The Effect of Adding Bioactive Glass Infused with Strontium on the Surface Hardness and Surface Roughness Properties of a Heat-Cured Acrylic-Based Soft Liner
Abstract
1. Introduction
2. Materials and Methods
2.1. Phosphate Bioactive Glass Preparation
2.2. Mold and Specimen Preparation
2.3. Surface Hardness Test
2.4. Surface Roughness Test
2.5. Additional Tests
2.5.1. Field Emission Scanning Electron Microscope (FESEM)
2.5.2. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
2.5.3. Statistics
3. Results
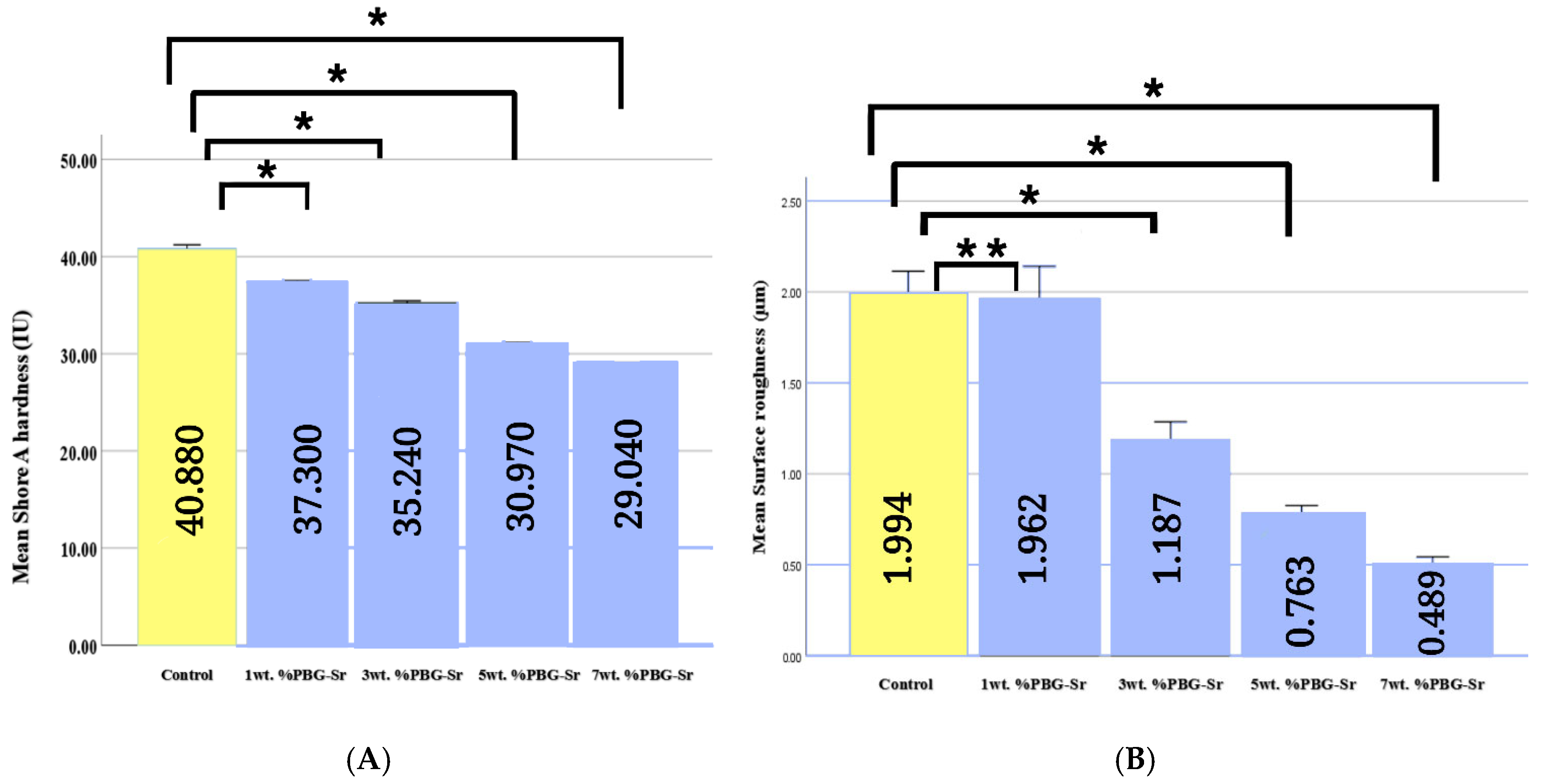
3.1. Surface Hardness and Surface Roughness
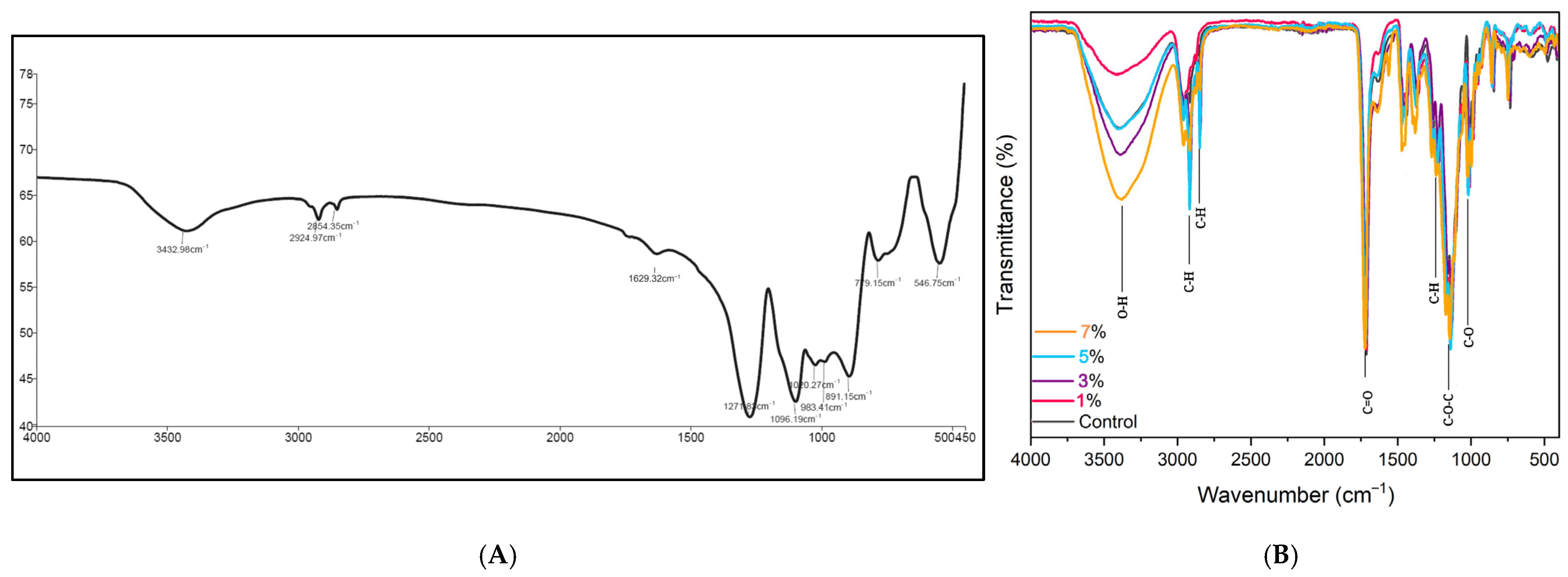
3.2. Fourier Transform Infrared Spectroscopy Analysis
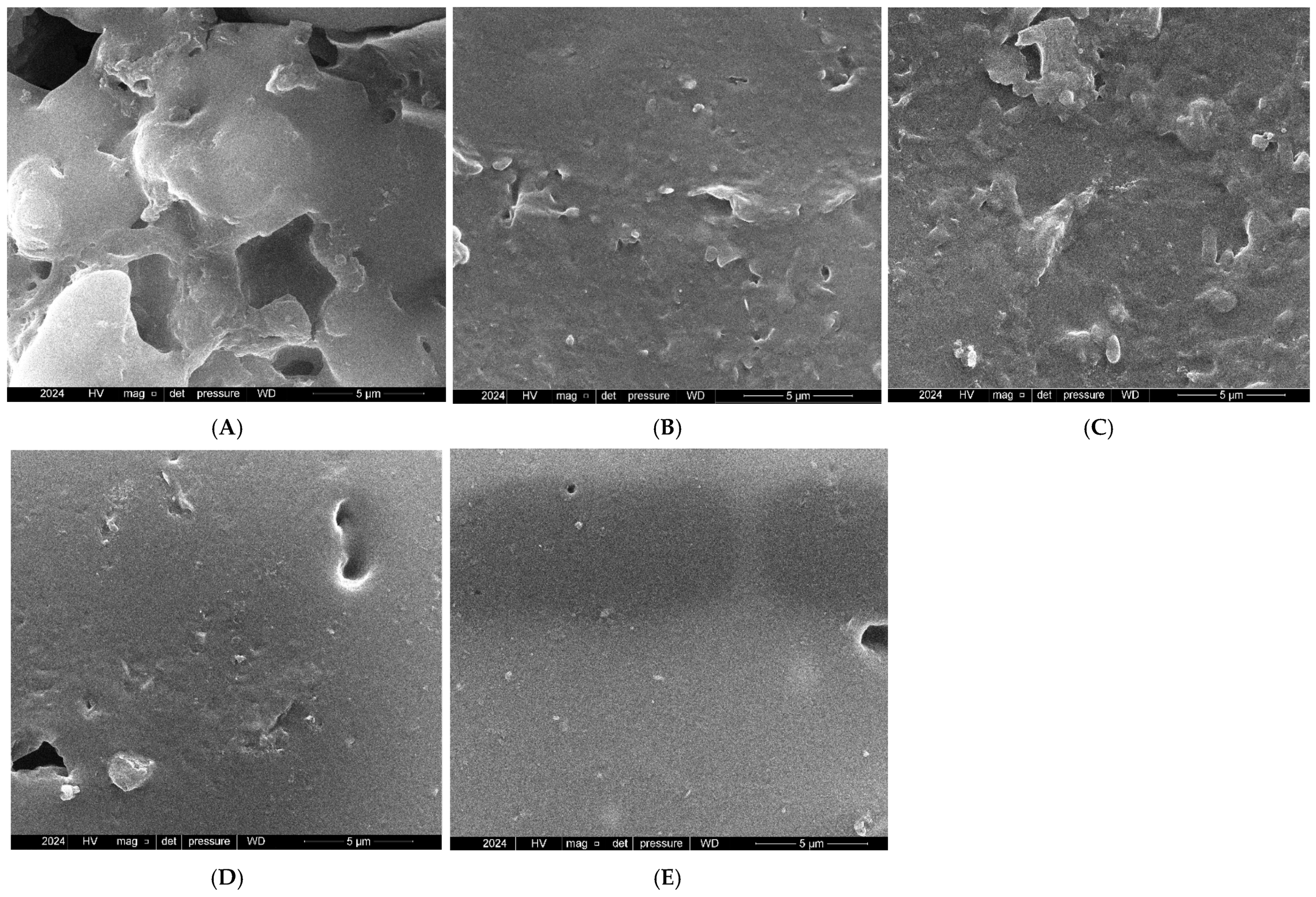
3.3. Field Emission Scanning Electron Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasegawa, Y.; Minakuchi, H.; Nishimura, M.; Nishio, K.; Yoshioka, F.; Ishii, T.; Watanabe, T.; Nishiyama, Y.; Sato, Y.; Yoshida, K.; et al. Effect of soft denture liners on complete denture treatments: A systematic review. J. Prosthodont. Res. 2024, 68, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Kumar, S.; Chowdhary, Z.; Bumb, P.; Kumar, C. Effect of Water Sorption and Solubility on Two Soft Denture Lining Materials Stored in Three Different Mediums. Int. J. Prosthodont. Restore Dent. 2023, 13, 129–136. [Google Scholar]
- Memon, M.R.; Memon, H.; Shoro, M.; Bhurgri, H.; Issrani, R.; Iqbal, A.; Khattak, O.; Altassan, M.; Almabadi, A.A.; Sultan, S.E.S.; et al. Effectiveness of Chitosan versus Natural Aloe Vera on Candida Adherence in Denture Soft Lining Material. Scientifica 2024, 2024, 9918914. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Alnazzawi, A.A.; Farghal, A.E.; Bakr, R.M.; Mahmoud, I.I. Impact of acrylic and Silicone-Based Soft-Liner materials on biting force and quality of life of the complete denture wearers: A randomized clinical trial. J. Clin. Med. 2023, 12, 2073. [Google Scholar] [CrossRef]
- Hamedirad, F.; Alikhasi, M.; Hasanzade, M. The effect of sandblasting on bond strength of soft liners to denture base resins: A Systematic Review and Meta-Analysis of In Vitro Studies. Int. J. Dent. 2021, 2021, 5674155. [Google Scholar] [CrossRef]
- Hashem, M.I. Advances in soft denture liners: An update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar]
- Jasim, B.S.; Alalwan, H.K.A.; Fatalla, A.A.; Al-Samaray, M.E. The impact of modified metallic nanoparticles on thermomechanical properties of PMMA soft liner. Nano Biomed. Eng. 2023, 15, 408–415. [Google Scholar] [CrossRef]
- Songsang, N.; Anunmana, C.; Pudla, M.; Eiampongpaiboon, T. Effects of Litsea cubeba Essential Oil Incorporated into Denture Soft Lining Materials. Polymers 2022, 14, 3261. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Omidvaran, A.; Eskandarion, S.; Shamshiri, A.R. Effect of Incorporation of Silver Nanoparticles on the Tensile Bond Strength of a Long term Soft Denture Liner. Eur. J. Dent. 2020, 14, 268–273. [Google Scholar] [CrossRef]
- Ahmed, A.Q.; Al-Hmedat, S.J.A.Z.; Hanweet, D.M.; Haider, J. Assessing the Antifungal Activity of a Soft Denture Liner Loaded with Titanium Oxide Nanoparticles (TiO2 NPs). Dent. J. 2023, 11, 90. [Google Scholar] [CrossRef]
- Waly, G.H. Effect of incorporating undoped or silver-doped photocatalytic titanium dioxide on the antifungal effect and dynamic viscoelastic properties of long-term acrylic denture liners. Future Dent. J. 2018, 4, 8–15. [Google Scholar] [CrossRef]
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Zmudzki, J.; Król, W.; Lukaszczyk, J. Antifungal activity of denture soft lining material modified by Silver Nanoparticles—A pilot study. Int. J. Mol. Sci. 2011, 12, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; Razak, N.A.B.A.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2021, 33, 3. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of human clinical trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef]
- Silva, A.V.; Gomes, D.D.S.; De Sousa Victor, R.; De Lima Santana, L.N.; Neves, G.A.; Menezes, R.R. Influence of strontium on the biological behavior of bioactive glasses for bone regeneration. Materials 2023, 16, 7654. [Google Scholar] [CrossRef]
- Go, H.B.; Lee, M.J.; Seo, J.Y.; Byun, S.Y.; Kwon, J.S. Mechanical properties and sustainable bacterial resistance effect of strontium-modified phosphate-based glass microfiller in dental composite resins. Sci. Rep. 2023, 13, 17763. [Google Scholar] [CrossRef]
- Gobbo, V.A.; Parihar, V.S.; Prato, M.; Kellomäki, M.; Vernè, E.; Spriano, S.; Massera, J. Surface modification of silicate, borosilicate and phosphate bioactive glasses to improve/control protein adsorption: PART I. Ceram. Int. 2022, 49, 1261–1275. [Google Scholar] [CrossRef]
- Ryu, J.H.; Mangal, U.; Lee, M.J.; Seo, J.Y.; Jeong, I.J.; Park, J.Y.; Na, J.Y.; Lee, K.J.; Yu, H.S.; Cha, J.K.; et al. Effect of strontium substitution on functional activity of phosphate-based glass. Biomater. Sci. 2023, 11, 6299–6310. [Google Scholar] [CrossRef]
- ISO 10139-2:2016; Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use. ISO: Geneva, Switzerland, 2016.
- Ivana, I.; Nasution, I.D.; Nasution, D.Y. Effect of sealer coating on hardness and water sorption data of soft denture lining materials. Data Brief 2021, 36, 107083. [Google Scholar] [CrossRef] [PubMed]
- Herla, M.; Boening, K.; Meissner, H.; Walczak, K. Mechanical and Surface Properties of Resilient Denture Liners Modified with Chitosan Salts. Materials 2019, 12, 3518. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, I.U.; Yanikoğlu, N.D.; Kul, E.; Duymuş, Z.Y.; Sağsöz, N.P. Effect of sealer coating and storage methods on the surface roughness of soft liners. J. Prosthet. Dent. 2015, 115, 371–376. [Google Scholar] [CrossRef]
- Stoch, P.; Stoch, A.; Ciecinska, M.; Krakowiak, I.; Sitarz, M. Structure of phosphate and iron-phosphate glasses by DFT calculations and FTIR/Raman spectroscopy. J. Non-Cryst. Solids 2016, 450, 48–60. [Google Scholar] [CrossRef]
- Stuart, B.W.; Stan, G.E.; Popa, A.C.; Carrington, M.J.; Zgura, I.; Necsulescu, M.; Grant, D.M. New solutions for combatting implant bacterial infection based on silver nano-dispersed and gallium incorporated phosphate bioactive glass sputtered films: A preliminary study. Bioact. Mater. 2021, 8, 325–340. [Google Scholar] [CrossRef]
- Rajkumar, G.; Rajendran, V.; Aravindan, S. Role of MgO on the HAp forming ability in phosphate based glasses. Ceram. Int. 2012, 38, 3781–3790. [Google Scholar] [CrossRef]
- Ghanavati, S.; Petit, L.; Massera, J. New Mg/Sr phosphate bioresorbable glass system with enhanced sintering properties. J. Non-Cryst. Solids 2023, 616, 122446. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, X.; Hu, M.; Meng, Y.; Zhao, J.; Tan, Y.; Luo, X.; Wang, C.; Ma, J.; Sun, Z.; et al. In Situ Enzymatic Reaction Generates Magnesium-Based Mineralized Microspheres with Superior Bioactivity for Enhanced Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2300727. [Google Scholar] [CrossRef]
- Elgharbawy, H.; Hemeda, O.M.; Henaish, A.M.A. Exploring the potential of new boron-phosphate bioactive glass doped with lanthanum and zinc oxides for bone regeneration: In vitro study. J. Mol. Struct. 2024, 1307, 137958. [Google Scholar] [CrossRef]
- Elangovan, U.; Anbarasu, K.; Shanmugam, R.; Veeramuthu, D.; John, J.G. Biological Properties of Mycosynthesized Novel Strontium Oxide Nanoparticles from Biomass Free Filtrate of Endophytic Fungus Aspergillus sp. LCJ315. BioNanoScience 2024, 14, 2145–2158. [Google Scholar] [CrossRef]
- Sun, L.; Li, S.; Yang, K.; Wang, J.; Li, Z.; Dan, N. Polycaprolactone strengthening keratin/bioactive glass composite scaffolds with double cross-linking networks for potential application in bone repair. J. Leather Sci. Eng. 2022, 4, 1. [Google Scholar] [CrossRef]
- Ikhmayies, S.J. Advances in Glass Research; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Mohan Babu, M.; Syam Prasad, P.; Hima Bindu, S.; Prasad, A.; Venkateswara Rao, P.; Putenpurayil Govindan, N.; Veeraiah, N.; Özcan, M. Investigations on Physico-Mechanical and Spectral studies of Zn2+ doped P2O5-Based bioglass System. J. Compos. Sci. 2020, 4, 129. [Google Scholar] [CrossRef]
- Popescu, M.C.; Froidevaux, J.; Navi, P.; Popescu, C.M. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2012, 1033, 176–186. [Google Scholar] [CrossRef]
- Aziz, R.; Sadoon, M. Surface hardness and fourier transform infrared spectroscopical characterization of bioactive modified chairside hard denture reliner. Al-Rafidain Dent. J. 2024, 24, 231–247. [Google Scholar] [CrossRef]
- Noori, A.A.; Jaber, M.A. Evaluation the effect of incorporation of different herbal extract powders (Either neem or Aloe vera) on thermal conductivity and shear bond strength of acrylic soft denture liner material. Tikrit J. Dent. Sci. 2023, 10, 35–46. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. Dyna 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Habibi, H.; Alam, A.S.; Prasetyawan, O.; Supriyadi, G. FTIR and SEM analysis of breakdown XLPE cable insulation. In Proceedings of the 2021 3rd International Conference on High Voltage Engineering and Power Systems (ICHVEPS), Bandung, Indonesia, 5–6 October 2021; pp. 314–317. [Google Scholar] [CrossRef]
- Alsaiari, M.; Khan, G.; Khan, M.A.; Liaqat, S.; Alkorbi, A.S.; Irfan, M.; Rizk, M.A.; Muhammad, N. Evaluation of the effect of lignin on mechanical, water sorption, and antifungal properties of silicone based denture soft liners. Silicon 2023, 15, 6121–6134. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Denture Liners: A Systematic Review relative to adhesion and mechanical properties. Sci. World J. 2019, 2019, 6913080. [Google Scholar] [CrossRef]
- Alsaiari, M.; Roghani, K.; Liaqat, S.; Alkorbi, A.S.; Sharif, F.; Irfan, M.; Rizk, M.A.; Uroos, M.; Ahmad, N.; Muhammad, N. Effect of ionic liquids on mechanical, physical, and antifungal properties and biocompatibility of a soft denture lining material. ACS Omega 2023, 8, 27300–27311. [Google Scholar] [CrossRef]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Preparation and characterization of acrylic resins with bioactive glasses. Sci. Rep. 2022, 12, 16624. [Google Scholar] [CrossRef]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.Q.; Ali, M.M.M. The Influence of Titanium Dioxide Nanoparticles Incorporation into Soft Denture Lining Material on Candida albicans Adherence and Some Mechanical and Physical Properties. J. Pure Appl. Microbiol. 2018, 12, 783–791. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Yuan, P.; Wang, L.; Li, X.; Lei, B. Multifunctional bioactive glass nanoparticles: Surface-Interface decoration and biomedical applications. Regen. Biomater. 2024, 11, rbae110. [Google Scholar] [CrossRef]
- Zhao, H.; Tsigkou, O.; Chen, X. The effect of bioactive glass particle size on viscosity, stickiness and packability of resin composites. Next Mater. 2024, 5, 100265. [Google Scholar] [CrossRef]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. 3D printed denture base material: The effect of incorporating TiO2 nanoparticles and artificial ageing on the physical and mechanical properties. Dent. Mater. 2023, 39, 1122–1136. [Google Scholar] [CrossRef]
- Lopes, P.; Garcia, M.; Fernandes, M.; Fernandes, M. Properties and osteoblast cytocompatibility of self-curing acrylic cements modified by glass fillers. J. Biomater. Appl. 2012, 28, 498–513. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.M. Bioactive glass nanoparticles: From synthesis to materials design for biomedical applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef]
- Nayak, C.; Balani, K. Effects of reinforcements and gamma-irradiation on wear performance of ultra-high molecular weight polyethylene as acetabular cup liner in hip-joint arthroplasty: A review. J. Appl. Polym. Sci. 2021, 138, 51275. [Google Scholar] [CrossRef]
- Jang, E.J.; Hong, Y.J.; Jeong, Y.H.; Kim, K.E.; Jo, E.S.; Lee, M.J.; Yang, S.Y. In vitro antifungal and physicochemical properties of polymerized acrylic resin containing strontium-modified phosphate-based glass. BMC Oral Health 2024, 24, 775. [Google Scholar] [CrossRef]
- Chladek, G.; Kalamarz, I.; Pakieła, W.; Barszczewska-Rybarek, I.; Czuba, Z.; Mertas, A. A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties. Materials 2024, 17, 902. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.; Kula, P.; Chladek, G. Review of the Anti-Candida albicans Activity and Physical Properties of Soft Lining Materials Modified with Polyene Antibiotics, Azole Drugs, and Chlorohexidine Salts. Materials 2024, 17, 5383. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Raszewski, Z.; Chojnacka, K.; Mikulewicz, M.; Kulbacka, J.; Alaqeely, R.; Mirdad, A.; Haider, J. Evaluating the Translucency, Surface Roughness, and Cytotoxicity of a PMMA Acrylic Denture Base Reinforced with Bioactive Glasses. J. Funct. Biomater. 2023, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.G.; De Sousa, E.J.B.; Hotta, J.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Surface properties of temporary soft liners modified by minimum inhibitory concentrations of antifungals. Braz. Dent. J. 2017, 28, 158–164. [Google Scholar] [CrossRef]
- Raghunath, S.M.; Jei, J.B.; Muthukumar, B. Characterization of nanoceria-modified silicone soft liners: Surface morphology, hardness, wettability, cytotoxicity, and antifungal properties in artificial saliva—An in vitro study. J. Oral Biol. Craniofacial Res. 2024, 14, 614–619. [Google Scholar] [CrossRef]
- Naser, H.J.; Abdul-Ameer, F.M. Evaluating the effect of lemongrass essential oil addition on some properties of heat cure acrylic Soft-Lining material. Med. J. Babylon. 2022, 19, 646–652. [Google Scholar] [CrossRef]
- Gad, M.M.; Abu-Rashid, K.; Alkhaldi, A.; Alshehri, O.; Khan, S.Q. Evaluation of the effectiveness of bioactive glass fillers against Candida albicans adhesion to PMMA denture base materials: An in vitro study. Saudi Dent. J. 2022, 34, 730–737. [Google Scholar] [CrossRef]
- Le, P.H.; Linklater, D.P.; Medina, A.A.; MacLaughlin, S.; Crawford, R.J.; Ivanova, E.P. Impact of multiscale surface topography characteristics on Candida albicans biofilm formation: From cell repellence to fungicidal activity. Acta Biomater. 2024, 177, 20–36. [Google Scholar] [CrossRef]
- Bail, M.; Jorge, J.H.; Urban, V.M.; Campanha, N.H. Surface roughness of acrylic and Silicone-Based soft liners: In vivo study in a Rat model. J. Prosthodont. 2013, 23, 146–151. [Google Scholar] [CrossRef]
- Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of antibacterial Silver-Releasing filler on the physicochemical properties of Poly(Methyl methacrylate) denture base material. Materials 2019, 12, 4146. [Google Scholar] [CrossRef]

| Properties | Groups | Sum of Squares | df | Mean Square | F | p-Value |
|---|---|---|---|---|---|---|
| Surface hardness | Between groups | 911.915 | 4 | 227.979 | 2428.177 | 0.000 |
| Within groups | 4.225 | 45 | 0.094 | |||
| Total | 916.140 | 49 | ||||
| Surface roughness | Between groups | 0.046 | 5 | 0.009 | 121.552 | 0.000 |
| Within groups | 0.004 | 54 | 0.000075 | |||
| Total | 0.050 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielewi, N.H.; Abdul-Ameer, F.M. The Effect of Adding Bioactive Glass Infused with Strontium on the Surface Hardness and Surface Roughness Properties of a Heat-Cured Acrylic-Based Soft Liner. Prosthesis 2025, 7, 69. https://doi.org/10.3390/prosthesis7040069
Ielewi NH, Abdul-Ameer FM. The Effect of Adding Bioactive Glass Infused with Strontium on the Surface Hardness and Surface Roughness Properties of a Heat-Cured Acrylic-Based Soft Liner. Prosthesis. 2025; 7(4):69. https://doi.org/10.3390/prosthesis7040069
Chicago/Turabian StyleIelewi, Nada Hussien, and Faiza M. Abdul-Ameer. 2025. "The Effect of Adding Bioactive Glass Infused with Strontium on the Surface Hardness and Surface Roughness Properties of a Heat-Cured Acrylic-Based Soft Liner" Prosthesis 7, no. 4: 69. https://doi.org/10.3390/prosthesis7040069
APA StyleIelewi, N. H., & Abdul-Ameer, F. M. (2025). The Effect of Adding Bioactive Glass Infused with Strontium on the Surface Hardness and Surface Roughness Properties of a Heat-Cured Acrylic-Based Soft Liner. Prosthesis, 7(4), 69. https://doi.org/10.3390/prosthesis7040069






