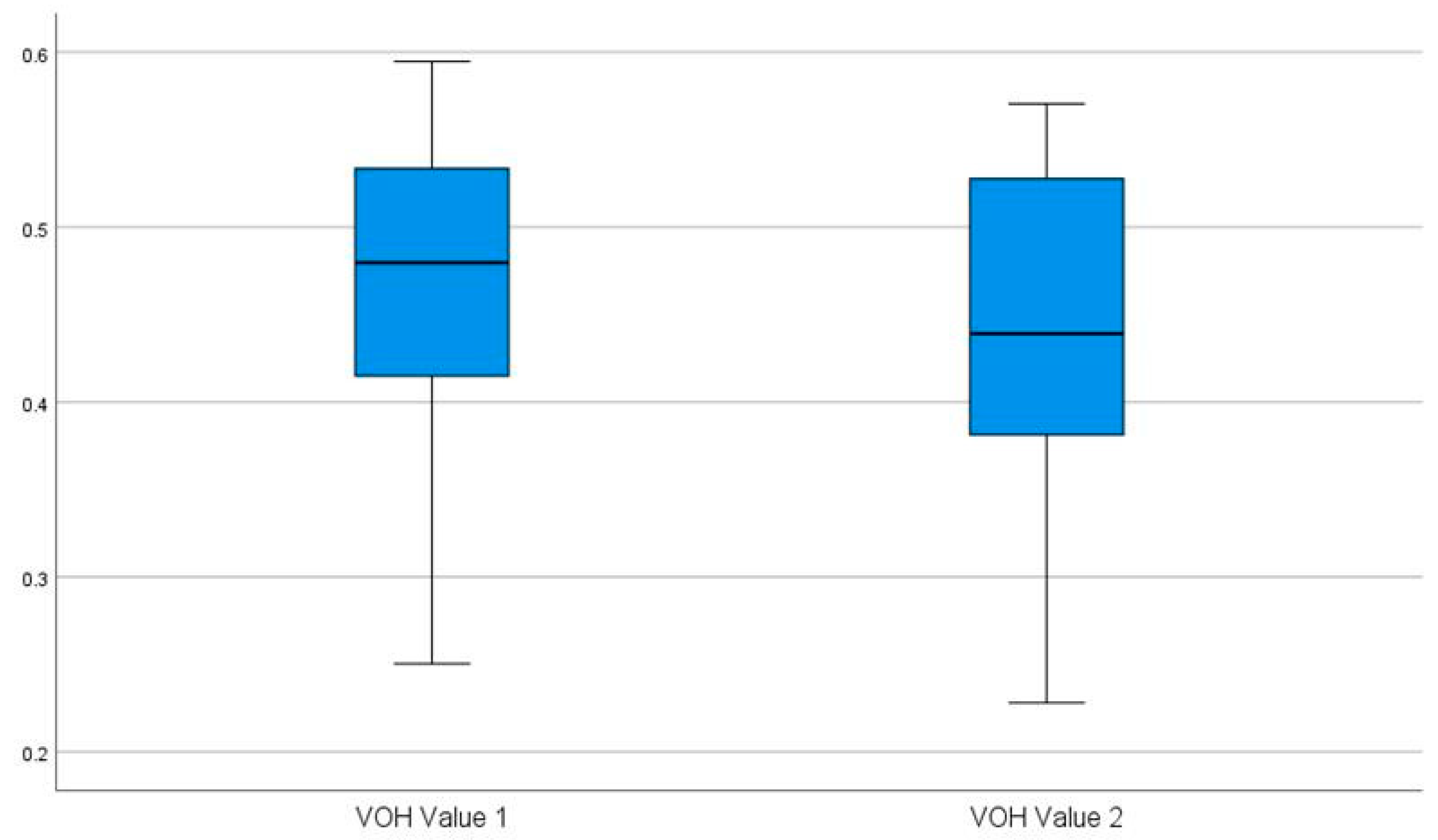
3.2.3. Wilcoxon Signed-Rank Test for Paired Samples of HOV Values
3.2.4. Correlation
4. Discussion
4.1. Discussion of Materials and Methods
4.2. Interpretation of Findings
4.2.1. Discussion of Objective Evaluations
4.2.2. Discussion of Subjective Evaluations
4.2.3. Discussion of the Correlation Between Objective and Subjective Evaluations
4.3. Limitations and Future Directions
5. Conclusions
- The determination of masticatory performance using the chewing gum test and colorimetric analysis effectively differentiated between pre- and postprosthetic masticatory efficiency.
- Regardless of objective changes, all patients reported higher satisfaction in their subjective assessment using the visual analog scale (VAS).
- Despite the statistically significant differences observed before and after prosthesis placement in terms of both VAS score and VOH values, no correlation was found between them.
- It appears that subjective assessment using VAS scores and objective assessment via VOH values measure different domains of masticatory performance.
- The use of colorimetric analysis and smartphone cameras offers a feasible and objective approach for assessing masticatory performance, supporting its potential integration in clinical settings for monitoring postprosthetic outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orchardson, R.; Cadden, S.W. Mastication and swallowing: 1. Functions, performance and mechanisms. Dent. Update 2009, 36, 327–330, 332–334, 337. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, A. Assessment of mastication with implications for oral rehabilitation: A review. J. Oral Rehabil. 2011, 38, 754–780. [Google Scholar] [CrossRef] [PubMed]
- Arce-Tumbay, J.; Sanchez-Ayala, A.; Sotto-Maior, B.S.; Senna, P.M.; Campanha, N.H. Mastication in subjects with extremely shortened dental arches rehabilitated with removable partial dentures. Int. J. Prosthodont. 2011, 24, 517–519. [Google Scholar] [PubMed]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef]
- Kumar, A.; Kothari, M.; Grigoriadis, A.; Trulsson, M.; Svensson, P. Bite or brain: Implication of sensorimotor regulation and neuroplasticity in oral rehabilitation procedures. J. Oral Rehabil. 2018, 45, 323–333. [Google Scholar] [CrossRef]
- Schimmel, M.; Leemann, B.; Herrmann, F.R.; Kiliaridis, S.; Schnider, A.; Müller, F. Masticatory function and bite force in stroke patients. J. Dent. Res. 2011, 90, 230–234. [Google Scholar] [CrossRef]
- Budtz-Jörgensen, E. Restoration of the partially edentulous mouth—A comparison of overdentures, removable partial dentures, fixed partial dentures and implant treatment. J. Dent. 1996, 24, 237–244. [Google Scholar] [CrossRef]
- Meena, A.; Jain, V.; Singh, N.; Arora, N.; Jha, R. Effect of implant-supported prosthesis on the bite force and masticatory efficiency in subjects with shortened dental arches. J. Oral Rehabil. 2014, 41, 87–92. [Google Scholar] [CrossRef]
- Jasser, E.; Salami, Z.; El Hage, F.; Makzoumé, J.; Boulos, P.J. Masticatory efficiency in implant-supported fixed complete dentures compared with conventional dentures: A randomized clinical trial by color-mixing analysis test. Int. J. Oral Maxillofac. Implants 2020, 35, 599–606. [Google Scholar] [CrossRef]
- Khalid, T.; Yunus, N.; Ibrahim, N.; Saleh, N.B.M.; Goode, D.; Masood, M. Assessment of masticatory function of mandibular implant-supported overdenture wearers: A 3-year prospective study. J. Prosthet. Dent. 2020, 124, 674–681. [Google Scholar] [CrossRef]
- Zhu, H.; Kang, Y.; Shan, X.; Ge, Y.; Cai, Z. Effect of dental rehabilitation on masticatory function following jaw reconstruction. Int. J. Oral Maxillofac. Implants 2022, 37, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Homsi, G.; Kumar, A.; Almotairy, N.; Wester, E.; Trulsson, M.; Grigoriadis, A. Assessment of masticatory function in older individuals with bimaxillary implant-supported fixed prostheses or with a natural dentition: A case-control study. J. Prosthet. Dent. 2023, 129, 871–877. [Google Scholar] [CrossRef]
- Pedroni-Pereira, A.; Marquezin, M.C.S.; Araujo, D.S.; Pereira, L.J.; Bommarito, S.; Castelo, P.M. Lack of agreement between objective and subjective measures in the evaluation of masticatory function: A preliminary study. Physiol. Behav. 2018, 184, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Shaddox, L.M.; Toda, C.; Paleari, A.G.; Pero, A.C.; Compagnoni, M.A. Methods for evaluation of masticatory efficiency in conventional complete denture wearers: A systematized review. Oral Health Dent. Manag. 2014, 13, 757–762. [Google Scholar] [PubMed]
- Kimoto, K.; Ogawa, T.; Garrett, N.R.; Toyoda, M. Assessment of masticatory performance: Methodologies and their application. Prosthodont. Res. Pract. 2004, 3, 33–45. [Google Scholar] [CrossRef]
- Halazonetis, D.J.; Schimmel, M.; Antonarakis, G.S.; Christou, P. Novel software for quantitative evaluation and graphical representation of masticatory efficiency. J. Oral Rehabil. 2013, 40, 329–335. [Google Scholar] [CrossRef]
- Schimmel, M.; Christou, P.; Miyazaki, H.; Halazonetis, D.; Herrmann, F.R.; Müller, F. A novel colourimetric technique to assess chewing function using two-coloured specimens: Validation and application. J. Dent. 2015, 43, 955–964. [Google Scholar] [CrossRef]
- Kaya, M.S.; Güçlü, B.; Schimmel, M.; Akyüz, S. Two-colour chewing gum mixing ability test for evaluating masticatory performance in children with mixed dentition: Validity and reliability study. J. Oral Rehabil. 2017, 44, 827–834. [Google Scholar] [CrossRef]
- Silva, L.C.; Nogueira, T.E.; Rios, L.F.; Schimmel, M.; Leles, C.R. Reliability of a two-colour chewing gum test to assess masticatory performance in complete denture wearers. J. Oral Rehabil. 2018, 45, 301–307. [Google Scholar] [CrossRef]
- Buser, R.; Ziltener, V.; Samietz, S.; Fontolliet, M.; Nef, T.; Schimmel, M. Validation of a purpose-built chewing gum and smartphone application to evaluate chewing efficiency. J. Oral Rehabil. 2018, 45, 845–853. [Google Scholar] [CrossRef]
- Schimmel, M.; Rachais, E.; Husain, N.A.H.; Müller, F.; Srinivasan, M.; Abou-Ayash, S. Assessing masticatory performance with a colour-mixing ability test using smartphone camera images. J. Oral Rehabil. 2022, 49, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.M.; Ng, S.K.; Corbet, E.F.; Leung, W.K. Non-surgical periodontal therapy improves oral health-related quality of life. J. Clin. Periodontol. 2012, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.K.; Betzler, C.; Kohnen, R.; Krisam, J.; Kim, T.S. Oral health-related quality of life in patients under supportive periodontal therapy. Acta Odontol. Scand. 2018, 76, 572–579. [Google Scholar] [CrossRef]
- Moya-Villaescusa, M.J.; Sánchez-Pérez, A.; Esparza-Marín, J.; Jornet-García, A.; Montoya-Carralero, J.M. Periodontal disease and nonsurgical periodontal therapy on the OHRQoL of the patient: A pilot study of case series. Dent. J. 2023, 11, 94. [Google Scholar] [CrossRef]
- Ali, Z.; Baker, S.R.; Shahrbaf, S.; Martin, N.; Vettore, M.V. Oral health-related quality of life after prosthodontic treatment for patients with partial edentulism: A systematic review and meta-analysis. J. Prosthet. Dent. 2019, 121, 59–68.e3. [Google Scholar] [CrossRef]
- Gonçalves, G.S.Y.; De Magalhães, K.M.F.; Rocha, E.P.; Dos Santos, P.H.; Assunção, W.G. Oral health-related quality of life and satisfaction in edentulous patients rehabilitated with implant-supported full dentures all-on-four concept: A systematic review. Clin. Oral Investig. 2022, 26, 83–94. [Google Scholar] [CrossRef]
- Parmar, R.P.; Bakutra, G.V.; Vishnoi, S.L.; Nadig, P.; Rana, R. Awareness and oral health related quality of life (OHRQoL) in patients with dental implants compared to tooth supported FPD: A questionnaire study. J. Oral Biol. Craniofac. Res. 2024, 14, 252–256. [Google Scholar] [CrossRef]
- Pavel, K.; Seydlova, M.; Dostalova, T.; Zdenek, V.; Chleborad, K.; Jana, Z.; Feberova, J.; Radek, H. Dental implants and improvement of oral health-related quality of life. Community Dent. Oral Epidemiol. 2012, 40 (Suppl. S1), 65–70. [Google Scholar] [CrossRef]
- Alzarea, B.K. Assessment and evaluation of quality of life (OHRQoL) of patients with dental implants using the oral health impact profile (OHIP-14)—A clinical study. J. Clin. Diagn. Res. 2016, 10, ZC57–ZC60. [Google Scholar] [CrossRef]
- Manly, R.S. Factors affecting masticatory performance and efficiency among young adults. J. Dent. Res. 1951, 30, 874–882. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, H. Factors affecting subjective and objective masticatory function in older adults: Importance of an integrated approach. J. Dent. 2021, 113, 103787. [Google Scholar] [CrossRef] [PubMed]
- Ek, S. Gender differences in health information behaviour: A Finnish population-based survey. Health Promot. Int. 2015, 30, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, C.M.; Abbink, J.H.; Van der Glas, H.W.; Janssen, N.G.; Van der Bilt, A. Mixing ability test compared with a comminution test in persons with normal and compromised masticatory performance. Eur. J. Oral Sci. 2009, 117, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, N.; Kalberer, N.; Müller, F.; Leles, C.R.; Schimmel, M.; Srinivasan, M. Comparison of smartphone-camera and conventional flatbed scanner images for analytical evaluation of chewing function. J. Oral Rehabil. 2020, 47, 1496–1502. [Google Scholar] [CrossRef]
- Nedeljković, Đ.; Lemić, A.M.; Pfićer, J.K.; Stančić, I.; Popovac, A.; Čelebić, A. Subjective and objective assessment of chewing performance in older adults with different dental occlusion. Med. Princ. Pract. 2023, 32, 110–116. [Google Scholar] [CrossRef]
- Amaral, C.F.; Pinheiro, M.A.; De Moraes, M.; Garcia, R.C.M.R. Psychometric analysis and masticatory efficiency of elderly people with single-implant overdentures. Int. J. Oral Maxillofac. Implants 2018, 33, 1383–1389. [Google Scholar] [CrossRef]
- Awad, A.G. ‘The patient’: At the center of patient-reported outcomes. Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 729–731. [Google Scholar] [CrossRef]
- Wadgave, U.; Khairnar, M.R. Parametric tests for Likert scale: For and against. Asian J. Psychiatr. 2016, 24, 67–68. [Google Scholar] [CrossRef]
- Huskisson, E.C.; Jones, J.; Scott, P.J. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol. Rehabil. 1976, 15, 185–187. [Google Scholar] [CrossRef]
- Peršić, S.; Palac, A.; Bunjevac, T.; Celebić, A. Development of a new chewing function questionnaire for assessment of a self-perceived chewing function. Community Dent. Oral Epidemiol. 2013, 41, 565–573. [Google Scholar] [CrossRef]
- Fan, Y.; Shu, X.; Lo, E.C.M.; Leung, K.C.M. Development and validation of a chewing function questionnaire for Chinese older adults. J. Dent. 2021, 104, 103520. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; John, M.T.; Inukai, M.; Aridome, K.; Igarahsi, Y. Validating an alternate version of the chewing function questionnaire in partially dentate patients. BMC Oral Health 2009, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Sischo, L.; Broder, H.L. Oral health-related quality of life: What, why, how, and future implications. J. Dent. Res. 2011, 90, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D. Derivation and validation of a short-form oral health impact profile. Community Dent. Oral Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef]
- John, M.T. Foundations of oral health-related quality of life. J. Oral Rehabil. 2020, 48, 355–359. [Google Scholar] [CrossRef]
- Collins, J. Improving patients’ oral health-related quality of life with aesthetic dentistry. Prim. Dent. J. 2013, 2, 61–65. [Google Scholar] [CrossRef]
- Vieira, R.A.; Melo, A.C.; Budel, L.A.; Gama, J.C.; De Mattias Sartori, I.A.; Thomé, G. Benefits of rehabilitation with implants in masticatory function: Is patient perception of change in accordance with the real improvement? J. Oral Implantol. 2014, 40, 263–269. [Google Scholar] [CrossRef]
- Homsi, G.; Karlsson, A.; Almotairy, N.; Trulsson, M.; Kumar, A.; Grigoriadis, A. Subjective and objective evaluation of masticatory function in patients with bimaxillary implant-supported prostheses. J. Oral Rehabil. 2023, 50, 140–149. [Google Scholar] [CrossRef]
- Geertman, M.E.; Slagter, A.P.; Van ‘t Hof, M.A.; Van Waas, M.A.; Kalk, W. Masticatory performance and chewing experience with implant-retained mandibular overdentures. J. Oral Rehabil. 1999, 26, 7–13. [Google Scholar] [CrossRef]



| Variable | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Number of present teeth | 26 | 25 | 27 | 0.78 |
| Number of missing teeth | 2 | 1 | 3 | 0.78 |
| Number of implants | 2.1 | 1 | 4 | 1.12 |
| VOH value of 1 | 0.462 | 0.250 | 0.595 | 0.094 |
| VOH value of 2 | 0.438 | 0.228 | 0.570 | 0.093 |
| VAS score before | 3.467 | 0.10 | 8.85 | 2.47 |
| VAS score after | 7.292 | 2.95 | 9.81 | 1.96 |
| Position | n | Pre-VOH (Mean) | Post-VOH (Mean) | ΔVOH | Pre-VAS (Mean) | Post-VAS (Mean) | ΔVAS |
|---|---|---|---|---|---|---|---|
| Anterior (AS/AI) | 7 | 0.472 | 0.444 | − 0.028 | 2.71 | 6.63 | + 3.92 |
| Posterior (PS/PI) | 23 | 0.417 | 0.417 | − 0.041 | 3.62 | 7.52 | + 3.90 |
| ID | Age | Sex | Implants | Position | ΔVOH | ΔVAS | Note |
|---|---|---|---|---|---|---|---|
| 12 | 49 | M | 2 | PI | − 0.033 | + 1.95 | Lowest satisfaction improvement |
| 27 | 63 | M | 1 | AS | −0.023 | + 9.6 | Larges perception gap |
| 10 | 79 | M | 3 | PS | −0.034 | + 0.96 | Minimal subjetive improvement |
| Implants | n | ΔVOH | ΔVAS | VOH Improvement vs Single (%) |
|---|---|---|---|---|
| 1 | 10 | −0.031 | + 3.5 | − |
| 2 | 8 | −0.038 | +3.8 | +22.6% |
| 3 | 7 | −0.042 | +4.1 | +35.5% |
| 4 | 5 | −0.047 | +4.3 | 51.6% |
| Sex | n | ΔVOH | ΔVAS | % |
|---|---|---|---|---|
| M | 16 | −0.037 | +3.7 | 12.3% |
| F | 14 | −0.039 | +4.2 | 15.8% |
| Variable | Value | gl | Sig. |
|---|---|---|---|
| Age in years | 0.986 | 30 | =0.949 |
| Number of present teeth | 0.808 | 30 | <0.001 * |
| Number of missing teeth | 0.808 | 30 | <0.001 * |
| Number of implants | 0.820 | 30 | <0.001 * |
| VAS score before | 0.938 | 30 | =0.079 * |
| VAS score after | 0.938 | 30 | =0.082 * |
| VOH value 1 | 0.934 | 30 | =0.063 * |
| VOH value 2 | 0.951 | 30 | =0.178 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Carralero, J.M.; Sánchez-Pérez, A.; Sánchez-Olaya, A.; Jornet-García, A.; Moya-Villaescusa, M.J. Masticatory Efficacy Following Implant Rehabilitation: Objective Assessment and Patient Perception Through Two-Color Mixing Test and Viewgum® Software. Prosthesis 2025, 7, 70. https://doi.org/10.3390/prosthesis7040070
Montoya-Carralero JM, Sánchez-Pérez A, Sánchez-Olaya A, Jornet-García A, Moya-Villaescusa MJ. Masticatory Efficacy Following Implant Rehabilitation: Objective Assessment and Patient Perception Through Two-Color Mixing Test and Viewgum® Software. Prosthesis. 2025; 7(4):70. https://doi.org/10.3390/prosthesis7040070
Chicago/Turabian StyleMontoya-Carralero, José María, Arturo Sánchez-Pérez, Alba Sánchez-Olaya, Alfonso Jornet-García, and María José Moya-Villaescusa. 2025. "Masticatory Efficacy Following Implant Rehabilitation: Objective Assessment and Patient Perception Through Two-Color Mixing Test and Viewgum® Software" Prosthesis 7, no. 4: 70. https://doi.org/10.3390/prosthesis7040070
APA StyleMontoya-Carralero, J. M., Sánchez-Pérez, A., Sánchez-Olaya, A., Jornet-García, A., & Moya-Villaescusa, M. J. (2025). Masticatory Efficacy Following Implant Rehabilitation: Objective Assessment and Patient Perception Through Two-Color Mixing Test and Viewgum® Software. Prosthesis, 7(4), 70. https://doi.org/10.3390/prosthesis7040070






