Current Progress in the Development of Resin Materials with Nanofillers for 3D Printing of Denture Base
Abstract
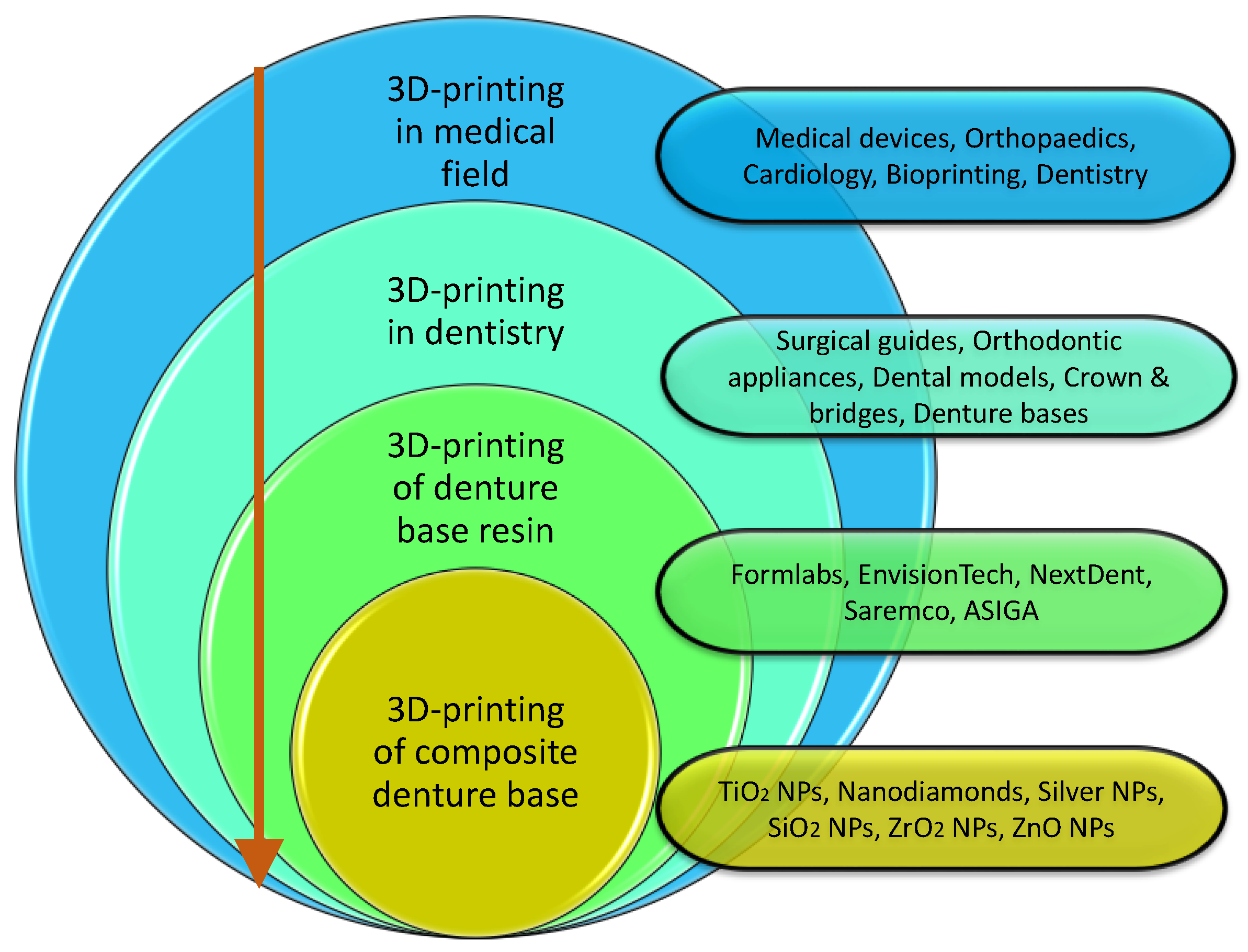
1. Introduction
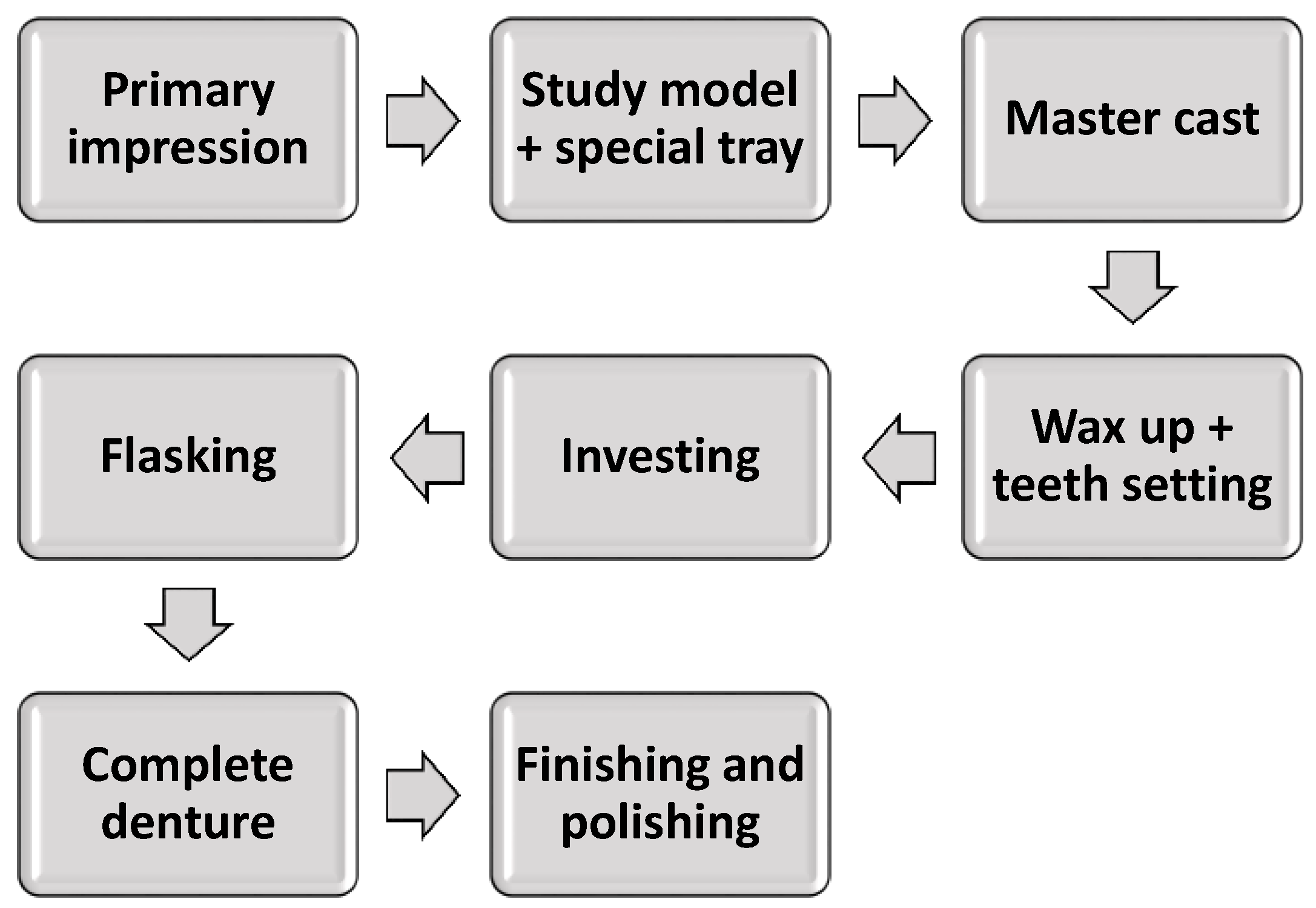
1.1. Denture Base Fabrication: Conventional vs. CAD/CAM Techniques
1.1.1. Conventional Fabrication Technique
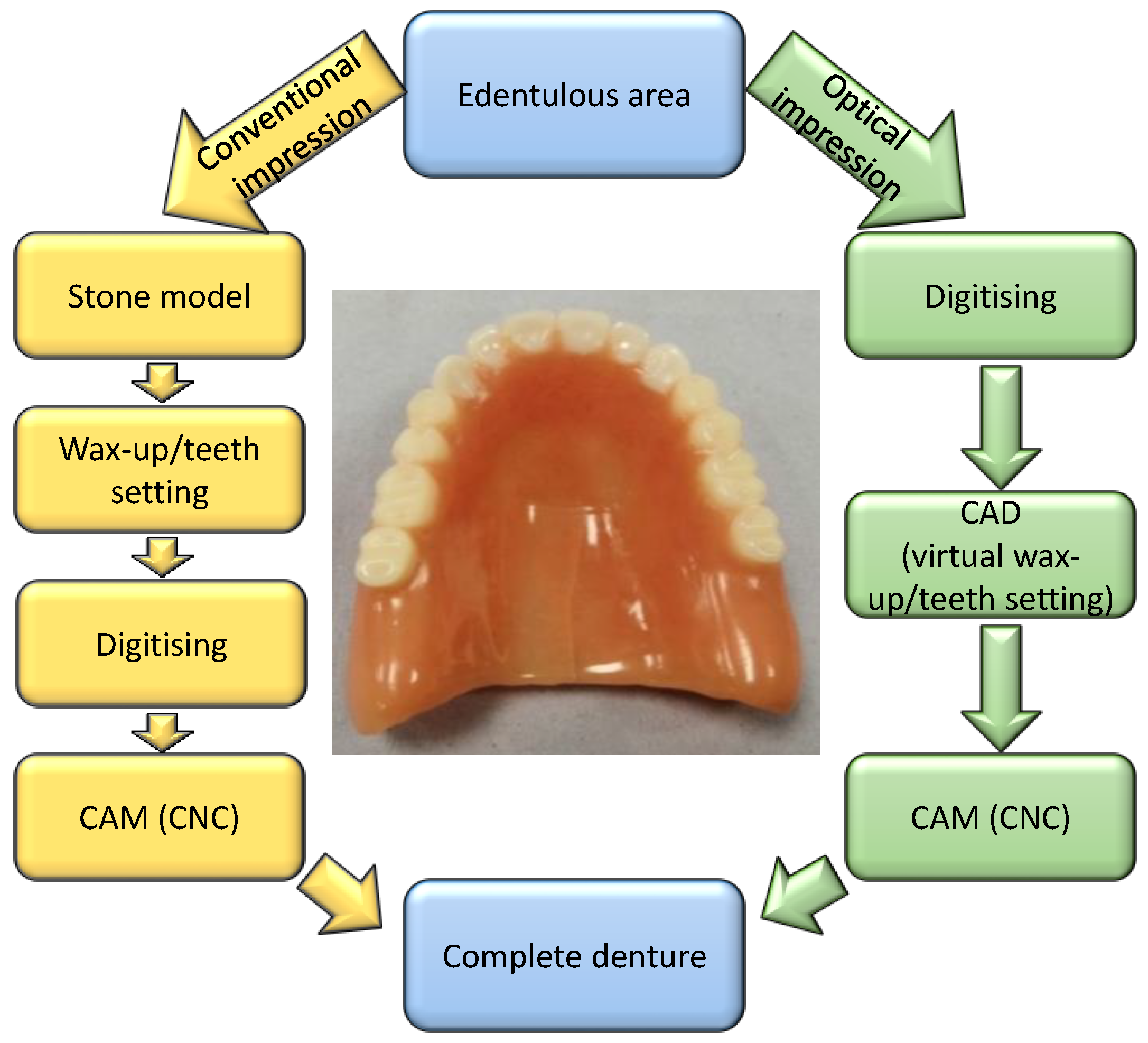
1.1.2. CAD/CAM Technology
- Data acquisition: this initial phase involves scanning the treatment site or stone model using a digitisation tool or scanner, capturing digital data that are then processed by a computer.
- Data processing: CAD/CAM software generates the data set required for the intended prosthesis.
- Manufacturing: The digital data are converted into the desired prosthetic. Once the prosthesis design is completed by the CAD, the information is sent to the production unit, which is controlled by the CAM software.
1.1.3. Comparative Analysis of Fabrication Methods
1.2. Three-Dimensional-Printing Technology in Dentistry
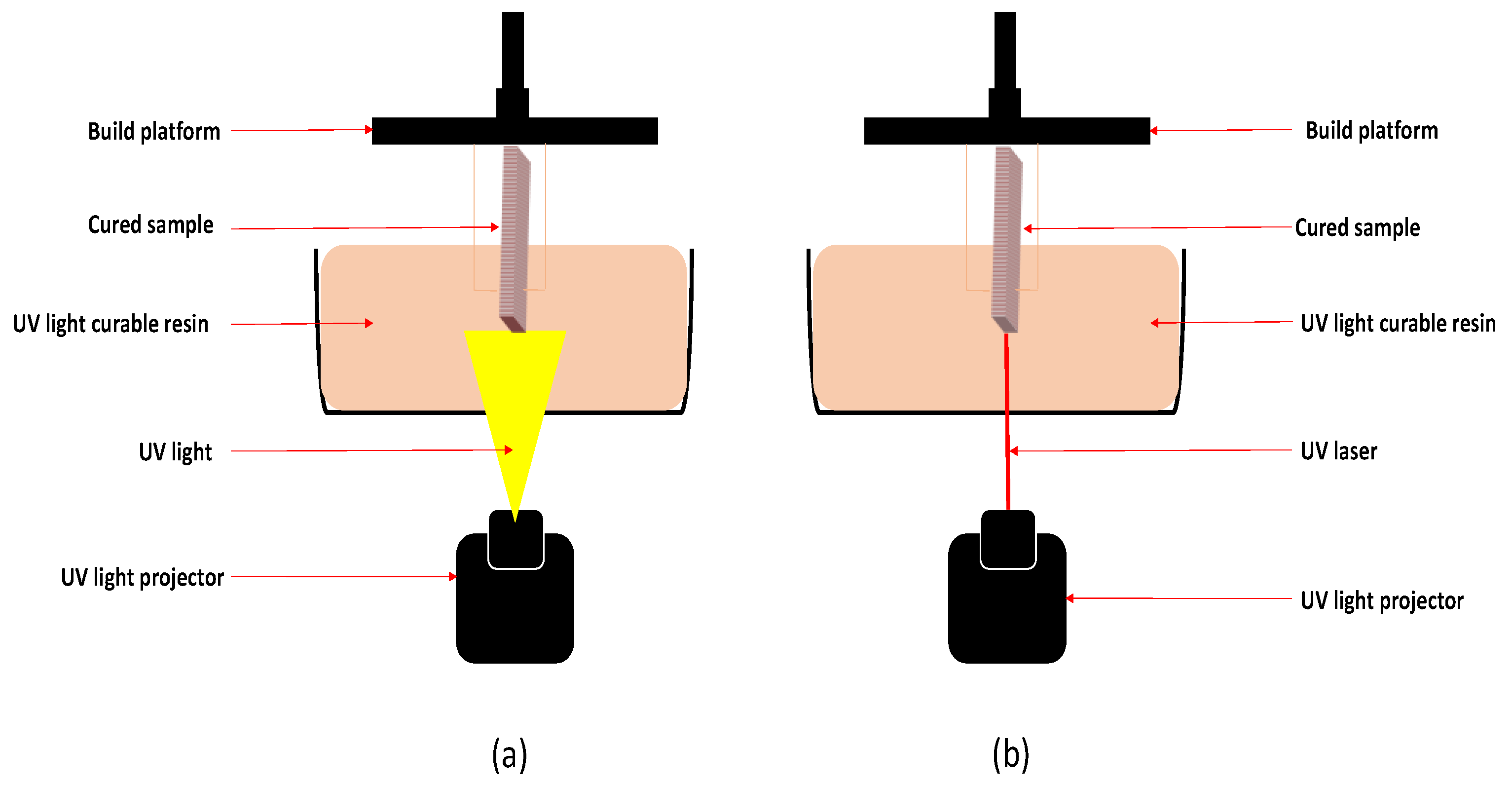
1.2.1. Stereolithography Technique (SLA)
1.2.2. DLP Techniques
1.2.3. Material Jetting (MJ, PP)
1.2.4. Material Extrusion (ME, FDM)
1.2.5. Selective Laser Sintering (SLS, SLM)
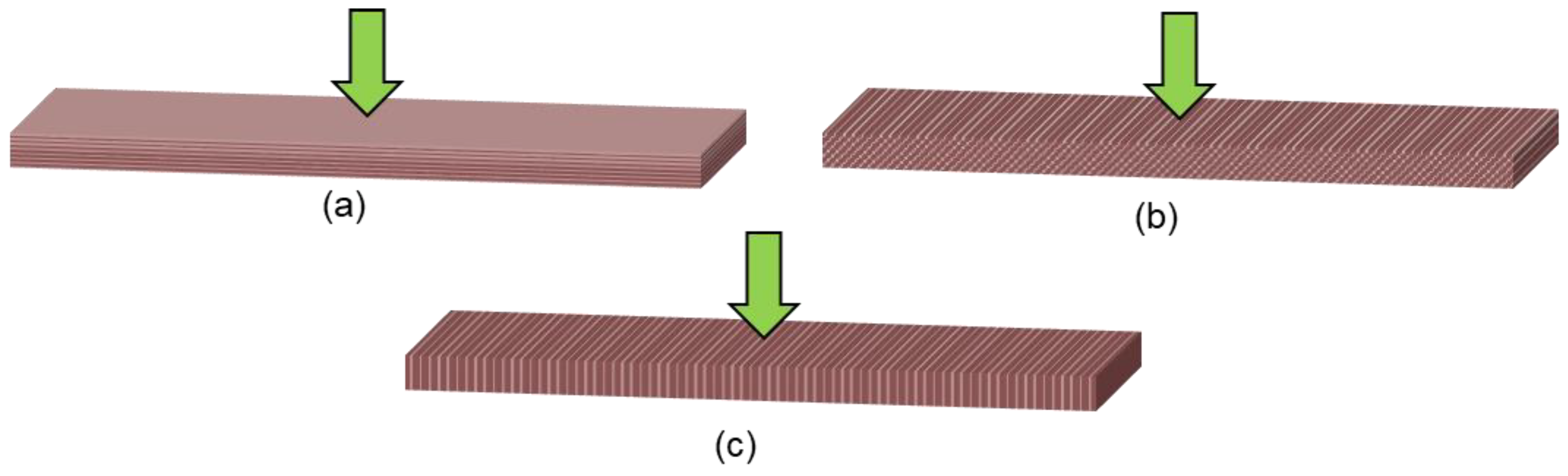
1.3. Effect of Printing Parameters
1.4. Aim
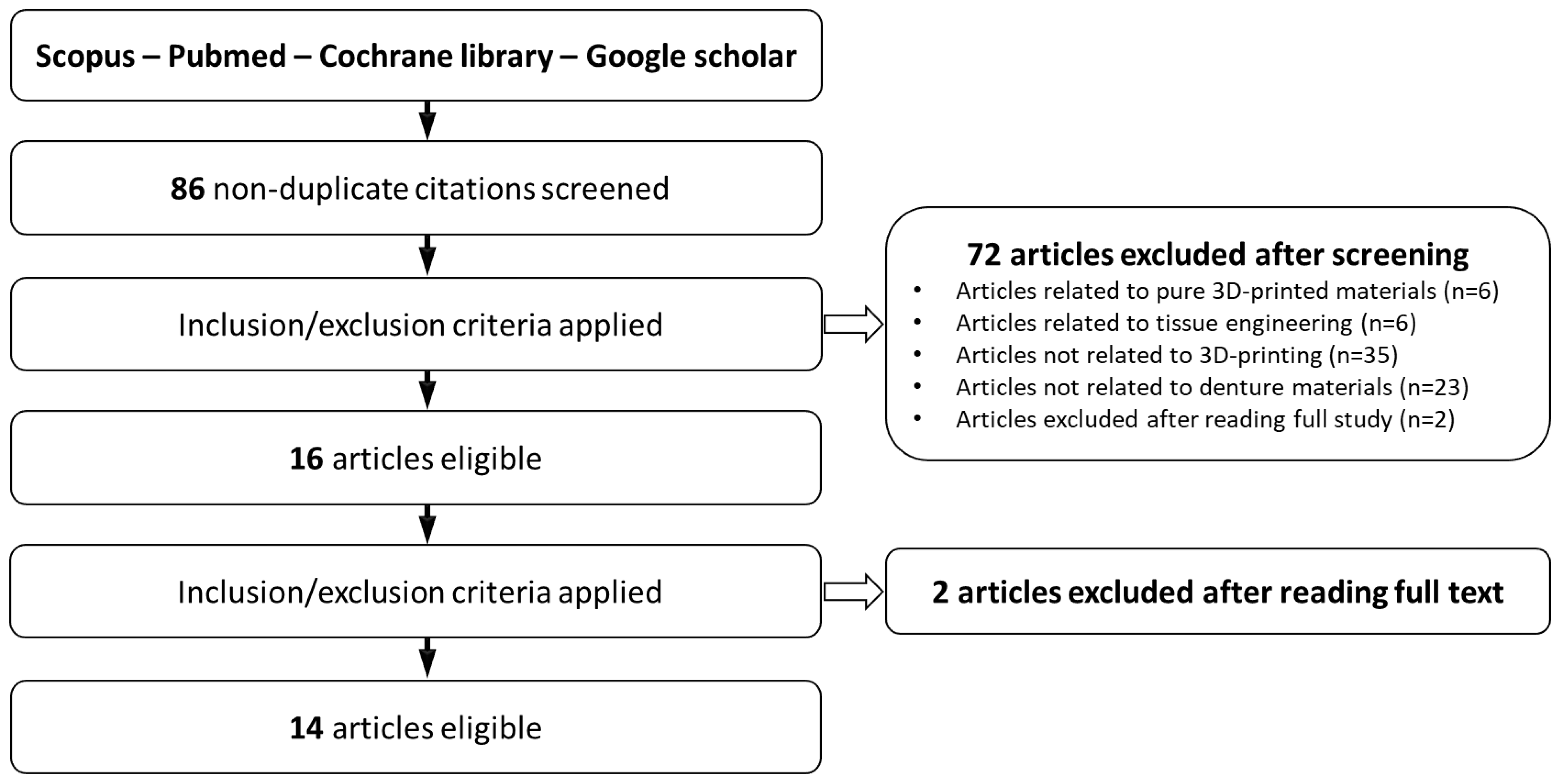
2. Methodology
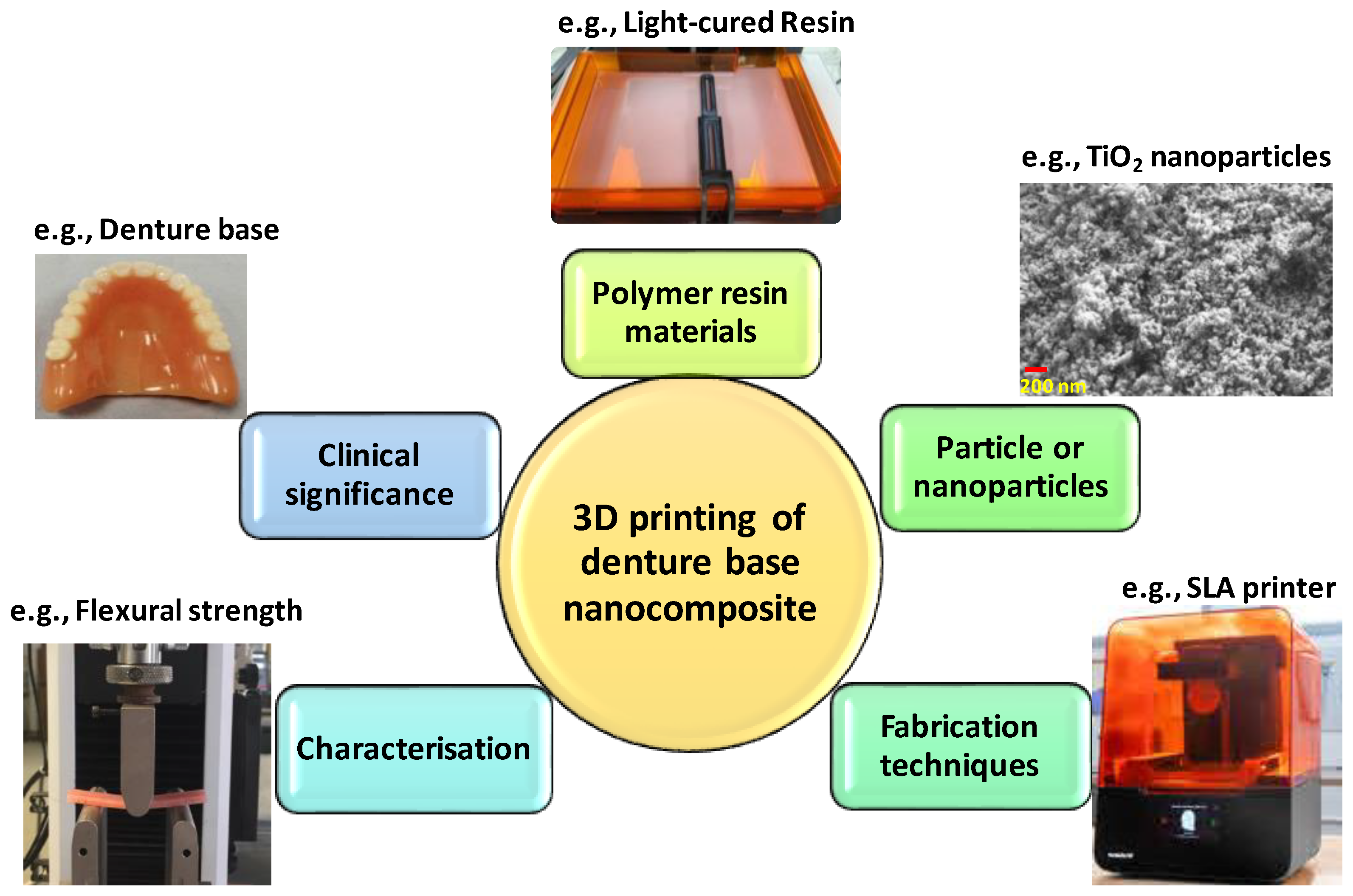
3. Three-Dimensional Printing of Denture Base Nanocomposites
3.1. Denture Base Polymers for 3D Printing
3.2. Denture Base Filler Particles Used for 3D Printing
3.2.1. Titanium Dioxide Nanoparticles and PEEK Microparticles
3.2.2. Nanodiamonds
3.2.3. Silver Nanoparticles (AgNPs)
3.2.4. Thermochromic Pigments
3.2.5. Zirconium Oxide
3.2.6. Silicon Dioxide
3.2.7. Zinc Oxide
3.3. Fabrication Techniques
3.3.1. Functionalisation
3.3.2. Mixing
3.3.3. 3D-Printing Techniques
3.4. Characterisation of the Composite 3D-Printed Denture Base Materials
3.4.1. Physical Characteristics
3.4.2. Mechanical Characteristics
3.4.3. Biological Characteristics
3.5. Clinical Significance
4. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-Printed Nanocomposite Denture-Base Resins: Effect of ZrO2 Nanoparticles on the Mechanical and Surface Properties In Vitro. Nanomaterials 2022, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Eakle, W.S.; Bastin, K.G. Dental Materials: Clinical Applications for Dental Assistants and Dental Hygienists; Elsevier Health Sciences: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Rueggeberg, F.A. From vulcanite to vinyl, a history of resins in restorative dentistry. J. Prosthet. Dent. 2002, 87, 364–379. [Google Scholar] [CrossRef]
- Tasaka, A.; Matsunaga, S.; Odaka, K.; Ishizaki, K.; Ueda, T.; Abe, S.; Yoshinari, M.; Yamashita, S.; Sakurai, K. Accuracy and retention of denture base fabricated by heat curing and additive manufacturing. J. Prosthodont. Res. 2019, 63, 85–89. [Google Scholar] [CrossRef]
- Batisse, C.; Nicolas, E. Comparison of CAD/CAM and Conventional Denture Base Resins: A Systematic Review. Appl. Sci. 2021, 11, 5990. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A comparison of the surface properties of CAD/CAM and conventional polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef]
- Ring, M.E. Dentistry: An Illustrated History; Abrams: New York, NY, USA, 1985. [Google Scholar]
- Murray, M.D.; Darvell, B.W. The Evolution of the Complete Denture Base—Theories of Complete Denture Retention—A Review. Aust. Dent. J. 1993, 38, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Schweiger, J.; Edelhoff, D. Digital dentistry: An overview of recent developments for CAD/CAM generated restorations. Br. Dent. J. 2008, 204, 505–511. [Google Scholar] [CrossRef]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Davidowitz, G.; Kotick, P.G. The use of CAD/CAM in dentistry. Dent. Clin. N. Am. 2011, 55, 559–570. [Google Scholar] [CrossRef]
- Bilgin, M.S.; Baytaroglu, E.N.; Erdem, A.; Dilber, E. A review of computer-aided design/computer-aided manufacture techniques for removable denture fabrication. Eur. J. Dent. 2016, 10, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Rekow, E.D.; Erdman, A.G.; Riley, D.R.; Klamecki, B. CAD/CAM for dental restorations—Some of the curious challenges. IEEE Trans. Biomed. Eng. 1991, 38, 314–318. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hotta, Y. CAD/CAM systems available for the fabrication of crown and bridge restorations. Aust. Dent. J. 2011, 56 (Suppl. S1), 97–106. [Google Scholar] [CrossRef]
- Cristache, C.M.; Totu, E.E.; Iorgulescu, G.; Pantazi, A.; Dorobantu, D.; Nechifor, A.C.; Isildak, I.; Burlibasa, M.; Nechifor, G.; Enachescu, M. Eighteen Months Follow-Up with Patient-Centered Outcomes Assessment of Complete Dentures Manufactured Using a Hybrid Nanocomposite and Additive CAD/CAM Protocol. J. Clin. Med. 2020, 9, 324. [Google Scholar] [CrossRef]
- Lee, S.; Hong, S.J.; Paek, J.; Pae, A.; Kwon, K.R.; Noh, K. Comparing accuracy of denture bases fabricated by injection molding, CAD/CAM milling, and rapid prototyping method. J. Adv. Prosthodont. 2019, 11, 55–64. [Google Scholar] [CrossRef]
- McLaughlin, J.B.; Ramos, V., Jr.; Dickinson, D.P. Comparison of Fit of Dentures Fabricated by Traditional Techniques Versus CAD/CAM Technology. J. Prosthodont. 2019, 28, 428–435. [Google Scholar] [CrossRef]
- Masri, G.; Mortada, R.; Ounsi, H.; Alharbi, N.; Boulos, P.; Salameh, Z. Adaptation of Complete Denture Base Fabricated by Conventional, Milling, and 3-D Printing Techniques: An In Vitro Study. J. Contemp. Dent. Pract. 2020, 21, 367–371. [Google Scholar] [PubMed]
- Prpic, V.; Schauperl, Z.; Catic, A.; Dulcic, N.; Cimic, S. Comparison of Mechanical Properties of 3D-Printed, CAD/CAM, and Conventional Denture Base Materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K.; Miettinen, V.; Alakuijala, P. Residual monomer content and its release into water from denture base materials. Dent. Mater. 1995, 11, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lin, Y.M.; Lai, Y.L.; Lee, S.Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of BisEMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2019, 123, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Devlin, H.; Watts, D.C. Acrylic ‘allergy’? Br. Dent. J. 1984, 157, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in computer-aided manufacturing in prosthodontics: A review of the available streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tesini, F.; Pozzan, M.C.; Zamperoli, E.M.; Carossa, M.; Catapano, S. Comparison of the accuracy between denture bases produced by subtractive and additive manufacturing methods: A pilot study. Prosthesis 2022, 4, 151–159. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A review of 3D printing in dentistry: Technologies, affecting factors, and applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef] [PubMed]
- Mubaraki, M.Q.; Moaleem, M.M.A.; Alzahrani, A.H.; Shariff, M.; Alqahtani, S.M.; Porwal, A.; Al-Sanabani, F.A.; Bhandi, S.; Tribst, J.P.M.; Heboyan, A. Assessment of Conventionally and Digitally Fabricated Complete Dentures: A Comprehensive Review. Materials 2022, 15, 3868. [Google Scholar] [CrossRef]
- ETEC. 2023. Available online: https://etec.desktopmetal.com/wp-content/uploads/2022/06/ETEC_PRD_SPEC_EnvisionOne_220606.pdf (accessed on 1 December 2023).
- Formlabs. Form2 3D-Printer. 2023. Available online: https://formlabs.com/uk/3d-printers/form-2/tech-specs/ (accessed on 1 December 2023).
- ASIGA. ASIGA MAX UV. 2023. Available online: https://www.asiga.com/max/ (accessed on 1 December 2023).
- 3D-Systems. NextDent 5100 3D-Printer. 2023. Available online: https://www.3dsystems.com/3d-printers/nextdent-5100 (accessed on 1 December 2023).
- Revilla-León, M.; Özcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of printing layer thickness and postprinting conditions on the flexural strength and hardness of a 3D-printed resin. BioMed Res. Int. 2022, 835, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 638,905, 1984. [Google Scholar]
- Al-Dulaijan, Y.A.; Alsulaimi, L.; Alotaibi, R.; Alboainain, A.; Alalawi, H.; Alshehri, S.; Khan, S.Q.; Alsaloum, M.; AlRumaih, H.S.; Alhumaidan, A.A. Comparative Evaluation of Surface Roughness and Hardness of 3D Printed Resins. Materials 2022, 15, 6822. [Google Scholar] [CrossRef] [PubMed]
- Infuehr, R.; Pucher, N.; Heller, C.; Lichtenegger, H.; Liska, R.; Schmidt, V.; Kuna, L.; Haase, A.; Stampfl, J. Functional polymers by two-photon 3D lithography. Appl. Surf. Sci. 2007, 254, 836–840. [Google Scholar] [CrossRef]
- Petrovic, V.; Vicente Haro Gonzalez, J.; Jordá Ferrando, O.; Delgado Gordillo, J.; Ramón Blasco Puchades, J.; Portolés Griñan, L. Additive layered manufacturing: Sectors of industrial application shown through case studies. Int. J. Prod. Res. 2011, 49, 1061–1079. [Google Scholar] [CrossRef]
- Singh, V. Rapid prototyping, materials for RP and applications of RP. Int. J. Eng. Res. Sci. 2013, 4, 473–480. [Google Scholar]
- Fahad, M.; Dickens, P.; Gilbert, M. Novel polymeric support materials for jetting based additive manufacturing processes. Rapid Prototyp. J. 2013, 19, 230–239. [Google Scholar] [CrossRef]
- Miller, F.P.; Vandome, A.F.; McBrewster, J. Fused Deposition Modeling; VDM Publishing: Saarbrücken, Germany, 2010; pp. 13–34. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing; Springer: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping: Principles and Applications (with Companion CD-ROM); World Scientific: Singapore, 2010. [Google Scholar]
- Torabi, K.; Farjood, E.; Hamedani, S. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J. Dent. 2015, 16, 1. [Google Scholar]
- Kruth, J.P.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Traini, T.; Mangano, C.; Sammons, R.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef]
- Averyanova, M. Quality control of dental bridges and removable prostheses manufactured using Phenix systems equipment. Proceedings of AEPR’12, 17th European Forum on Rapid Prototyping and Manufacturing, Paris, France, 12–14 June 2012. [Google Scholar]
- Abou Tara, M.; Eschbach, S.; Bohlsen, F.; Kern, M. Clinical outcome of metal-ceramic crowns fabricated with laser-sintering technology. Int. J. Prosthodont. 2011, 24, 46. [Google Scholar] [PubMed]
- Bencharit, S.; Staffen, A.; Yeung, M.; Whitley, D.; Laskin, D.M.; Deeb, G.R. In Vivo Tooth-Supported Implant Surgical Guides Fabricated with Desktop Stereolithographic Printers: Fully Guided Surgery Is More Accurate Than Partially Guided Surgery. J. Oral Maxil. Surg. 2018, 76, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Deeb, G.R.; Allen, R.K.; Hall, V.P.; Whitley, D.; Laskin, D.M.; Bencharit, S. How Accurate Are Implant Surgical Guides Produced With Desktop Stereolithographic 3-Dimentional Printers? J. Oral Maxil. Surg. 2017, 75, 2559.e2551–2559.e2558. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.E.; Bergauer, B.; Adler, W.; Wichmann, M.; Nickenig, H.J. The impact of the fabrication method on the three-dimensional accuracy of an implant surgery template. J. Cranio Maxill. Surg. 2017, 45, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Vayrynen, V.O.E.; Tanner, J.; Vallittu, P.K. The anisotropicity of the flexural properties of an occlusal device material processed by stereolithography. J. Prosthet. Dent. 2016, 116, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of build direction on the mechanical properties of 3D-printed complete coverage interim dental restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, E324–E333. [Google Scholar] [CrossRef]
- Park, J.M.; Ahn, J.S.; Cha, H.S.; Lee, J.H. Wear Resistance of 3D Printing Resin Material Opposing Zirconia and Metal Antagonists. Materials 2018, 11, 1043. [Google Scholar] [CrossRef]
- Chockalingam, K.; Jawahar, N.; Chandrasekhar, U. Influence of layer thickness on mechanical properties in stereolithography. Rapid Prototyp. J. 2006, 12, 106–113. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.; Cristache, C.; Işildak, İ.; Yildirim, R.; Burlibasa, M.; Niğde, M.; Burlibasa, L. Preliminary studies on citotoxicity and genotoxicity assessment of the PMMA-TiO2 nanocompozites for stereolithographic complete dentures manufacturing. Mater. Sci. Med. 2018, 69, 1160–1165. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.Z.; Jia, Y.G.; Lu, B.H.; Ren, L. TiO2 and PEEK Reinforced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Li, K.; Lu, B.; Ren, L. Carboxylic acid-functionalized TiO2 nanoparticle-loaded PMMA/PEEK copolymer matrix as a dental resin for 3D complete denture manufacturing by stereolitographic technique. Int. J. Food Prop. 2018, 21, 2557–2565. [Google Scholar] [CrossRef]
- Mangal, U.; Seo, J.Y.; Yu, J.; Kwon, J.S.; Choi, S.H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials 2020, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Mangal, U.; Min, Y.J.; Seo, J.Y.; Kim, D.E.; Cha, J.Y.; Lee, K.J.; Kwon, J.S.; Choi, S.H. Changes in tribological and antibacterial properties of poly(methyl methacrylate)-based 3D-printed intra-oral appliances by incorporating nanodiamonds. J. Mech. Behav. Biomed. Mater. 2020, 110, 103992. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. A Study of 3D-Printable Reinforced Composite Resin: PMMA Modified with Silver Nanoparticles Loaded Cellulose Nanocrystal. Materials 2018, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zheng, S.; Chen, S.; Zhao, L.; Huang, X.; Huang, L.; Kang, S. Surface silanization and grafting reaction of nano-silver loaded zirconium phosphate and properties strengthen in 3D-printable dental base composites. J. Mech. Behav. Biomed. Mater. 2020, 110, 103864. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.; Li, Y.; Li, B.; Zhu, P. 3D-printable thermochromic acrylic resin with excellent mechanical performance. J. Appl. Polym. Sci. 2020, 137, 48277. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Harbi, F.A.; Akhtar, S.; Fouda, S.M. 3D-Printable Denture Base Resin Containing SiO2 Nanoparticles: An In Vitro Analysis of Mechanical and Surface Properties. J. Prosthodont. 2022, 31, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Aneja, S.; Kassar, M.; Leung, R.; Nguyen, A.; Tran, S.; Shrestha, B.; Fawzy, A. Silver-loaded mesoporous silica nanoparticles enhanced the mechanical and antimicrobial properties of 3D printed denture base resin. J. Mech. Behav. Biomed. 2022, 134, 105421. [Google Scholar] [CrossRef] [PubMed]
- Khattar, A.; Alsaif, M.H.; Alghafli, J.A.; Alshaikh, A.A.; Alsalem, A.M.; Almindil, I.A.; Alsalman, A.M.; Alboori, A.J.; Al-Ajwad, A.M.; Almuhanna, H.M. Influence of ZrO2 Nanoparticle Addition on the Optical Properties of Denture Base Materials Fabricated Using Additive Technologies. Nanomaterials 2022, 12, 4190. [Google Scholar] [CrossRef] [PubMed]
- Al-Douri, M.E.; Sadoon, M.M. Flexural Strength, Hardness and Surface Roughness of 3D Printed Denture Base Resin Reinforced by Zinc Oxide Nanoparticles. J. Res. Med. Dent. Sci. 2023, 11, 194–200. [Google Scholar]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA Denture Base Materials Containing Titanium Dioxide Nanoparticles: A Literature Review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Napankangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of Nanodiamond Addition on Flexural Strength, Impact Strength, and Surface Roughness of PMMA Denture Base. J. Prosthodont. 2019, 28, e417–e425. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Fouda, S.M.; Napankangas, R.; Raustia, A. Flexural and Surface Properties of PMMA Denture Base Material Modified with Thymoquinone as an Antifungal Agent. J. Prosthodont. 2020, 29, 243–250. [Google Scholar] [CrossRef]
- Cierech, M.; Osica, I.; Kolenda, A.; Wojnarowicz, J.; Szmigiel, D.; Lojkowski, W.; Kurzydlowski, K.; Ariga, K.; Mierzwinska-Nastalska, E. Mechanical and Physicochemical Properties of Newly Formed ZnO-PMMA Nanocomposites for Denture Bases. Nanomaterials 2018, 8, 305. [Google Scholar] [CrossRef]
- Chaijareenont, P.; Takahashi, H.; Nishiyama, N.; Arksornnukit, M. Effect of different amounts of 3-methacryloxypropyltrimethoxysilane on the flexural properties and wear resistance of alumina reinforced PMMA. Dent. Mater. J. 2012, 31, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, L.; Shirkavand, S.; Akbari, F.; Sabzikari, N. Tensile strength and impact strength of color modified acrylic resin reinforced with titanium dioxide nanoparticles. J. Clin. Exp. Dent. 2017, 9, e661–e665. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.; Yang, Y.; Jiang, Z.; Deng, Y.; Song, S.; Yang, W.; Chen, Z.G. Bioinspired, biocompatible and peptide-decorated silk fibroin coatings for enhanced osteogenesis of bioinert implant. J. Biomater. Sci. Polym. Ed. 2018, 29, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.L.; Carbone, S.; Abdelgaied, A.; Fisher, J.; Jennings, L.M. Influence of contact pressure, cross-shear and counterface material on the wear of PEEK and CFR-PEEK for orthopaedic applications. J. Mech. Behav. Biomed. Mater. 2016, 63, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Visser, J.M.; Polikeit, A. The long-term mechanical integrity of non-reinforced PEEK-OPTIMA polymer for demanding spinal applications: Experimental and finite-element analysis. Eur. Spine J. 2006, 15, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lumkemann, N.; Eichberger, M.; Stawarczyk, B. Different PEEK qualities irradiated with light of different wavelengths: Impact on Martens hardness. Dent. Mater. 2017, 33, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al Ghamdi, M.A.; Khan, S.Q.; Akhtar, S.; Al Eraky, D.M.; Al-Harbi, F.A. Effect of Low Nanodiamond Concentrations and Polymerization Techniques on Physical Properties and Antifungal Activities of Denture Base Resin. Polymers 2021, 13, 4331. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; A Al Ghamdi, M.; Khan, S.Q.; Akhtar, S.; Ali, M.S.; Al-Harbi, F.A. Flexural properties, impact strength, and hardness of nanodiamond-modified PMMA denture base resin. Int. J. Biomater. 2022, 2022, 6583084. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Elbadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Du, Z.; Zhu, S.; Geng, H.; Zhang, X.; Cai, Q.; Yang, X. Composite resin reinforced with silver nanoparticles-laden hydroxyapatite nanowires for dental application. Dent. Mater. 2017, 33, 12–22. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural strength and hardness of filler-reinforced PMMA targeted for denture base application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing fracture toughness and impact strength of PMMA reinforced with nano-particles and fibre as advanced denture base materials. Materials 2021, 14, 4127. [Google Scholar] [CrossRef]
- Gad, M.M.A.; Abualsaud, R.; Al-Thobity, A.M.; Almaskin, D.F.; AlZaher, Z.A.; Abushowmi, T.H.; Qaw, M.S.; Akhtar, S.; Al-Harbi, F.A. Effect of SiO2 nanoparticles addition on the flexural strength of repaired acrylic denture base. Eur. J. Dent. 2020, 14, 019–023. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Bahador, A.; Khalil, S.; Shahroudi, A.S.; Kassaee, M.Z. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Salahuddin, N.; El-Kemary, M.; Ibrahim, E. Reinforcement of polymethyl methacrylate denture base resin with ZnO nanostructures. Int. J. Appl. Ceram. Technol. 2018, 15, 448–459. [Google Scholar] [CrossRef]
- Vikram, S.; Chander, N.G. Effect of zinc oxide nanoparticles on the flexural strength of polymethylmethacrylate denture base resin. Eur. Oral Res. 2020, 54, 31–35. [Google Scholar] [CrossRef]
- Pritchard, G. Plastics Additives: An A-Z Reference; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Karami, P.; Salkhi Khasraghi, S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites—A comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef]
- Pentecost, A.; Gour, S.; Mochalin, V.; Knoke, I.; Gogotsi, Y. Deaggregation of nanodiamond powders using salt- and sugar-assisted milling. ACS Appl Mater Interfaces 2010, 2, 3289–3294. [Google Scholar] [CrossRef]
- Harini, P.; Mohamed, K.; Padmanabhan, T.V. Effect of Titanium dioxide nanoparticles on the flexural strength of polymethylmethacrylate: An in vitro study. Indian J. Dent. Res. 2014, 25, 459–463. [Google Scholar] [CrossRef]
- BS EN ISO 10477; Dentistry—Polymer-Based Crown and Veneering Materials. International Organization for Standardization: Geneva, Switzerland, 2020.
- BS EN ISO 20795-1; Dentistry-Base Polymers–Part 1: Denture Base Polymers. ISO: Geneva, Switzerland, 2013.
- Tsuji, M.; Ueda, T.; Sawaki, K.; Kawaguchi, M.; Sakurai, K. Biocompatibility of a titanium dioxide-coating method for denture base acrylic resin. Gerodontology 2016, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Lopez-Marin, L.M.; Nunez-Anita, R.E.; Hernandez-Padron, G.; Castano, V.M. Biocompatible Metal-Oxide Nanoparticles: Nanotechnology Improvement of Conventional Prosthetic Acrylic Resins. J. Nanomater. 2011, 2011, 941561. [Google Scholar] [CrossRef]
- BS EN ISO 10993–5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Cristache, C.M.; Totu, E.E.; Grosu, A.R.; Ene, O.; Beuran, I.A.; Burlibasa, M. Nanocomposite for rapid prototyped complete denture. Rev. Chim. 2019, 70, 387–392. [Google Scholar] [CrossRef]
- Rekow, E.D. Digital dentistry: The new state of the art—Is it disruptive or destructive? Dent. Mater. 2020, 36, 9–24. [Google Scholar] [CrossRef]

| Related Factors | Conventional Method | Subtractive Manufacturing | Additive Manufacturing |
|---|---|---|---|
| Human errors |  |  |  |
| Clinical chair time |  |  |  |
| Number of visits |  |  |  |
| Digital archiving | N/A |  |  |
| Patient-centred outcomes |  |   |   |
| Mechanical properties |  |  |  |
| Physical properties |  |  |  |
| Accuracy of the fitted surface |  |   |   |
| Dimensional stability |  |  |  |
| Shrinkage |  |  |  |
| Resin type | PMMA | PMMA | Dimethacrylate-based polymers |
| Total cost of the treatment |  |   |  |
| Total cost in 10 years |  |  |   |
| New training |  |  |  |
| Parameters | 3D Printers | |||
|---|---|---|---|---|
| Formlabs Form2 | NextDent 5100 | EnvisionTEC One | ASIGA Max UV | |
| Country | USA | Netherland | USA | Australia |
| Printer technology | SLA | DLP | DLP | DLP |
| Light source | UV laser | LED light projector | LED light projector | LED light projector |
| Light source wavelength (nm) | 405 | 405 | 385 | 385 |
| Light source resolution (micron) | N/A (laser) | 65 | 93 | 62 |
| Laser power (mW) | 250 | n/a | n/a | n/a |
| Layer thickness (micron) | 25–300 | n/a | n/a | n/a |
| Parameters | Post-Cure Boxes | ||
|---|---|---|---|
| Formlabs | NextDent | EnvisionTec | |
| Country | USA | The Netherlands | USA |
| Number of light sources | 13 | 12 | 36 |
| LED power per light source (W) | 39 | 18 | n/a |
| LED wavelength (nm) | 405 | 300–550 | 390–420 |
| Maximum temperature | 80 °C | 60–80 °C | 70 °C |
| Printing Parameters | Effects |
|---|---|
| Printing orientations | Horizontal orientation improves the flexural strength [58,59] and the compressive strength [58]. Vertical orientation improves the flexural strength [57,60] and the flexural modulus [57]. Horizontal orientation improves the accuracy of the print [61]. |
| Position on the build platform | Printing in the middle of the build platform improves the accuracy of the print [60]. |
| Layer thickness | Thinner layering improves the tensile and the impact strength [62]. |
| Reference | Added Particles/Weight Percentage | 3D-Printing Technology | Characterisation | Specimen Shape and Size | Clinical Significance |
|---|---|---|---|---|---|
| Totu et al. [63,64] | TiO2 (0.1–2.5 wt%) Particle size: 56–170 nm | DLP | Biological/Antimicrobial: Candida species: C. scotti Bacterial species: Staphylococcus aureus Cytotoxicity: fibroblasts | N/A | The denture made with this material can resist fungal growth. |
| Microscopy: SEM, EDX | |||||
| Chemical: FTIR | |||||
| Chen et al. [65,66] | TiO2/PEEK (1–4 wt%) Particle size: 30–40 nm | SLA/DLP | Biological/Antimicrobial: Candida species: C. scotti; Bacterial species: E. coli, Staph aureus; Cytocompatibility/Cytotoxicity | N/A | The denture made with this material has better mechanical properties and can resist bacterial and fungal growth. |
| Microscopy: SEM; TEM | |||||
| Chemical: FTIR; XRD | |||||
| Mechanical: Flexural strength and modulus; Impact strength | −64 × 10 × 3.3 mm | ||||
| Mangal et al. [67,68] | Nanodiamonds (0.1 wt%) Particles size: 4–255 nm | DLP | Biological/Antimicrobial: Bacterial species: Streptococcus mutans | −20 × 20 × 3.5 mm | 3D-printed objects made with composite materials have better mechanical and physical quality compared to the original material. Resistance to S. mutans is proven. |
| Physical: Surface roughness; Accuracy; Water sorption and solubility, Hydrophilicity | −15 × 1 mm disc | ||||
| Chemical: FTIR | N/A | ||||
| Microscopy: SEM; TEM | |||||
| Mechanical: Flexural strength and modulus; Impact strength; Hydrothermal fatigue; Surface microhardness | −64 × 10 × 3.3 mm −64 × 12.7 × 3.2 mm | ||||
| Tribological: Wear resistance | N/A | ||||
| Chen et al. [69] | Cellulose nanocrystals (CNCs)-silver nanoparticles (AgNPs) (0.05, 0.1, 0.15, 0.2 and 0.25 wt%) | DLP | Biological/Antimicrobial: Bacterial species: Staphylococcus aureus, E. coli Cytocompatibility/Cytotoxicity: fibroblasts | N/A | Composite resin (CNCs-AgNPs 0.10–0.25 wt%) exhibits strong antibacterial activity with no discernible cytotoxic impact. Due to the uniform distribution of AgNPs throughout the resin matrix, the group with CNCs-AgNPs of 0.10 wt% exhibited the best mechanical qualities. |
| Chemical: FTIR | −64 × 10 × 3.3 mm | ||||
| Microscopy: TEM | N/A | ||||
| Radiation: XPS | |||||
| Mechanical: Flexural strength, elastic modulus and impact strength | 64 × 10 × 3.3 mm | ||||
| Liao et al. [70] | P-6S-NP3 (1–3 wt%) Particle size: 3100–3500 nm 6S-NP3 (3 wt%) Particle size: up to 4100 nm | DLP | Biological/Antimicrobial: Bacterial species: Staphylococcus aureus, E. coli | 64 mm × 10 mm × 3.3 mm | 3D-printed objects made with composite material have better mechanical and physical quality compared to the original resin. Resistance to S. aureus and E. coli is proven. |
| Physical: Water contact angle | |||||
| Chemical: FTIR; XRD | |||||
| Microscopy: SEM | |||||
| Mechanical: Flexural strength and modulus; Impact strength | |||||
| Duan et al. [71] | Thermochromic pigments (0.3–1.0 wt%) Particle size: 3–4 µm | SLA | Physical: Conversion rate; Colour change behaviour | −13 × 5 mm cylinder Eiffel tower structure sample | Objects or appliances printed with this composite material have better mechanical properties and provide a thermosensor function to them |
| Chemical: FTIR | |||||
| Microscopy: SEM | |||||
| Mechanical: Tensile strength; Young’s modulus; Elongation at break | |||||
| Alshaikh et al. [2] | ZrO2 nanoparticles (0.5–5.0 wt%) Particles size = 40 nm | DLP | Flexure strength and elastic modulus | 64 × 10 × 3.3 mm | The ZrO2NPs supplement enhanced the 3D-printed resins’ mechanical properties. |
| Impact strength | 50 × 6 × 4 mm | ||||
| Surface roughness and hardness | 15 × 2 mm | ||||
| Gad et al. [72] | SiO2 Nanoparticles (0.25 wt% and 0.50 wt%) | DLP | Physical: Surface roughness and hardness | 64 × 10 × 3.3 mm | While the inclusion of Silicon dioxide nanoparticles did not result in any significant changes in the surface roughness, the result revealed an overall increase in the mechanical properties of the modified 3D-printed resin. |
| Mechanical: Flexural strength, Impact strength | 10 × 2 mm | ||||
| Aati et al. [73] | Ag/MSN (0.0–2.0 wt%) Size = 3 nm | DLP | Biological/Antifungal: Fungal species: C. albicans Cytotoxicity: human oral fibroblasts | N/A | The addition of Ag/MSN significantly enhanced the mechanical and antimicrobial properties without showing adverse effect on human fibroblasts. |
| Physical: Surface hardness, surface roughness, water sorption and solubility, FTIR | −15 × 1 mm disc | ||||
| Microscopy: TEM, AFM | N/A | ||||
| Mechanical: Flexural strength, fracture toughness | −65 × 10 × 3.3 mm −39 × 8.0 × 4.0 mm | ||||
| Khattar et al. [74] | ZrO2 nanoparticles (0.5–5.0 wt%) Size = 40 nm | DLP | Biological/Antifungal: Fungal species: C. albicans | −15 × 2 mm | Incorporating ZrO2 nanoparticles into 3D-printed resin at a low concentration (0.5 wt%) leads to a significant reduction in the adhesion of C. albicans without affecting the surface roughness of the printed material. |
| Physical: surface roughness | |||||
| Al-Douri et al. [75] | ZnO nanoparticles (2.0, 3.0, and 4.0 wt%) | DLP | Physical: Surface hardness, surface roughness | N/A | The addition of ZnO improved the flexural strength and decreased the surface roughness, while the surface hardness was not affected. |
| Mechanical: flexural strength | −64 × 10 × 3.3 mm |
| Commercial Name | Digital Denture | Denture 3D+ | Denture Base II | DentureTEC | Denture |
|---|---|---|---|---|---|
| Company | Formlabs | NextDent | DENTCA | Saremco | Detax |
| Printing wavelength (nm) | 405 | 405 | 385/405 | 385 | 385 |
| Printing temperature (°C) | 31 | 18–28 | 18–30 | 35 | 23–25 |
| Post-cure time (minutes) | 30 | 30 | 20–60 | 4000 flashes (N* gas) | 2000 flashes (N* gas) |
| Post-cure temperature (°C) | 80 | 60 | >60 | Not needed | Not needed |
| Post-cure wavelength (nm) | 405 | 300–550 | 320–405 | 320–500 | 320–500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. Current Progress in the Development of Resin Materials with Nanofillers for 3D Printing of Denture Base. Prosthesis 2024, 6, 770-797. https://doi.org/10.3390/prosthesis6040055
Altarazi A, Haider J, Alhotan A, Silikas N, Devlin H. Current Progress in the Development of Resin Materials with Nanofillers for 3D Printing of Denture Base. Prosthesis. 2024; 6(4):770-797. https://doi.org/10.3390/prosthesis6040055
Chicago/Turabian StyleAltarazi, Ahmed, Julfikar Haider, Abdulaziz Alhotan, Nikolaos Silikas, and Hugh Devlin. 2024. "Current Progress in the Development of Resin Materials with Nanofillers for 3D Printing of Denture Base" Prosthesis 6, no. 4: 770-797. https://doi.org/10.3390/prosthesis6040055
APA StyleAltarazi, A., Haider, J., Alhotan, A., Silikas, N., & Devlin, H. (2024). Current Progress in the Development of Resin Materials with Nanofillers for 3D Printing of Denture Base. Prosthesis, 6(4), 770-797. https://doi.org/10.3390/prosthesis6040055










