AI-Enhanced Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control Incorporating Anthropometric Measures
Abstract
1. Introduction
- (a)
- A lack of direct user control: the current force control mechanism hinders natural interaction by not allowing users to exert direct control over the force.
- (b)
- Neglecting influential factors: omitting factors such as the fat level and muscle mass undermines the accuracy of EMG-based control mechanisms.
- (c)
- Maintenance and durability concerns: regular maintenance and wear and tear impact the longevity of force feedback components, including contact and non-contact sensors.
- (d)
- Subjectivity in force feedback: customizable control options are necessary to cater to individual variances in force intensity, timing, and sensory preferences.
- (e)
- Power demands and device weight: the integration of force feedback mechanisms imposes power requirements and increases the weight of prosthetic devices, posing challenges to battery life and practicality.
- (f)
- Insufficient fine motor control: the existing force feedback control in prosthetic hands may not deliver the required precision for delicate manipulation and fine motor control tasks.
- (a)
- This study thoroughly examines the influence of body composition factors—such as the fat level, muscle mass, and subcutaneous fat—on EMG signal quality, identifying these as crucial parameters for accurate EMG-based control. Through bio-impedance analysis (BIA) for body fat measurement, the research minimizes manual errors, ensuring reliable inputs for the model. A regression model is developed to explore the correlations between EMG data, the force, and additional recorded features, enabling the identification and assessment of key variables.
- (b)
- By using EMG as the primary control mechanism, the approach reduces dependence on external sensors, which enhances durability and minimizes maintenance. Additionally, the study demonstrates that optional sensors can be selectively integrated for safety or specific adjustments, ensuring flexibility in control systems for prosthetic applications.
- (c)
- The maximum voluntary contraction (MVC) is used as a baseline, enabling consistent force control across diverse individuals. By investigating the EMG output at varied grip force levels, including 25% of the MVC, the study illustrates that, with training, users can achieve refined motor control even at low force levels, supporting realistic and adaptive control for prosthetic use.
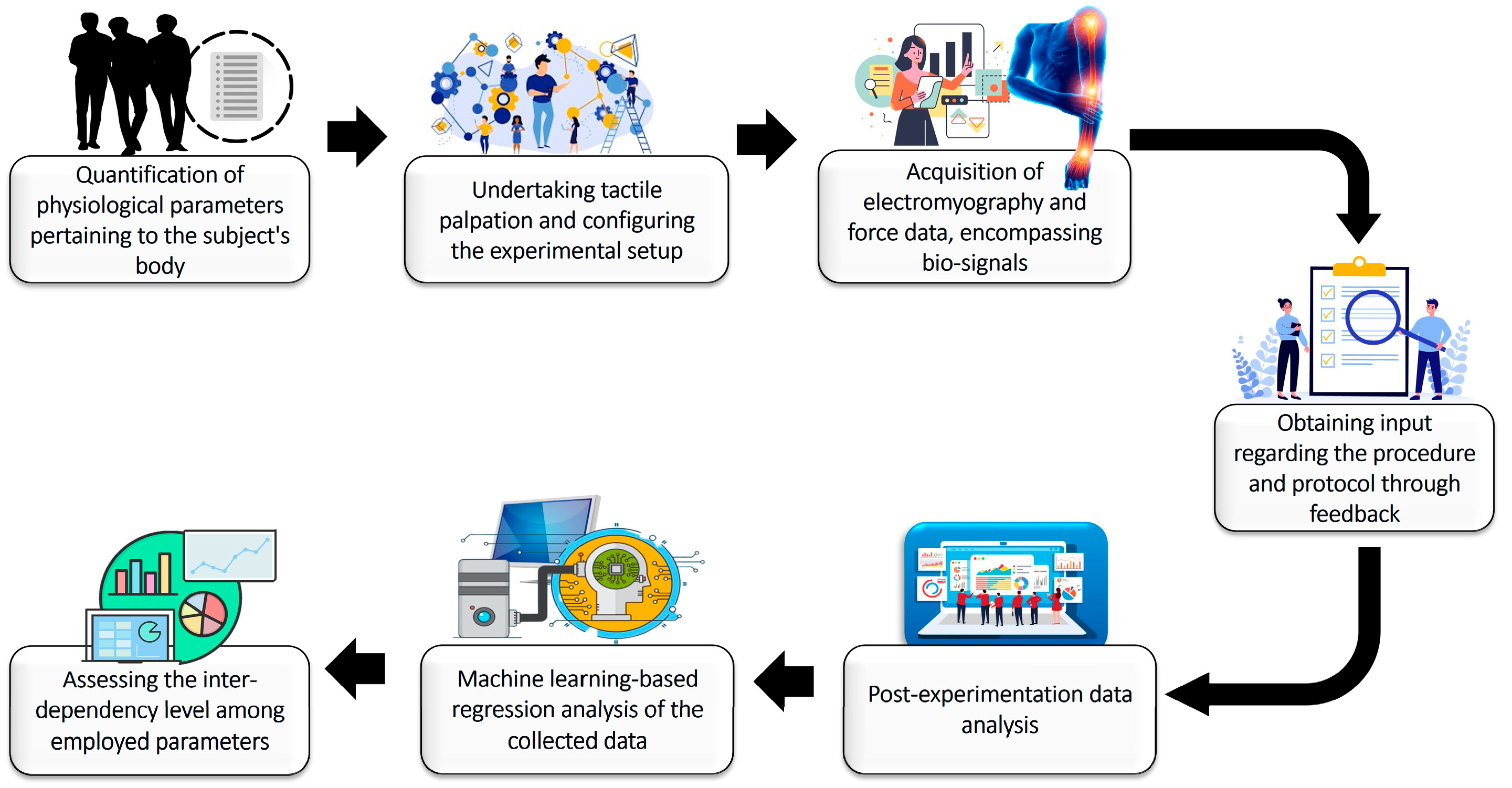
2. Materials and Methods
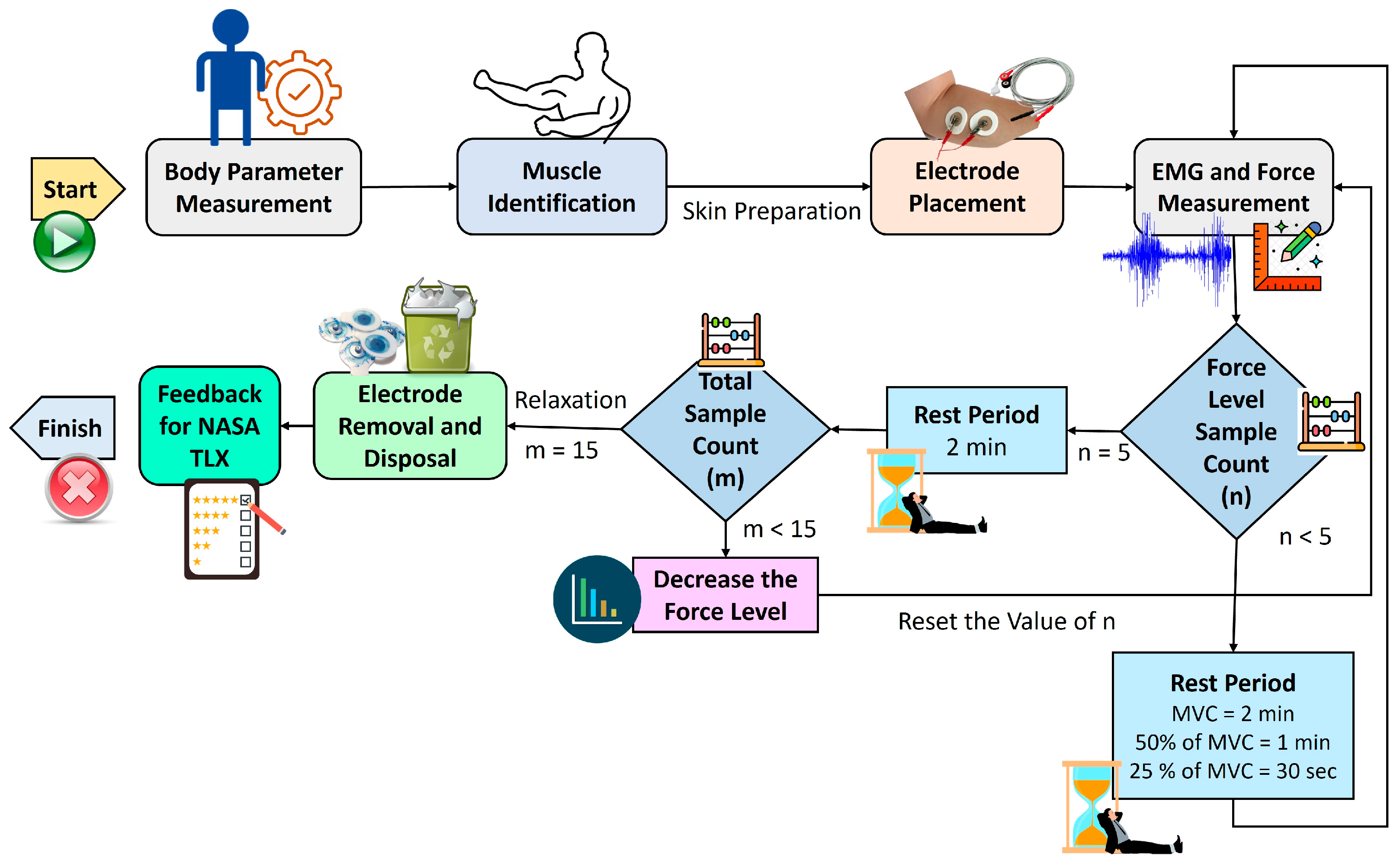
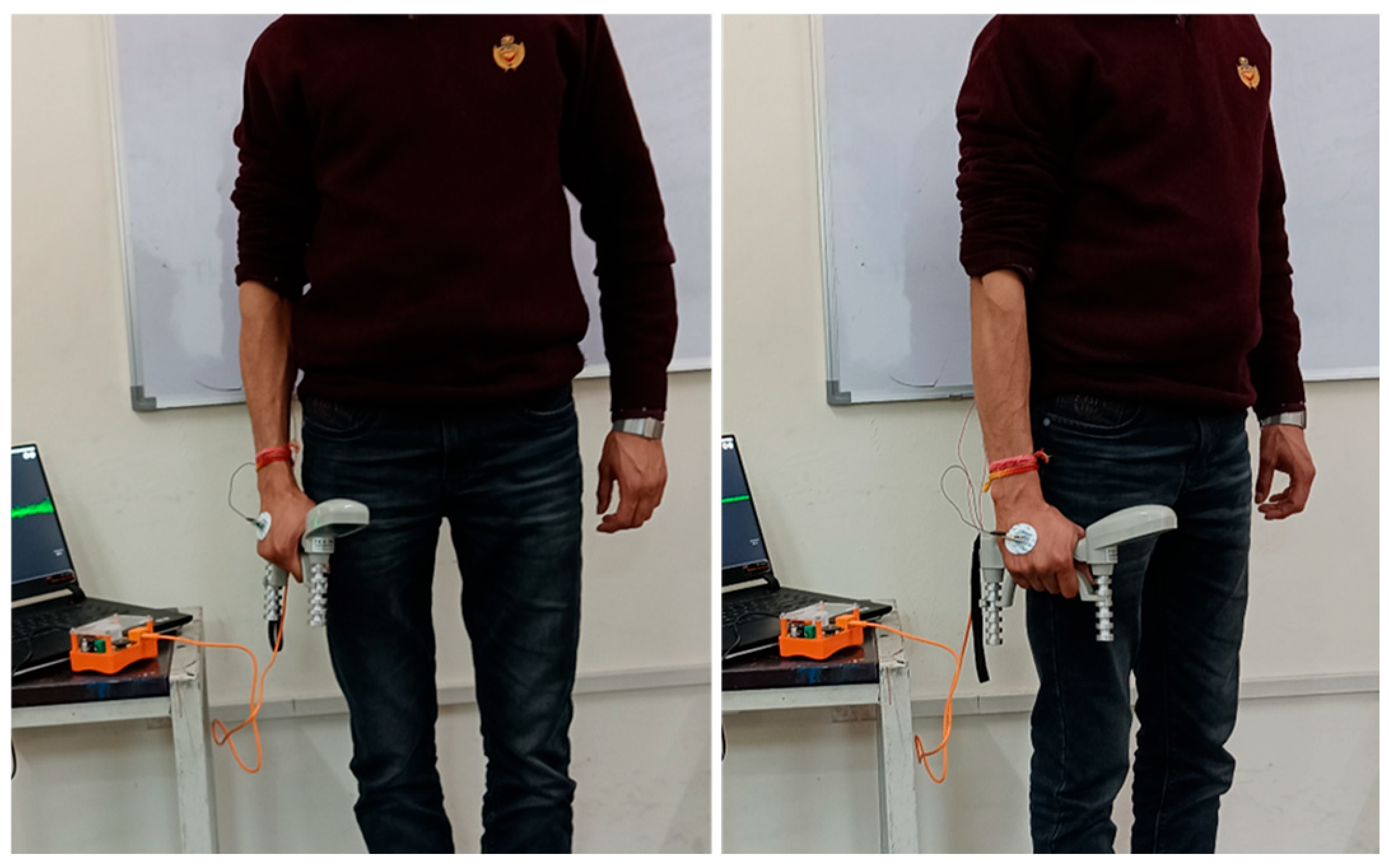
2.1. Experimental Setup
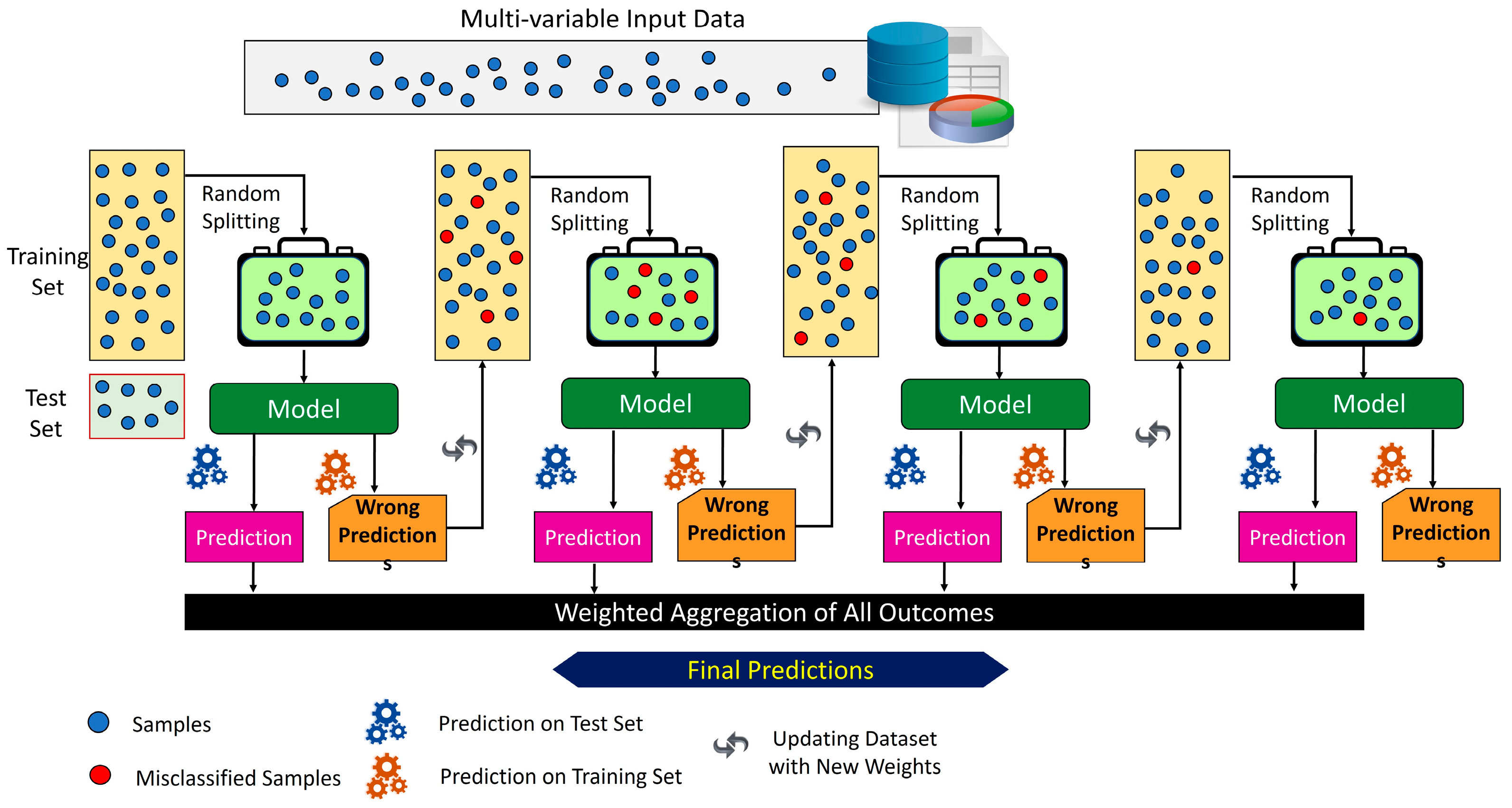
2.2. Proposed Methodology
- (a)
- General data: gender, age, height, and weight, with height and weight measured using a stadiometer and weight scale.
- (b)
- Body composition parameters: body fat level, muscle mass, and subcutaneous fat, assessed with a BIA scale according to specific protocols provided to the subjects.
- (c)
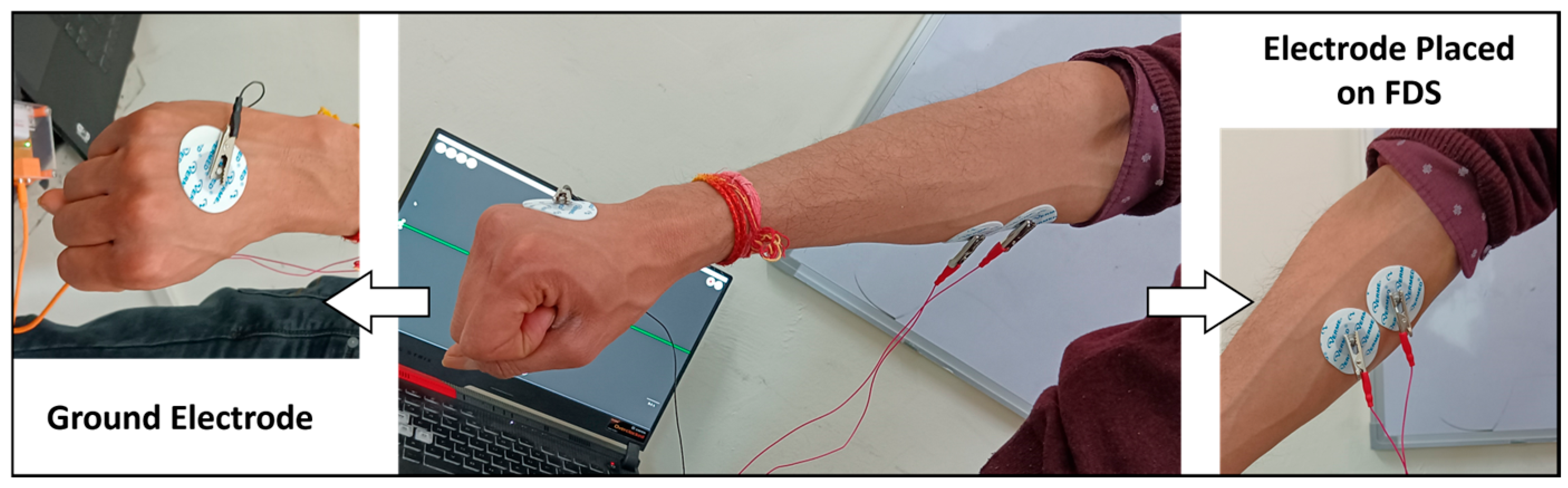
- EMG data: Two electrodes were affixed to the skin over the FDS muscle, with another electrode serving as the ground. These electrodes were connected to the EMG setup, which was then linked to the data acquisition system.
- (d)
- Force data: captured using a dynamometer, with subjects performing the MVC to determine the highest force achievable, which was then divided into 50% and 25% of the MVC.
- (i)
- 2 min between MVC trials.
- (ii)
- 1 min 30 s for 50% and 25% MVC trials.
2.3. Machine Learning-Based Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control
3. Results
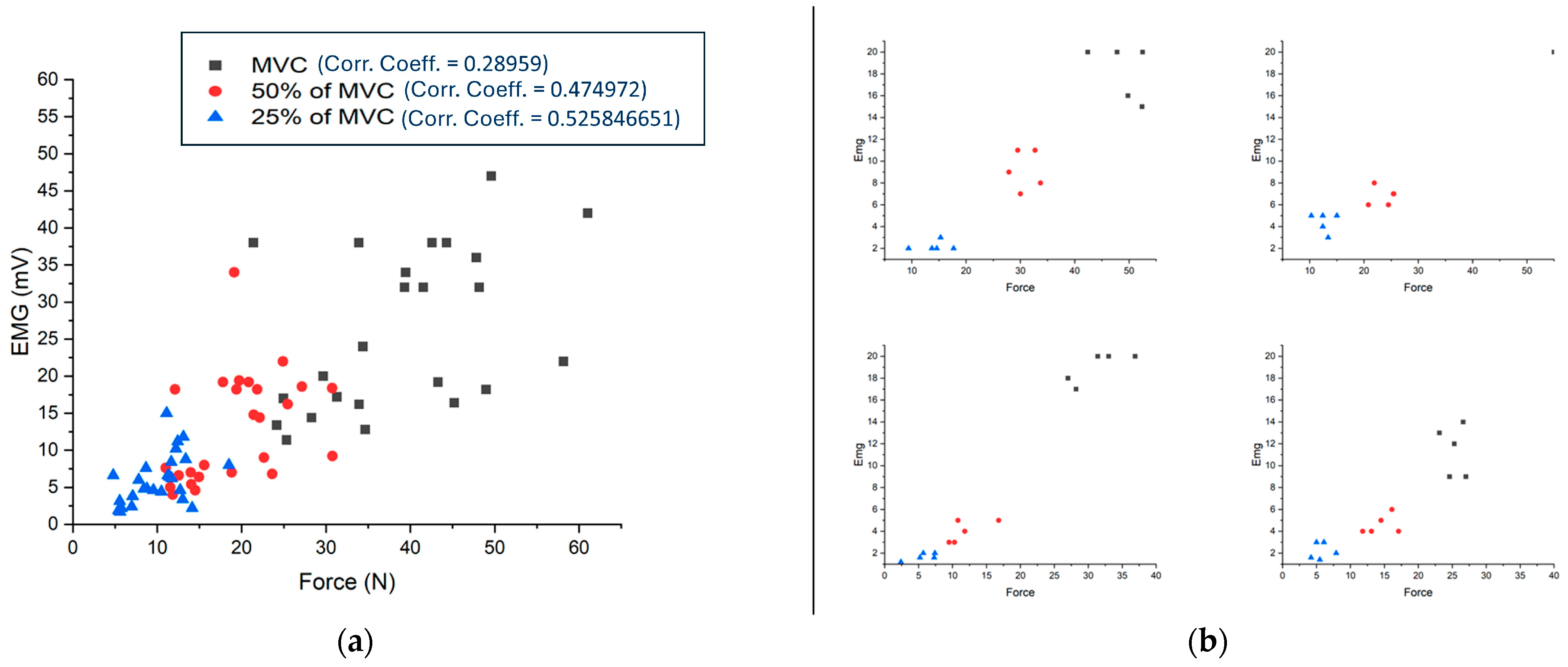
3.1. EMG Values vs. Force
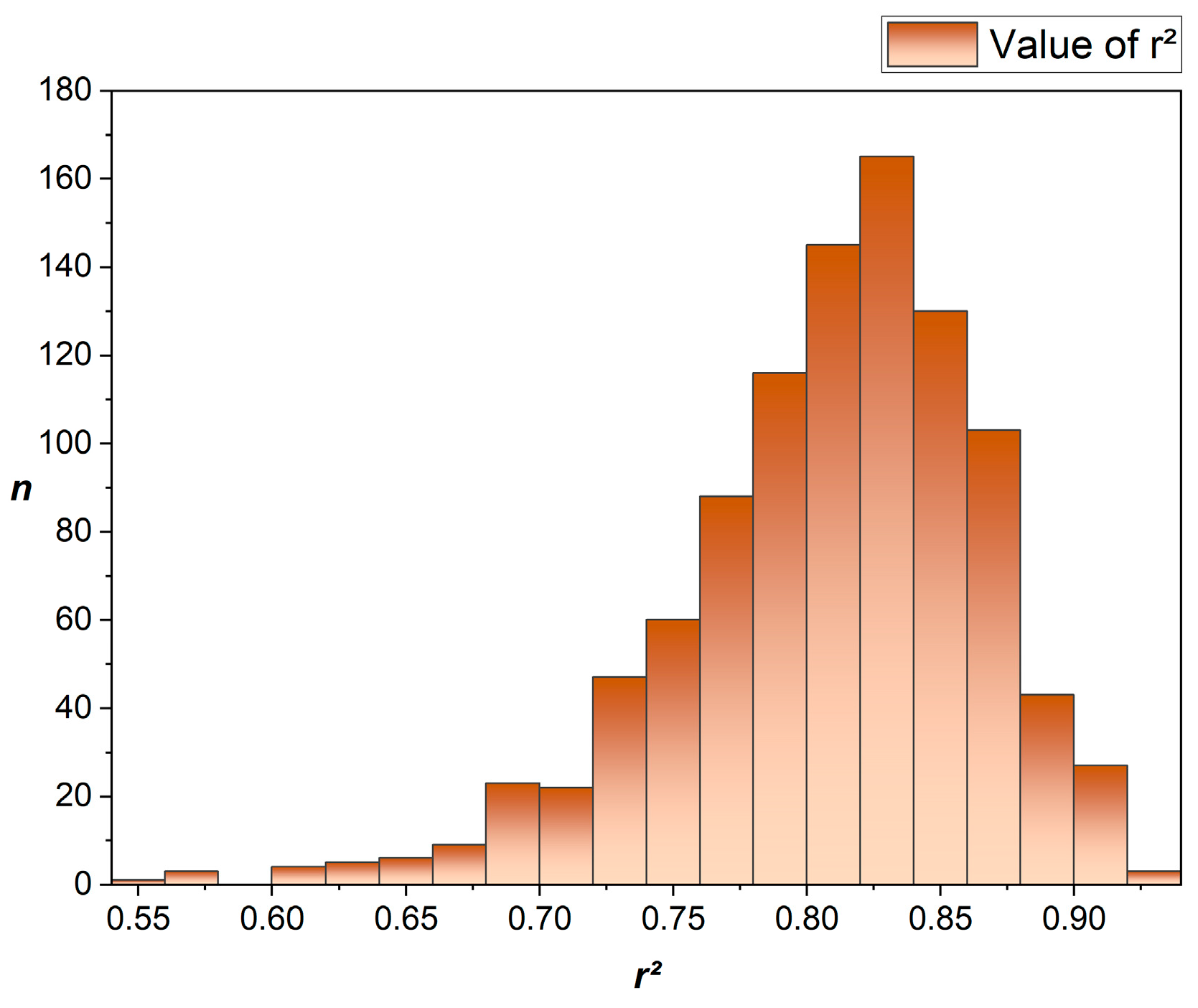
3.2. Machine Learning-Based Regression Model
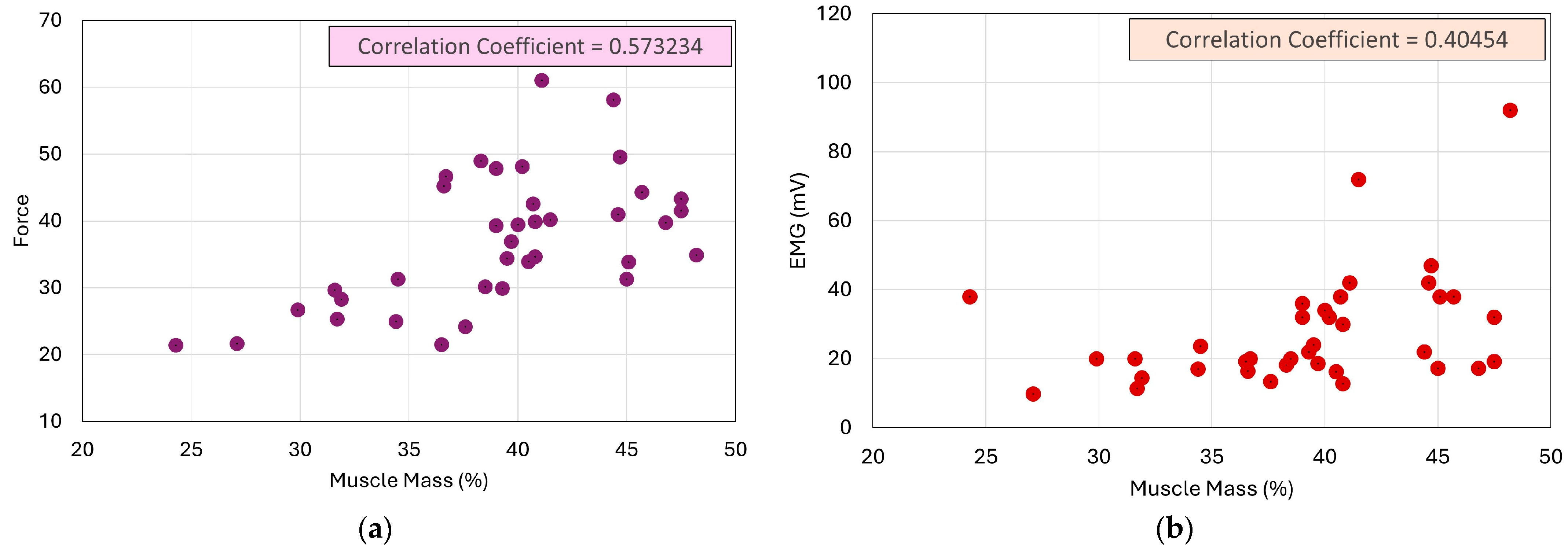
3.3. Impact of Body Parameters on EMG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Enoka, R.M. Physiological validation of the decomposition of surface EMG signals. J. Electromyogr. Kinesiol. 2019, 46, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Rojer, A.G.; D’andrea, L.; Otten, R.H.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.; Maier, A.B. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef] [PubMed]
- Wimalasena, L.N.; Braun, J.F.; Keshtkaran, M.R.; Hofmann, D.; Gallego, J.; Alessandro, C.; Tresch, M.C.; E Miller, L.; Pandarinath, C. Estimating muscle activation from EMG using deep learning-based dynamical systems models. J. Neural Eng. 2022, 19, 036013. [Google Scholar] [CrossRef] [PubMed]
- Subasi, A.; Qaisar, S.M. Surface EMG signal classification using TQWT, Bagging and Boosting for hand movement recognition. J. Ambient. Intell. Humaniz. Comput. 2022, 13, 3539–3554. [Google Scholar] [CrossRef]
- Mann, S.; Panduro, M.B.; Paarup, H.M.; Brandt, L.; Søgaard, K. Surface electromyography of forearm and shoulder muscles during violin playing. J. Electromyogr. Kinesiol. 2021, 56, 102491. [Google Scholar] [CrossRef]
- Sri-Iesaranusorn, P.; Chaiyaroj, A.; Buekban, C.; Dumnin, S.; Pongthornseri, R.; Thanawattano, C.; Surangsrirat, D. Classification of 41 Hand and Wrist Movements via Surface Electromyogram Using Deep Neural Network. Front. Bioeng. Biotechnol. 2021, 9, 548357. [Google Scholar] [CrossRef]
- Rubin, D.I. Needle electromyography: Basic concepts. Handb. Clin. Neurol. 2019, 160, 243–256. [Google Scholar] [CrossRef]
- Kumar, D.K.; Jelfs, B.; Sui, X.; Arjunan, S.P. Prosthetic hand control: A multidisciplinary review to identify strengths, shortcomings, and the future. Biomed. Signal Process. Control 2019, 53, 101588. [Google Scholar] [CrossRef]
- Enders, H.; Nigg, B.M. Measuring human locomotor control using EMG and EEG: Current knowledge, limitations and future considerations. Eur. J. Sport Sci. 2016, 16, 416–426. [Google Scholar] [CrossRef]
- Kimura, J.; Machida, M.; Ishida, T.; Yamada, T.; Rodnitzky, R.L.; Kudo, Y.; Suzuki, S. Relation between size of compound sensory or muscle action potentials, and length of nerve segment. Neurology 1986, 36, 647. [Google Scholar] [CrossRef]
- Kramer, M.; Schmid, I.; Sander, S.; Högel, J.; Eisele, R.; Kinzl, L.; Hartwig, E. Guidelines for the intramuscular positioning of EMG electrodes in the semispinalis capitis and cervicis muscles. J. Electromyogr. Kinesiol. 2003, 13, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 934, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanzarella, S.; Muceli, S.; Santello, M.; Farina, D. Synergistic Organization of Neural Inputs from Spinal Motor Neurons to Extrinsic and Intrinsic Hand Muscles. J. Neurosci. 2021, 41, 6878–6891. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; van der Smagt, P. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Gijsberts, A.; Castellini, C.; Caputo, B.; Hager, A.-G.M.; Elsig, S.; Giatsidis, G.; Bassetto, F.; Müller, H. Electromyography data for non-invasive naturally-controlled robotic hand prostheses. Sci. Data 2014, 1, 140053. [Google Scholar] [CrossRef]
- Osborn, L.E.; Iskarous, M.M.; Thakor, N.V. Sensing and Control for Prosthetic Hands in Clinical and Research Applications. Wearable Robot. 2020, 1, 445–468. [Google Scholar] [CrossRef]
- Sánchez-Velasco, L.E.; Arias-Montiel, M.; Guzmán-Ramírez, E.; Lugo-González, E. A Low-Cost EMG-Controlled Anthropomorphic Robotic Hand for Power and Precision Grasp. Biocybern. Biomed. Eng. 2020, 40, 221–237. [Google Scholar] [CrossRef]
- Asogbon, M.G.; Samuel, O.W.; Geng, Y.; Oluwagbemi, O.; Ning, J.; Chen, S.; Ganesh, N.; Feng, P.; Li, G. Towards resolving the co-existing impacts of multiple dynamic factors on the performance of EMG-pattern recognition based prostheses. Comput. Methods Programs Biomed. 2020, 184, 105278. [Google Scholar] [CrossRef]
- Aly, H.; Youssef, S.M. Bio-signal based motion control system using deep learning models: A deep learning approach for motion classification using EEG and EMG signal fusion. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 991–1002. [Google Scholar] [CrossRef]
- Deng, H.; Xu, X.; Zhuo, W.; Zhang, Y. Current-Sensor-Based Contact Stiffness Detection for Prosthetic Hands. IEEE Access 2020, 8, 29456–29466. [Google Scholar] [CrossRef]
- Jiang, X.; Merhi, L.-K.; Xiao, Z.G.; Menon, C. Exploration of Force Myography and surface Electromyography in hand gesture classification. Med. Eng. Phys. 2017, 41, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Sahi, A.K.; Sharma, N.; Sharma, S. Force myography controlled multifunctional hand prosthesis for upper-limb amputees. Biomed. Signal Process. Control 2020, 62, 102122. [Google Scholar] [CrossRef]
- Gentile, C.; Cordella, F.; Rodrigues, C.R.; Zollo, L. Touch-and-slippage detection algorithm for prosthetic hands. Mechatronics 2020, 70, 102402. [Google Scholar] [CrossRef]
- Badawy, A.; Alfred, R. Myoelectric prosthetic hand with a proprioceptive feedback system. J. King Saud Univ. Eng. Sci. 2020, 32, 388–395. [Google Scholar] [CrossRef]
- Phinyomark, A.; Campbell, E.; Scheme, E. Surface Electromyography (EMG) Signal Processing, Classification, and Practical Considerations. In Biomedical Signal Processing; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–29. [Google Scholar] [CrossRef]
- Meldrum, D.; Cahalane, E.; Conroy, R.; Fitzgerald, D.; Hardiman, O. Maximum voluntary isometric contraction: Reference values and clinical application. Amyotroph. Lateral Scler. 2007, 8, 47–55. [Google Scholar] [CrossRef]
- Koral, J.; Oranchuk, D.J.; Wrightson, J.G.; Twomey, R.; Millet, G.Y. Mechanisms of neuromuscular fatigue and recovery in unilateral versus bilateral maximal voluntary contractions. J. Appl. Physiol. 2020, 128, 785–794. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Falla, D.; Dall’Alba, P.; Rainoldi, A.; Merletti, R.; Jull, G. Location of innervation zones of sternocleidomastoid and scalene muscles-a basis for clinical and research electromyography applications. Clin. Neurophysiol. 2002, 113, 57–63. [Google Scholar] [CrossRef]
- Schetz, M. Assessment of Volume Status. Crit. Care Nephrol. 2009, 95, 499–504. [Google Scholar] [CrossRef]
- Barry, D. Sarcopenia. In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1781–1803.e19. [Google Scholar] [CrossRef]
- He, B.; Armaghani, D.J.; Lai, S.H.; Mohamad, E.T. Application of an expert extreme gradient boosting model to predict blast-induced air-overpressure in quarry mines. In Applications of Artificial Intelligence in Mining, Geotechnical and Geoengineering; Elsevier: Amsterdam, The Netherlands, 2024; pp. 269–289. [Google Scholar] [CrossRef]
- Dev, V.A.; Eden, M.R. Gradient Boosted Decision Trees for Lithology Classification. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–118. [Google Scholar] [CrossRef]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef]
- Marzullo, T.C.; Gage, G.J. The SpikerBox: A Low Cost, Open-Source BioAmplifier for Increasing Public Participation in Neuroscience Inquiry. PLoS ONE 2012, 7, e30837. [Google Scholar] [CrossRef] [PubMed]
- Braarud, P.Ø. An efficient screening technique for acceptable mental workload based on the NASA Task Load Index—Development and application to control room validation. Int. J. Ind. Ergon. 2020, 76, 102904. [Google Scholar] [CrossRef]
- Blackwell, J.R.; Kornatz, K.W.; Heath, E.M. Effect of grip span on maximal grip force and fatigue of flexor digitorum superficialis. Appl. Ergon. 1999, 30, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Madarshahian, S.; Letizi, J.; Latash, M.L. Synergic control of a single muscle: The example of flexor digitorum superficialis. J. Physiol. 2021, 599, 1261–1279. [Google Scholar] [CrossRef]
- Duque, J.; Masset, D.; Malchaire, J. Evaluation of handgrip force from EMG measurements. Appl. Ergon. 1995, 26, 61–66. [Google Scholar] [CrossRef]
- Lehman, G.J.; McGill, S.M. The importance of normalization in the interpretation of surface electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Maciukiewicz, J.M.; Lulic, T.; MacKay, K.L.; Meszaros, K.A.; Dickerson, C.R. The influence of posture variation on electromyographic signals in females obtained during maximum voluntary isometric contractions: A shoulder example. J. Electromyogr. Kinesiol. 2022, 62, 102319. [Google Scholar] [CrossRef]
- Nordander, C.; Willner, J.; Hansson, G.-A.; Larsson, B.; Unge, J.; Granquist, L.; Skerfving, S. Influence of the subcutaneous fat layer, as measured by ultrasound, skinfold calipers and BMI, on the EMG amplitude. Eur. J. Appl. Physiol. 2003, 89, 514–519. [Google Scholar] [CrossRef]
- Tarulli, A.; Chin, A.; Lee, K.; Rutkove, S.B. Impact of skin-subcutaneous fat layer thickness on electrical impedance myography measurements: An initial assessment. Clin. Neurophysiol. 2007, 118, 2393–2397. [Google Scholar] [CrossRef]
- Nojima, I.; Suwa, Y.; Sugiura, H.; Noguchi, T.; Tanabe, S.; Mima, T.; Watanabe, T. Smaller muscle mass is associated with increase in EMG–EMG coherence of the leg muscle during unipedal stance in elderly adults. Hum. Mov. Sci. 2020, 71, 102614. [Google Scholar] [CrossRef]
- Krings, B.M.; Shepherd, B.D.; Swain, J.C.; Turner, A.J.; Chander, H.; Waldman, H.S.; McAllister, M.J.; Knight, A.C.; Smith, J.W. Impact of Fat Grip Attachments on Muscular Strength and Neuromuscular Activation During Resistance Exercise. J. Strength Cond. Res. 2021, 35, S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Abd, M.A.; Ingicco, J.; Hutchinson, D.T.; Tognoli, E.; Engeberg, E.D. Multichannel haptic feedback unlocks prosthetic hand dexterity. Sci. Rep. 2022, 12, 2323. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Colgate, J.E. Haptic Feedback Enhances Grip Force Control of sEMG-Controlled Prosthetic Hands in Targeted Reinnervation Amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Basumatary, H.; Hazarika, S.M. A Decade of Haptic Feedback for Upper Limb Prostheses. IEEE Trans. Med. Robot. Bionics 2023, 5, 793–810. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, Q.; Liu, H.; Shull, P.B. A novel, co-located EMG-FMG-sensing wearable armband for hand gesture recognition. Sens. Actuators A Phys. 2020, 301, 111738. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, Z.; Zhao, R.; Ni, Y.; Jing, X.; Gao, S. A Review of EMG-, FMG-, and EIT-Based Biosensors and Relevant Human–Machine Interactivities and Biomedical Applications. Biosensors 2022, 12, 516. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Chen, Z.; Li, J. A Novel EMG-Based Variable Impedance Control Method for a Tele-Operation System Under an Unstructured Environment. IEEE Access 2022, 10, 89509–89518. [Google Scholar] [CrossRef]
- Borzelli, D.; D’Avella, A.; Gurgone, S.; Gastaldi, L. Unconstrained and constrained estimation of a linear EMG-to-force mapping during isometric force generation. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, B.; Ling, Z.; Chen, J.; Guo, L.; Xiong, D.; Wang, J. Quantitative Evaluation System of Wrist Motor Function for Stroke Patients Based on Force Feedback. Sensors 2022, 22, 3368. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Boyd, W.J.; Martinez-Luna, C.; Dai, C.; Wang, H.; Wang, H.; Huang, X.; Farrell, T.R.; Clancy, E.A. Virtual regression-based myoelectric hand-wrist prosthesis control and electrode site selection using no force feedback. Biomed Signal Process Control. 2023, 82, 104602. [Google Scholar] [CrossRef]
- Barontini, F.; Van Straaten, M.; Catalano, M.G.; Thoreson, A.; Lopez, C.; Lennon, R.; Bianchi, M.; Andrews, K.; Santello, M.; Bicchi, A.; et al. Evaluating the effect of non-invasive force feedback on prosthetic grasp force modulation in participants with and without limb loss. PLoS ONE 2023, 18, e0285081. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Sung, M.; Park, J.; Kim, S.; Choi, Y. Development of EMG-FMG Based Prosthesis With PVDF-Film Vibrational Feedback Control. IEEE Sens. J. 2021, 21, 23597–23607. [Google Scholar] [CrossRef]
- Flanders, M.; Soechting, J.F. Arm muscle activation for static forces in three-dimensional space. J. Neurophysiol. 1990, 64, 1818–1837. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, V.; Yue, G.H.; Ranganathan, V.K.; Liu, J.Z.; Sahgal, V. Relationship between motor activity-related cortical potential and voluntary muscle activation. Exp. Brain Res. 2000, 133, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, C.; Wu, D.; Xu, X.; Wang, S.; Yao, J. Recognition of Human Motion Intentions Based on Bayesian-Optimized XGBOOST Algorithm. J. Sens. 2022, 2022, 3015645. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, L.; Zhang, S.; Zhang, D.; Liu, F.; Wang, H.; Xie, P. A Model for Lumbar EMG Signal Recognition Based on Stacking Integration Learning. IEEE Sens. J. 2023, 23, 3766–3775. [Google Scholar] [CrossRef]
- Ptaszkowski, K.; Wlodarczyk, P.; Paprocka-Borowicz, M. The Relationship Between the Electromyographic Activity Of Rectus And Oblique Abdominal Muscles And Bioimpedance Body Composition Analysis-A Pilot Observational Study. Diabetes Metab. Syndr. Obes. 2019, 12, 2033–2040. [Google Scholar] [CrossRef]
- Gallón, V.M.; Vélez, S.M.; Ramírez, J.; Bolaños, F. Comparison of machine learning algorithms and feature extraction techniques for the automatic detection of surface EMG activation timing. Biomed. Signal Process. Control 2024, 94, 106266. [Google Scholar] [CrossRef]
- Larivière, C.; Arsenault, A.; Gravel, D.; Gagnon, D.; Loisel, P.; Vadeboncoeur, R. Electromyographic assessment of back muscle weakness and muscle composition: Reliability and validity issues. Arch. Phys. Med. Rehabil. 2002, 83, 1206–1214. [Google Scholar] [CrossRef]
- Jochumsen, M.; Waris, A.; Kamavuako, E.N. The effect of arm position on classification of hand gestures with intramuscular EMG. Biomed. Signal Process. Control 2018, 43, 1–8. [Google Scholar] [CrossRef]
- Funk, D.A.; An, K.N.; Morrey, B.F.; Daube, J.R. Electromyographic analysis of muscles across the elbow joint. J. Orthop. Res. 1987, 5, 529–538. [Google Scholar] [CrossRef]
- Doheny, E.P.; Lowery, M.M.; FitzPatrick, D.P.; O’malley, M.J. Effect of elbow joint angle on force–EMG relationships in human elbow flexor and extensor muscles. J. Electromyogr. Kinesiol. 2008, 18, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Alici, G. Non-Invasive Human-Machine Interface (HMI) Systems with Hybrid On-Body Sensors for Controlling Upper-Limb Prosthesis: A Review. IEEE Sens. J. 2022, 22, 10292–10307. [Google Scholar] [CrossRef]
- Wolf, E.J.; Cruz, T.H.; Emondi, A.A.; Langhals, N.B.; Naufel, S.; Peng, G.C.Y.; Schulz, B.W.; Wolfson, M. Advanced technologies for intuitive control and sensation of prosthetics. Biomed. Eng. Lett. 2020, 10, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Stafford, N.; Huang, S.; Hu, X.; Ferris, D.P.; Huang, H. Myoelectric control of robotic lower limb prostheses: A review of electromyography interfaces, control paradigms, challenges and future directions. J. Neural Eng. 2021, 18, 041004. [Google Scholar] [CrossRef]
- Pribis, P.; Burtnack, C.A.; Mckenzie, S.O.; Thayer, J. Trends in Body Fat, Body Mass Index and Physical Fitness Among Male and Female College Students. Nutrients 2010, 2, 1075–1085. [Google Scholar] [CrossRef]
- Aimé, J.; Simoneau, D.; Bouchard, C. Skeletal Muscle Metabolism and Body Fat Content in Men and Women. Obes. Res. 1995, 3, 23–29. [Google Scholar] [CrossRef]



| Subject | Force | EMG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MVC | 50% of MVC | 25% of MVC | MVC | 50% of MVC | 25% of MVC | |||||||
| Avg. | St. D. | Avg. | St. D. | Avg. | St. D. | Avg. | St. D. | Avg. | St. D. | Avg. | St. D. | |
| 1 | 31.30 | 3.5316 | 11.84 | 2.5897 | 5.60 | 1.8188 | 17.20 | 4.2615 | 4 | 0.8944 | 1.68 | 0.2993 |
| 2 | 25.32 | 1.4077 | 14.52 | 1.9271 | 5.74 | 1.2468 | 11.40 | 2.0591 | 4.6 | 0.8000 | 2.2 | 0.6812 |
| 3 | 48.98 | 3.7269 | 30.76 | 2.1332 | 14.14 | 2.7163 | 18.20 | 2.2271 | 9.2 | 1.6000 | 2.2 | 0.4000 |
| 4 | 34.88 | 2.5545 | 19.14 | 3.2818 | 11.10 | 1.4724 | 92.00 | 7.4833 | 34 | 10.1980 | 15 | 1.7889 |
| 5 | 39.30 | 2.9772 | 24.90 | 3.0535 | 11.46 | 1.5409 | 32.00 | 11.6619 | 22 | 4.0000 | 6.4 | 2.0591 |
| 6 | 42.56 | 1.0519 | 19.38 | 2.7469 | 13.10 | 1.3609 | 38.00 | 9.7980 | 18.2 | 2.4000 | 11.8 | 2.7129 |
| 7 | 48.16 | 1.6255 | 21.44 | 3.8333 | 11.66 | 0.8333 | 32.00 | 11.6619 | 14.8 | 3.4293 | 8.4 | 0.4899 |
| 8 | 39.44 | 4.3930 | 21.84 | 3.1576 | 12.22 | 3.0740 | 34.00 | 8.0000 | 18.2 | 2.2271 | 10.2 | 3.3106 |
| 9 | 47.82 | 3.4580 | 27.14 | 5.9241 | 9.54 | 2.9268 | 36.00 | 10.1980 | 18.6 | 5.7480 | 4.6 | 1.4967 |
| 10 | 43.28 | 0.9765 | 20.84 | 1.8139 | 13.38 | 0.8258 | 19.20 | 1.6000 | 19.2 | 1.6000 | 8.8 | 0.7483 |
| 11 | 40.20 | 0.5762 | 19.72 | 2.9735 | 12.42 | 1.0647 | 72.00 | 7.4833 | 19.4 | 1.2000 | 11.2 | 2.6382 |
| 12 | 61.02 | 3.1783 | 30.74 | 1.2419 | 18.50 | 1.4927 | 42.00 | 4.0000 | 18.4 | 1.3565 | 8 | 1.5492 |
| 13 | 24.96 | 1.4193 | 12.54 | 1.7351 | 7.08 | 1.1143 | 17.00 | 2.4495 | 6.6 | 1.3565 | 3.8 | 1.3267 |
| 14 | 44.28 | 2.8812 | 22.16 | 1.8800 | 11.26 | 1.9376 | 38.00 | 4.0000 | 14.4 | 1.3565 | 6.6 | 1.9596 |
| 15 | 58.14 | 2.3922 | 23.62 | 1.9177 | 12.70 | 1.5310 | 22.00 | 4.0000 | 6.8 | 0.7483 | 4.6 | 1.0198 |
| 16 | 24.16 | 1.9262 | 11.54 | 2.3922 | 5.56 | 0.8188 | 13.40 | 2.1541 | 5 | 1.0955 | 3.14 | 1.8217 |
| 17 | 49.58 | 2.1821 | 25.48 | 2.3718 | 11.72 | 2.1028 | 47.00 | 6.0000 | 16.2 | 3.1241 | 6.2 | 0.9798 |
| 18 | 28.28 | 0.4534 | 11.02 | 0.5671 | 7.80 | 0.8718 | 14.40 | 3.2619 | 7.6 | 1.0198 | 6 | 0.8944 |
| 19 | 45.20 | 1.5020 | 18.82 | 2.0153 | 10.48 | 1.5131 | 16.40 | 0.4899 | 7 | 0.6325 | 4.4 | 0.8000 |
| 20 | 29.66 | 1.1002 | 14.02 | 2.6806 | 5.40 | 0.9839 | 20.00 | 0.0000 | 5.4 | 1.4967 | 1.88 | 0.1600 |
| 21 | 34.38 | 2.1637 | 13.96 | 1.8424 | 8.78 | 1.1196 | 24.00 | 4.8990 | 7 | 0.0000 | 4.8 | 1.1662 |
| 22 | 33.92 | 1.7882 | 14.94 | 3.5086 | 6.98 | 1.3732 | 16.20 | 1.8330 | 6.4 | 1.8547 | 2.4 | 0.4899 |
| 23 | 34.64 | 1.1218 | 15.56 | 0.7228 | 8.42 | 0.5192 | 12.80 | 1.9391 | 8 | 0.8944 | 4.8 | 0.4000 |
| 24 | 33.88 | 1.3526 | 17.80 | 1.0696 | 8.66 | 1.0365 | 38.00 | 7.4833 | 19.2 | 1.6000 | 7.6 | 0.8000 |
| 25 | 21.40 | 0.7616 | 12.12 | 1.1686 | 4.78 | 0.2400 | 38.00 | 7.4833 | 18.2 | 2.2271 | 6.6 | 0.8000 |
| 26 | 41.52 | 1.4105 | 22.66 | 2.1786 | 13.02 | 1.0980 | 32.00 | 9.7980 | 9 | 1.0955 | 3.4 | 0.8000 |
| 27 | 39.88 | 1.3673 | 21.22 | 1.0438 | 14.42 | 0.8976 | 30.00 | 0.0000 | 16.4 | 1.0198 | 9 | 1.0955 |
| 28 | 46.66 | 3.8836 | 20.08 | 1.4497 | 12.86 | 1.4569 | 20.00 | 0.0000 | 9.6 | 0.4899 | 7.2 | 0.9798 |
| 29 | 30.14 | 1.0480 | 14.16 | 1.7693 | 8.14 | 1.4787 | 20.00 | 0.0000 | 7.4 | 1.0198 | 4 | 1.0955 |
| 30 | 26.70 | 1.4199 | 15.44 | 0.5314 | 6.14 | 0.9091 | 20.00 | 0.0000 | 10.4 | 1.0198 | 4.8 | 0.9798 |
| 31 | 21.52 | 1.9031 | 13.22 | 1.4892 | 7.96 | 0.8114 | 19.20 | 1.6000 | 13.6 | 1.7436 | 8.6 | 1.2000 |
| 32 | 31.28 | 3.5068 | 16.82 | 2.6589 | 9.18 | 1.3732 | 23.60 | 5.2764 | 9.6 | 1.0198 | 4 | 1.2649 |
| 33 | 21.67 | 2.3056 | 11.24 | 2.2879 | 7.02 | 0.9806 | 9.80 | 2.4000 | 4.6 | 0.4899 | 2.6 | 0.4899 |
| 34 | 36.94 | 4.0008 | 20.16 | 2.0195 | 12.44 | 1.7614 | 18.60 | 1.9596 | 16 | 0.6325 | 7.2 | 0.9798 |
| 35 | 40.98 | 4.8200 | 25.30 | 1.9860 | 13.80 | 1.3130 | 42.00 | 9.7980 | 18.8 | 1.6000 | 9.4 | 1.3565 |
| 36 | 29.92 | 2.9708 | 15.26 | 2.7594 | 6.56 | 1.7636 | 22.00 | 6.8118 | 8.6 | 1.3565 | 3 | 0.8944 |
| 37 | 39.76 | 2.4113 | 22.32 | 2.7931 | 11.36 | 1.5161 | 17.20 | 3.4871 | 9.8 | 1.6000 | 3.8 | 0.9798 |
| Reference | Year of Study | Number of Subjects | Muscle of Interest | Body Parameters | Number of Force States | Machine Learning | Force–EMG Relationship Characterization |
|---|---|---|---|---|---|---|---|
| [46] | 2022 | 12 | – | No | 2 | – | Concurrent application of two distinct amplitude forces via a robotic hand |
| [51] | 2022 | – | – | No | 3 | ✓ | The implementation of a user impedance control strategy |
| [52] | 2022 | 5 | Upper limb muscle and OpenSim upper limb model | No | – | Linear Mapping | Comparing force estimation between constrained and unconstrained environments |
| [53] | 2022 | 35 | Wrist motor muscle | No | – | ✓ | Implementation of force feedback for the purpose of post-stroke rehabilitation |
| [54] | 2023 | 15 | Site selection via calibration period | No | – | – | Selection of the ideal number of electrodes and optimal placement area |
| [55] | 2023 | 24 | Upper arm | No | – | – | The application of force feedback using a wearable haptic device |
| [56] | 2021 | – | – | No | – | ✓ | The utilization of EMG as a command input for a vibration sensory methodology |
| Current Study | 2023 | 37 | Flexor digitorum superficialis (FDS) | Fat level, muscle mass, subcutaneous fat | 3 | ✓ | Incorporating physiological parameters, an examination of the relationship between force and EMG measurements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, D.C.; Kumar, P.; Joshi, R.C.; Mitra, S. AI-Enhanced Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control Incorporating Anthropometric Measures. Prosthesis 2024, 6, 1459-1478. https://doi.org/10.3390/prosthesis6060106
Joshi DC, Kumar P, Joshi RC, Mitra S. AI-Enhanced Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control Incorporating Anthropometric Measures. Prosthesis. 2024; 6(6):1459-1478. https://doi.org/10.3390/prosthesis6060106
Chicago/Turabian StyleJoshi, Deepak Chandra, Pankaj Kumar, Rakesh Chandra Joshi, and Santanu Mitra. 2024. "AI-Enhanced Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control Incorporating Anthropometric Measures" Prosthesis 6, no. 6: 1459-1478. https://doi.org/10.3390/prosthesis6060106
APA StyleJoshi, D. C., Kumar, P., Joshi, R. C., & Mitra, S. (2024). AI-Enhanced Analysis to Investigate the Feasibility of EMG Signals for Prosthetic Hand Force Control Incorporating Anthropometric Measures. Prosthesis, 6(6), 1459-1478. https://doi.org/10.3390/prosthesis6060106






