Abstract
Purpose: In incomplete spinal cord injury (SCI), there is a partial decrease in motor or sensory or autonomic function. Mainly due to the motor impairment in SCI, a muscle–machine interface is a tool that can bring functional benefits to this population. Objective: To investigate the feasibility of the non-invasive myoelectric signal–functional electrical stimulation (MES-FES) interface on the response of the quadriceps muscle in an individual with incomplete SCI. Methods: This is a quasi-experimental, uncontrolled, longitudinal case report study carried out with an individual with incomplete SCI in the chronic phase. The assessments performed before (pre) and after eight (post8) interventions were neuromuscular assessment (surface electromyography (EMG) in rectus femoris (RF) and vastus lateralis (VL) muscles); muscle strength (load cell); knee extension range of motion (goniometry); spasticity (Modified Ashworth Scale); and quality of life (Spinal Cord Injury Quality-of-Life Questionnaire (SCI-QoL.Br)). The MES-FES interface was associated with physical therapy exercises on the extension knee joint muscle group. Results: Improvement in neuromuscular activation (normalized increase in EMGRMS of 2% (RF) and 3.3% (VL)) and synchronism of the motor units (normalized reduction in EMGMDF of 22.8% (RF) and 5.9% (VL)); 1.4 kgf increase in quadriceps strength; 10.6° increase in knee joint extension amplitude; 1 point spasticity reduction; improved quality of life, confirmed by a 12-point reduction in the SCI-QoL.Br score. Moreover, along with interventions, the participant increased the correct FES activation rate, indicating a user learning curve ( = 0.78, p-value = 0.04). Conclusions: The MES-FES interface associated with physical therapy promotes neuromuscular and quality of life improvements in the SCI participant.
1. Introduction
Spinal cord injury (SCI) is defined by the partial decrease or total loss of motor or sensory or autonomic function of the spinal cord below the level of the lesion, resulting from the interruption of the nervous tracts. The worldwide incidence of SCI is estimated to be between 250,000 and 500,000 per year [1]. The SCI can be classified as complete or incomplete. In complete SCI, there is sensory loss and total motor paralysis below the level of the injury, including the sacral roots, due to the complete interruption of the nervous tracts. On the other hand, in incomplete SCI, there is the preservation of muscle groups or sensitive areas that were not affected [2].
Due to motor impairment in SCI, functionality is restricted in activities of daily living related to standing and walking. Associated with the motor condition, autonomic changes, such as intestinal, urinary, and sexual dysfunctions, considerably impact the quality of life of people with SCI. The assessment of the quality of life in this population is of great importance, as it expands the decisions of the health team, thus enabling a more significant promotion of the individual’s well-being. One of the scales used to assess the quality of life of individuals with SCI is the Spinal Cord Injury Quality-of-Life Questionnaire (SCI QoL.Br).
Surface functional electrical stimulation (FES) is a low-frequency alternating current whose main objective is to generate a contraction response in the muscle’s motor units through electrical stimulation. FES has been widely used in cases of SCI, proving to be effective in improving muscle health, mainly by partially reversing or delaying muscle atrophy and intramuscular fat content, normalizing glucose uptake and metabolism, and increasing the generation of force, thus contributing positively to secondary outcomes such as metabolic diseases and musculoskeletal deficiencies, as well as improving physical capacity and rehabilitation [3].
Neuroplasticity is a broad term that refers to the many ways in which the nervous system can adapt to changes in environmental conditions that occur daily in the lives of individuals. This neural reorganization is a primary objective of neural recovery to assist in the recovery of physical functionality, which can be influenced by experience, behavior, and practice of activities [4]. Then, neuroplasticity allows the central nervous system to develop skills and remember information, reorganizing neuronal networks in response to environmental stimulation and recovering from brain injuries and SCI [5]. According to Balbinot et al. [6], the myoelectrical surface signal (MES) can capture the residual motor command of the muscle in great detail, even below the lesion level with apparently absent motor activities. Therefore, the MES detects the minimum contraction the individual performs and triggers the FES, which, in turn, performs the desired movement.
Therefore, this study aimed to investigate the viability of the MES-FES interface on the quadriceps femoris muscle response in an individual with incomplete SCI. As a hypothesis, the interventions are expected to be viable within a clinical or home environment and improve the neuromuscular activation of the quadriceps femoris.
2. Materials and Methods
The ethics committee approved the research involving human beings at the State University of Londrina (CEP-UEL) n° 4,060,700. Due to the characteristic of the study being quasi-experimental, seeking to evaluate the application system as a method of physical rehabilitation, the project was not submitted to the clinical trials committee. All steps were performed in the neural engineering and rehabilitation laboratory (LENeR), located in the Anatomy Department of UEL—Brazil.
2.1. Case Report
The participant is a female, 32 years old, with a clinical diagnosis of SCI due to an automobile accident in March 2019. According to the initial assessment by ASIA, the participant was classified in T5 “A” in the spinal shock phase. In the first days after the accident, magnetic resonance imaging (MRI) was performed, and signal alteration was observed in the spinal cord at the T3–T4 level, suggestive of edema. Approximately six months after SCI, some lower limb movements started to return (which at first were null), which happened slowly and progressively. The MRI was repeated in July 2021, with corresponding signs of myelomalacia and degeneration of the lateral corticospinal tracts at the T3–T4 level. Despite the persisting spinal cord alterations, the participant has an incomplete SCI, with a neurological level T5—classified as “D” on the ASIA Impairment Scale (AIS). She uses a wheelchair for locomotion, drives independently, and remains orthostatic only with significant support. Regarding physiological functions, she has urinary incontinence, bowel continence, and a normal menstrual cycle.
2.2. Assessments
The assessments were performed one week before (pre) the first intervention and one week after the eighth (post8) intervention.
2.2.1. Neuromuscular Assessment—Surface Electromyography (EMG)
For neuromuscular evaluation (as shown in Figure 1) a Bitalino®, electromyograph, MuscleBIT model, Bluetooth, with a resolution of 10 bits per channel, was used. The program used to acquire the signal was OpenSignals®, controlled by a smartphone with the Android system®. The participant was instructed to perform the trichotomy of the evaluated region. This process could occur at her home or in the laboratory, along with skin asepsis.
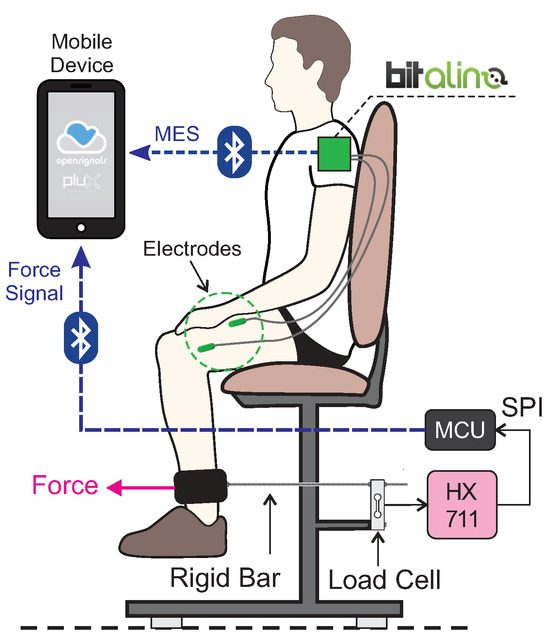
Figure 1.
Illustration of assessment equipment. Equipment representing Bitalino® biosignalsplux, PLUX wireless biosignals S.A. (Lisbon, Portugal), taken from https://bitalino.com/products/musclebit-bt on 11 July 2021.
Four channels (3M® Ag/AgCl electrodes) were used bilaterally on the rectus femoris and vastus lateralis muscles, with the reference electrode positioned on the right iliac crest. The acquisition frequency was 1 kHz. The participant was asked to perform the knee joint extension movement through the verbal command “extend your leg as much as you can” for approximately 5 s. This activity was performed twice with an interval of approximately 10 s between the repetitions. Simultaneously, a digital output in the program OpenSignals® was activated for demarcation and further processing. The same evaluation was performed on the biceps brachii muscle on the right side as a normalization criterion.
A customized routine of the free program Octave® was used to process the electromyographic signals. The filtering was a fourth-order Butterworth with a pass band 10–450 Hz, and a reject band over the mains harmonics (60, 120, 180, 240, 300, 360, and 420 Hz). From the 5 s of activity, two intermediates were selected, where the root mean squared (RMS) value descriptors were extracted (), as well as median frequency (), time domain, and frequency domain. The average of the two activities was taken for each descriptor. Wavelet processing could also be applied, depending on the algorithm used in [7].
Maximum voluntary isometric contraction is one of the most used ways to assess the EMG signal. On the other hand, people with SCI often do not have any voluntary contractions. Unlike other neurological conditions, such as after a stroke, where the affected side can be compared with the healthy side, this is impossible in SCI. However, in Fukuda et al. [8] EMG signals were captured from 24 young female participants, from 20 ± six years old, physically active, with a body mass index between 18.5 kg/m2 and 25 kg/m2. The muscles analyzed were the rectus femoris and the biceps brachii through the . Activities were performed during maximum voluntary isometric contraction and holding dumbbells of (I) 2 kg, (II) 4 kg, (III) 6 kg, and (IV) 8 kg. Normalizing the mean values along the activities of the rectus femoris by the biceps brachii, a value of 0.54 was found. Thus, the normal value during a contraction of the rectus femoris muscle is approximately 54% of the biceps brachii muscle. Based on this study, signal normalization was performed by the biceps brachii muscle on the right side (left when the right side is not accessible), as shown in Equation (1):
in which is the rectus femoris muscle or the vastus lateralis muscle. is the normalized data with a percentage value of the biceps brachii muscle in the pre-assessment () and is the pre or post8 evaluation.
2.2.2. Strength Assessment
The acquisition of the strength signal was performed simultaneously with the acquisition of the neuromuscular signal (Section 2.2.1). As instrumentation, a load cell was used; brand Alfa Instrumentos Eletrônicos® model GL20; aluminum measuring up to 20 kgf. The load cell was fixed to the adapted chair and connected to the distal third of the participant’s right leg. (Figure 1). The connection was made through a rigid threaded bar attached to a PVC (polyvinyl chloride) structure wrapped in foam. The analog signal produced at the load cell output was conditioned by the integrated circuit HX711 (it has a 24-bit analog/digital converter and SPI output interface) and sent directly to the microcontroller unit (MCU) through SPI communication.
2.2.3. Range of Motion Assessment
In the pre and post8 intervention periods, the range of motion (ROM) of the right knee extension was evaluated using goniometry. The initial knee position was 90∘ (considered as an angle of 0∘), with the participant actively performing the maximum knee extension. The goniometer was aligned using the following reference points: (I) axis on the knee joint line; (II) fixed arm parallel to the lateral surface of the femur pointed at the greater trochanter; and (III) movable arm parallel to the lateral face of the fibula, pointing to the lateral malleolus. The evaluation was repeated three consecutive times by the same evaluator, considering the average of the values.
2.2.4. Spasticity Assessment
The assessment of spasticity was performed in the pre and post8 intervention periods by the same evaluator, in addition to before and after each session, using the Modified Ashworth Scale (MAS). This assessment consisted of passive mobilization of knee extension, with the participant sitting on the chair, with the knee initially starting from flexion of approximately 90°. The MAS score ranges from 0 to 4, where: 0: normal tone; 1: increased tone at the beginning or end of the range of motion; 1+: increased tone in less than half of the range of motion, manifested by abrupt tension and followed by minimal resistance; 2: increased tone in more than half of the range of motion; 3: parts in flexion or extension and moved with difficulty; 4: rigid parts in flexion or extension.
2.2.5. Quality-of-Life Assessment
In the pre and post8 intervention periods, the quality of life in the SCI condition was evaluated using the Spinal Cord Injury Quality-of-Life Questionnaire (SCI-QoL.Br). The questionnaire is divided into five domains: general health status, social relationships, functional independence, accessibility, and emotional aspects [9]. The purpose of applying this questionnaire was to verify the interference of interventions in the participant’s quality of life.
2.2.6. Assessment of Current Motivation
At the beginning of each session, the rating scale was applied concerning the participant’s current emotional state, following the Likert scale [10], which ranges from 1 to 5, with one being very discouraged and five being very excited. The objective of this evaluation was to verify the interference of motivation in the performance of the intervention.
2.3. Interventions
MES-FES Interface
The MES-FES interface comprises a surface electromyography (EMG) acquisition system, a surface electrical stimulation module, and a microcontrolled system. The system was mounted on a 3D-printed plastic support (PLA filament). Figure 2 illustrates the application of the MES-FES interface during interventions.
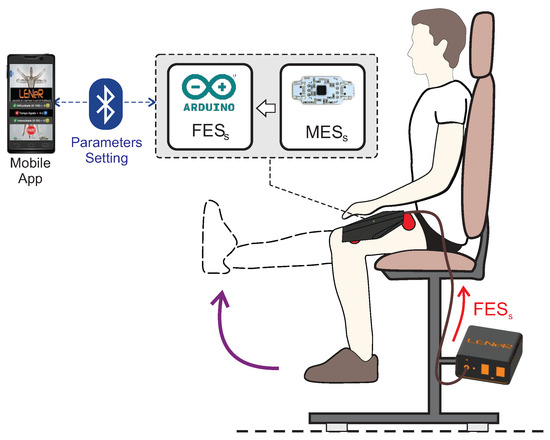
Figure 2.
Illustration of the equipment used during the protocol.
The system acquires the MES through a differential instrumentation amplifier, which amplifies the potential measured between a pair of electrodes positioned at a distance of ∼2 cm. A third electrode is connected to the reference to ensure signal stability.
A microcontroller converts the analog differential signal into a digital quantity. The MES is digitally filtered (second-order Butterworth bandpass type 10–40 Hz) to select the band of interest and eliminate noise and motion artifacts.
A moving average algorithm smooths the signal to avoid signal fluctuations. This information detects movement intent and generates an electrical stimulation trigger command. Then, the electrical stimulation triggering algorithm is started when the algorithm detects a signal variation.
To improve detection sensitivity, an adapted linear threshold based on the Taeger–Kaiser Energy Operator (TKEO) is applied to the rectified raw vector [11]. The start of FES is detected by comparing the signal to a threshold using Equation (2):
in which and are the integrated mean absolute values (MAVs) of two consecutive windows of 10 samples, k is a constant based on the baseline noise level ( in the present study), and the threshold, which is related to the difficulty of activating the FES driver and must be individually adjusted.
The command initiates a sequence of generations of biphasic pulses sent to the electrical stimulation system. The system receives a sequence of low-voltage pulses and amplifies them through a driver. The amplified signals are isolated by a transformer that generates the electrical stimulation pulses.
For artificial muscle activation [12], an electrical stimulator was customized exclusively for this work, following the criteria proposed in the IEC 60601-2-10 standard [13]. The electrodes were fixed according to the methodology proposed by [14]. In this work, two electrodes (self-adhesive with 5 × 9 cm) were placed (the electrodes are for individual use) on the anterior region of the participant’s right thigh (the electrodes were positioned after skin preparation (shaving and cleaning with alcohol, INPM)). One of the electrodes was positioned with the lower edge 3 cm above the base of the patella and the other over the femoral triangle (this point is found by palpating the femoral artery in the inguinal region) [15] to stimulate the quadriceps femoris muscle via the femoral nerve [16]. According to [14], after fixing the electrodes, an interval of approximately 10 min was respected to stabilize the electrode–skin impedance.
An adaptive threshold is calculated based on the percentage change in mean absolute values (MAVs) of consecutive windows to avoid movement artifacts, electrode displacement, or limb movements. is a value over the physiological range. To calibrate this value, a healthy individual performs a maximal voluntary contraction of the forearm muscles during wrist and hand extension. Therefore, the user’s paretic muscles have a lower value. If the instant value is less than and greater than , FES is activated.
The electrical stimulation system is a biphasic wave generator that drives an amplifier. The biphasic wave has a fixed period with a variable period of active duration (work cycle percentage). The intensity variation is of the type pulse width modulation (PWM), varying the total period . The period pulse (biphasic) is fixed and determined by the user/therapist, up to the pulse period (inactive). To generate the ramp effect of the FES, the frequency variation was performed between 20 and 40 Hz (up ramp) and between 40 and 20 Hz (down ramp), both of approximately 1 s. The application defined the on-time interval of FES.
An application was developed for (I) ease of use, and visualization of its functions; (II) free access; (III) available for Android®; (IV) connected via Bluetooth to the electrical stimulation circuit to activate the FES. The application is based on a single screen that presents the options to control the “difficulty”, “on-time”, “intensity”, and the “stop” function. The difficulty varies from 0 to 100. The higher the difficulty, the more difficult it will be to activate the FES. In other words, the greater the difficulty, the greater the muscle contraction will be necessary to activate the FES. The on-time function varies between 1 s and no maximum limit. It refers to the time in seconds that the FES will remain on to provide the electrical stimulus. The longer the time, the longer the stimulus will be maintained. The intensity can vary from 0 to 20 (arbitrary values), and the greater intensity, the greater electrical stimulus, and consequently, the greater muscle contraction (actual intensity is based on pulse duration modulation (work cycle percentage)). The stop function must be used in cases where it is necessary to deactivate the FES, thus reducing its intensity to 0.
At the beginning and end of each intervention, stretching of the hamstrings and triceps surae of the stimulated limb was performed, being performed in five initial and five final series, both associated with activation of the FES at low intensity (the intensity was adjusted according to the participant’s sensory tolerance) and maintained for the 30 s; stretching was performed to prevent injuries such as strain, dislocation, tendon rupture, among others. Then, active attempts at knee extension began, with a progressive increase in the number of attempts, intensity, and duration of the stimulus at each stage throughout the interventions. These attempts were divided into four stages: (I) small-range-of-motion (ROM) knee extension attempts, the limb started from approximately 45° of knee flexion; (II) attempts at greater ROM, the limb started from approximately 90° of knee flexion; (III) attempts at greater ROM associated with light manual resistance applied by the researcher during the electrical stimulus, partially resisting the knee extension movement; and (IV) attempts at greater ROM associated with high manual resistance applied by the researcher, totally resisting the movement. All attempts were aimed at reaching the maximum extension of the knee joint, and all were analyzed. The percentage of errors and hits was calculated, considering “error” any event not desired by the participant, for example, when the participant wanted to extend the knee but it did not occur, or when knee extension occurred without the participant wanting this movement. In contrast, the “hit” was when what the participant wanted at that moment happened.
2.4. Feasibility of the Intervention
The feasibility of the intervention was assessed by the following items:
- Intervention duration;
- Learning curve of the MES-FES interface by the participant: Number of hits and errors as a percentage of FES activation.
2.5. Data Analysis
The analysis presented in this case study is descriptive in nature. However, a customized routine of the open-source software Octave® version 5.2.0 was used for learning curve statistical tests. Due to the small sample, we assumed that our data were non-parametric and performed the Spearman’s correlation (and p-value) test for the eight sessions with the hits percentage of FES activation.
3. Results and Discussion
The present study applied a protocol of eight interventions, using the MES-FES interface once a week, in an individual with incomplete SCI.
3.1. Neuromuscular Assessment
The neuromuscular evaluation showed that in the rectus femoris muscle there was a normalized increase of 2% in the values of EMGRMS and a normalized reduction of 22.8% in EMGMDF after eight interventions (Table 1). Differences were also found in the evaluation of the vastus lateralis muscle, with a normalized increase of 3.3% in the EMGRMS values and a normalized reduction of 5.9% in EMGMDF. Figure 3 shows the temporo-spectral response pre and post8 EMG interventions for the rectus femoris and vastus lateralis muscles, respectively.

Table 1.
Results of EMG. RF: rectus femoris muscle, VL: vastus lateralis muscle. Normalized values according to Equation (1).
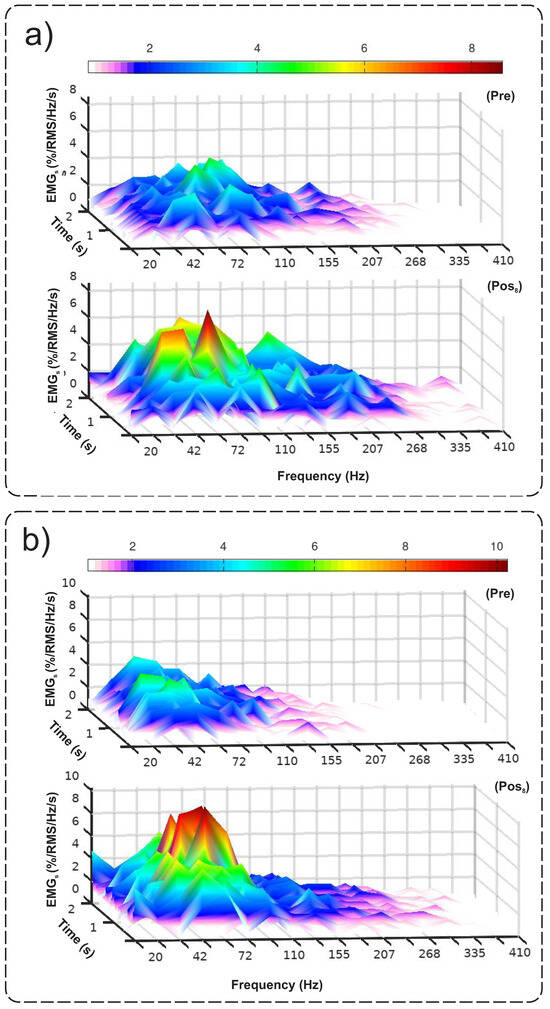
Figure 3.
Response of the (a) rectus femoris and (b) vastus lateralis muscles through the Cauchy wavelet transform.
Figure 3a,b refer to the rectus femoris and vastus lateralis muscles, respectively. Both indicate an increase in the magnitude of the signal, as evidenced by the increase in the EMGRMS. In the frequency representation, it is possible to verify an increase in the magnitude of the signal for the frequency bands between 42 and 72 Hz, corroborated by the reduction in the EMGMDF. This represents a greater synchronism in the recruitment of motor units, in addition to the improvement in the recruitment organization, with the consequent advance of motor learning. Corroborating this finding, Zhu et al. [17] investigated the changes in the paretic abductor pollicis brevis muscle after a stroke through evaluation with EMG, with two months of physical therapy rehabilitation. As a result, they obtained an increase in the values of EMGRMS and a reduction in EMGMDF of the paretic abductor pollicis brevis muscle after the interventions. Also, the EMGMDF values of the paretic muscle were in agreement with the values of the uncompromised contralateral muscle (healthy) [17]. Therefore, the results of the present study, similar to those of Zhu et al. [17], may indicate that the reduction in the value of EMGMDF occurs due to the improvement in motor control. No studies were found that correlated the relationship between EMGMDF reduction and motor control response in individuals with SCI.
3.2. Strength Assessment
After eight interventions, the load cell recorded an increase of 1.4 kgf in the contraction force of the right quadriceps femoris muscle, as shown in Table 2.

Table 2.
Pre and post8 interventions evaluation.
Given this, a study carried out with individuals with SCI (N = 12 complete and two incomplete lesions) by Bélanger et al. [18] analyzed the effect of FES and resistance training on muscle atrophy. For this analysis, the left quadriceps femoris was stimulated to contract against an isokinetic (resisted) load. The right was contracted against gravity (no resistance) for 1 h/day, 5 days/week, for 24 weeks. Strength was assessed through muscle torques measured at 90° from knee flexion to full extension, using a Cybex® isodynamometer set at 30°/s. Strength and endurance were tested with a 40 Hz train lasting 2 s, repeated every 5 s for 4 min. Their results showed that the loss in quadriceps femoris strength could be partially reversed by regular training assisted by FES, showing that the initial strength gain was substantially faster in the legs that worked against resistance, 8.1% per week, compared to the side without resistance (4.5% per week). Both sides stabilized at the end of 24 weeks of training [18]. Unlike the study by Bélanger et al. [18], which used applied resistance as a progression, the current study used an increment in the number of repetitions that gradually increased during the sessions. Furthermore, despite the difference in load increment, both showed a gain in quadriceps femoris contraction force. Although muscle fatigue was not evaluated, it was possible to notice during the interventions a greater muscular resistance of the stimulated quadriceps femoris, thus allowing the participant to perform a more significant number of attempts at knee extension without impairing her performance.
3.3. Assessment of Range of Motion
At the end of the interventions, there was a gain of 10.6° in knee joint extension range of motion (see Table 2). Similar results were found in a study by Granat et al. [19], which included six individuals with incomplete SCI in a gait program with FES. Despite the ROM of knee extension not being a primary outcome, there was a gain in the total range of extension of the right knee in one of the participants, who had a 5° flexion contracture at ten years duration, with consequent improvement in orthostatic posture. This improvement, evidenced in the present study by the increase in ROM, is explained by the reciprocal inhibition at the spinal level during the use of FES in the spastic muscle group [20].
3.4. Assessment of Spasticity
The present study resulted in a 1 point EMA reduction in spasticity in the knee flexor muscles (Table 2). Another result pointed out was the absence of a relationship between the effect of the intervention and the plantar flexor muscle spasticity because the spasticity of this muscle group remained the same.
According to the systematic review by Bekhet et al. [21], using neuromuscular electrical stimulation or FES provides an effective rehabilitation strategy in managing spasticity after SCI. As of the 23 analyzed studies, 15 reported a 45% to 60% reduction in spasticity. However, most studies demonstrated an acute effect, as the reduction in spasticity lasted, on average, less than 24 h after the intervention. As in the review by Bekhet et al. [21], it can be stated that the spasticity reduction in this study also had an acute effect. However, this neuromodulation did not persist until the subsequent intervention (one-week interval).
3.5. Quality-of-Life Assessment
Table 3 presents the sum of the scores of the domains of the Spinal Cord Injury Quality-of-Life Questionnaire (SCI QoL.Br) in the pre- and post-intervention periods.

Table 3.
Results of Spinal Cord Injury Quality-of-Life Questionnaire. In parantheses, the minimum–maximum values.
As shown in Table 3, there was a reduction in the scores in all domains, except for accessibility, because it had the lowest possible score in the pre period and at the end. Hence, a reduction of 12 points in the general sum of the five domains is observed, which is positive because the lower the score of the questionnaire, the better the individual’s quality of life is considered to be. With that, the study by Martino-Cinnera et al. [22] aimed to investigate how clinical variables influence the quality of life during subacute ischemic stroke rehabilitation. The rehabilitation was performed daily (40 min, twice per day) for 60 days, including exercise to recover upper and lower limb motor function, gait, and balance skills [22]. That study showed that lower limb motor recovery impacts the quality of life more than upper limb motor recovery [22]. Thus, despite the impairment in stroke involving the upper and lower limbs, the quality of life seems to have a more significant impact when the lower limb recovers. These results may justify an improvement in the quality of life of the participant in the present study as a consequence of the improvement of the lower limbs’ motor condition, given its importance for the return of functional activities, such as orthostatism and gait.
3.6. Current Motivation Assessment
The score of the current motivation scale for all sessions was five. That is, the participant was always humorous and considered very excited. Therefore, it was impossible to determine whether there was a relationship between the current state of motivation and performance during interventions. Regardless of the difference in performance throughout interventions, the current motivation was maintained. Unlike the present study, the research developed by Meadows et al. [23] identified a relationship between motivation and motor performance. This was explained by faster activation of the motor cortex (brain region linked to the frontal lobe) in cases where the individual is more motivated to perform a determinate activity.
3.7. Interface MES-FES
In another study by our research group, published by Fumagali and Krueger [24], the modulatory effect of the MES-FES interface on hand opening in four individuals with post-stroke neurological sequelae was investigated, more than six months after the injury. They showed that the use of the MES-FES interface, in 8 to 16 sessions lasting approximately 30 min each session, proved to be efficient in the evaluation of EMG, with an increase in the features EMGRMS and EMGMDF of the wrist and fingers extensor muscles only in the short term. In the follow-up evaluation, there was a regression in the values. The present study presented similar results to Fumagali and Krueger’s [24] regarding the increase in the EMGRMS descriptor. However, we obtained different results regarding the EMGMDF feature, which showed a decrease (this being positive), due to a more significant effect on motor learning. However, we did not carry out a follow-up evaluation. Thus, it was impossible to confirm whether this research’s results will be maintained in the short, medium, or long term.
MES-FES interfaces seek to mimic neuromuscular activation and promote neuroplasticity. Other interfaces with objectives similar to those of the MES-FES interface have been studied, such as brain–computer interfaces (BCI). BCI systems can decode brain signals and translate them into computer commands to control external devices or neural rehabilitation systems and could become a promising alternative neurorehabilitation approach [25]. A study by Marquez-Chin et al. [26] reported the therapeutic effects of integrating brain–computer interface technology and functional electrical stimulation therapy to restore upper limb reaching movements in a 64-year-old man with severe left hemiplegia after a hemorrhagic stroke. The results suggest functional electrical stimulation therapy with movement intention, using EEG indicators signaling motor intent, may restore reaching movements even 6 years after a stroke.
3.8. Intervention Viability
3.8.1. Duration of MES-FES Interface Protocol Steps
The following time intervals were recorded during each intervention: (I) Physical assembly of the FES and connection to the electrical stimulation circuit, with an average duration of 157 s; (II) intervention (comprises the initial and final stretch and four steps of knee extension attempts) with an average duration of 47 min. The total period of the protocol was the sum of I and II, totaling an average of 50 min. In this way, the present protocol is viable for clinical and home applicability, including stretching and muscle strengthening, with a similar duration to conventional therapy.
3.8.2. Learning Curve of the MES-FES Interface by User
During each intervention, the percentage of hits and errors of the activation of the FES was quantified (see Figure 4).
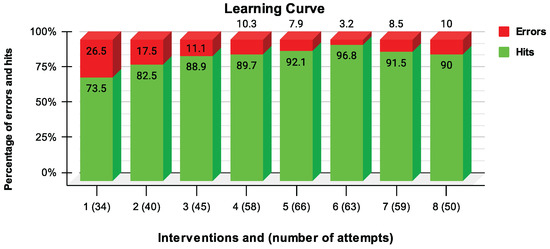
Figure 4.
Number of hits (green color) and errors (red color) as a percentage of FES activation.
Figure 4 indicates the learning effect of using the interface, comparing the first sessions with the last ones. The Spearman’s test, for the eight sessions with the hits percentage of FES activation, corroborates the correlation between the data ( = 0.78, p-value = 0.04). In addition, throughout the interventions, the participant showed greater autonomy and mastery in activating the MES-FES interface in a more independent and coordinated way. The study of Dutta et al. [27] included an individual with incomplete SCI who was able to stand but could not initiate a step. Six weeks of gait training were performed with 2 h sessions thrice a week. They used an implanted FES system consisting of intramuscular stimulation electrodes. They were bilaterally inserted into active quadriceps femoris, iliopsoas, tensor fascia lata, and tibialis anterior connected to an eight-channel implanted pulse generator. The participant used a switch to stop the FES system in case a step was manually activated when none was intended. At this moment, one of the outcomes was analyzed. Thus, the false positive rate was calculated during the swing phase assisted by FES throughout the interventions, resulting in a reduction to 1%. This false positive rate from the study by Dutta et al. [27] is similar to the error rate of activation of the MES-FES interface. In both studies, the rate was reduced, and the results corroborate the effect of motor learning, thus minimizing the probability of unexpectedly starting an unwanted activity.
According to Atkins and Bickel [3], the FES performs the electrical stimulation of the muscles affected by SCI, causing them a contraction in a coordinated way, aiming at the performance of a movement or functional task. For an individual to perform the activities of daily living in the best way, aiming at better functionality in their performance, among other observations, it is necessary that the muscles contract in a coordinated way during the performance of the desired activity. For this effect, motor learning has great importance. It directly relates to the execution of the desired activity through the various practical learning attempts, which tend to be developed over time, as shown in this study.
3.9. Study Limitations
Among the limitations of the study are (I) a restricted number of interventions, leading to the possibility of not having reached the ceiling effect; (II) not having assessed quadriceps spasticity; (III) the protocol applied was not standardized, making it difficult to compare it with the protocols of other studies; and (IV) the participant started treatment with urogynecological physiotherapy. Thus, it was not possible to prove whether the secondary improvements, such as bladder and sexual function, were justified by the interventions or by physiotherapeutic treatment.
4. Conclusions
The initial hypothesis of this study was evidenced by (I) an improvement in neuromuscular activation (normalized increase in EMGRMS of 2% (RF) and 3.3% (VL)) and synchronism of motor units (normalized reduction in EMGMDF of 22.8% (RF) and 5.9% (VL)); (II) an increase of 1.4 kgf; (III) an increase of 10.6° in the range of motion; and (IV) reduction in spasticity (1 point). Therefore, there was an improvement in the quality of life. As a criterion for the viability of the MES-FES interface, an average duration of 50 min was obtained, equivalent to the time of a conventional therapy session. The research participant showed greater mastery in using the interface, evidenced by the increase in the rate of correct answers (73.5–96.8%) throughout the interventions ( = 0.78, p-value = 0.04). With these results, applying the MES-FES interface to the quadriceps muscle group in individuals with incomplete SCI is a novelty, which is essential for functional activities involving the lower limbs. Therefore, it is suggested that the MES-FES interface be applied during activities such as standing and walking. Studies that used this same interface involved functional activities with upper limbs in a different population (stroke patients). Furthermore, the use of the interface can be extrapolated beyond the clinical environment. The interface can also be used as an assistive technology device for home use, and future studies could investigate its use in daily living activities.
Author Contributions
Conceptualization, E.K. and D.B.R.; methodology, D.B.R., E.K., L.G.S., M.V.G.M., R.B.d.S., D.P.C., P.B.J. and J.J.A.M.J.; writing—original draft preparation, D.B.R. and E.K.; writing—review and editing, L.G.S., M.V.G.M., R.B.d.S., D.P.C., P.B.J., J.J.A.M.J. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received a CNPq (National Council for Scientific and Technological Development) undergraduate research scholarship.
Institutional Review Board Statement
The present study was approved by the Ethics Committee and Research Involving Human Beings of the State University of Londrina, number 4,060,700, on 1 June 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SCI | Spinal cord injury |
| BCI | Brain–computer interface |
| EEG | Electroencephalography |
| MES | Myoelectric signal |
| NES | Neuroelectrical signal |
| FES | Functional electrical stimulation |
| EMG | Electromyography |
| RMS | Root mean squared |
| MDF | Median frequency |
| MAS | Modified Ashworth Scale |
| ERD | Event-related desynchronization |
| ERS | Event-related synchronization |
| MF | Magnetic field |
| SMF | Static magnetic field |
| MI | Motor imagery |
| CSP | Common spatial pattern |
| LDA | Linear discriminant analysis |
| VRPN | Virtual reality peripheral network |
| ASIA | American Spinal Cord Injury Association |
| AIS | ASIA Impairment Scale |
| ROM | Range of motion |
| BB | Biceps brachii |
References
- Willis, M.D.; Robertson, N.P. Technology-assisted recovery of walking after spinal cord injury. J. Neurol. 2019, 266, 1046–1048. [Google Scholar] [PubMed]
- Cerezetti, C.R.N.; Nunes, G.R.; Cordeiro, D.R.C.L.; Tedesco, S. Lesão medular traumática e estratégias de enfrentamento: Revisão crítica. O Mundo Saúde 2012, 36, 318–326. [Google Scholar] [CrossRef]
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231. [Google Scholar] [CrossRef] [PubMed]
- de Pinho Borella, M.; Sacchelli, T. Os efeitos da prática de atividades motoras sobre a neuroplasticidade. Rev. Neurociênc. 2009, 17, 161–169. [Google Scholar] [CrossRef][Green Version]
- Johnston, M.V. Plasticity in the developing brain: Implications for rehabilitation. Dev. Disabil. Res. Rev. 2009, 15, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, G.; Li, G.; Wiest, M.J.; Pakosh, M.; Furlan, J.C.; Kalsi-Ryan, S.; Zariffa, J. Properties of the surface electromyogram following traumatic spinal cord injury: A scoping review. J. Neuroeng. Rehabil. 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.; Magri, L.; Botelho, A.; Bach, F.; Rebellato, C.; Fracaro, L.; Fragoso, F.; Villanova, J., Jr.; Brofman, P.; Popović-Maneski, L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci. Lett. 2019, 696, 38–45. [Google Scholar] [CrossRef]
- Fukuda, T.Y.; Echeimberg, J.O.; Pompeu, J.E.; Lucareli, P.R.G.; Garbelotti, S.; Gimenes, R.O.; Apolinário, A. Root mean square value of the electromyographic signal in the isometric torque of the quadriceps, hamstrings and brachial biceps muscles in female subjects. J. Appl. Res. 2010, 10, 32–39. [Google Scholar]
- Feniman, S.P.; Cardoso, J.R.; Villegas, I.L.P.; Faganello, L.; Bela, D.; Santos, S.M.S.; Lavado, E.L. Desenvolvimento e validação de um questionário de qualidade de vida em indivíduos com lesão da medula espinal. CEP 2016, 86038, 440. [Google Scholar]
- Likert, R. A technique for the measurement of attitudes. Arch. Psychol. 1932, 22 140, 55. [Google Scholar]
- Solnik, S.; Rider, P.; Steinweg, K. Teager–Kaiser energy operator signal conditioning improves EMG onset detection. Eur. J. Appl. Physiol. 2010, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-Neto, G.; Scheeren, E.; Krueger, E.; Nohama, P.; Button, V.L.S.N. The Influence of Window Length Analysis on the Time and Frequency Domain of Mechanomyographic and Electromyographic Signals of Submaximal Fatiguing Contractions. Open J. Biophys. 2013, 3, 178–190. [Google Scholar] [CrossRef]
- IEC 60601-2-10:2012; Medical Electrical Equipment—Part 2-10: Particular Requirements for the Basic Safety and Essential Performance of nerve and Muscle Stimulators. International Electrotechnical Commission: Geneva, Switzerland, 2012.
- Krueger, E.; Scheeren, E.; Nogueira-Neto, G.; Nohama, P. Mechanomyography energy decreases during muscular fatigue in paraplegics. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society—EMBC 2014, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 5824–5827. [Google Scholar] [CrossRef]
- Rabischong, E. Surface action potentials related to torque output in paraplegics’ electrically stimulated quadriceps muscle. Med. Eng. Phys. 1996, 18, 538–547. [Google Scholar] [CrossRef]
- Schiefer, M.A.; Triolo, R.J.; Tyler, D.J. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 195–204. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Tang, X.; Chen, X.; Gao, X. Examining and monitoring paretic muscle changes during stroke rehabilitation using surface electromyography: A pilot study. Math. Biosci. Eng. 2020, 17, 216–234. [Google Scholar] [CrossRef]
- Bélanger, M.; Stein, R.B.; Wheeler, G.D.; Gordon, T.; Leduc, B. Electrical stimulation: Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch. Phys. Med. Rehabil. 2000, 81, 1090–1098. [Google Scholar] [CrossRef]
- Granat, M.; Ferguson, A.; Andrews, B.; Delargy, M. The role of functional electrical stimulation in the rehabilitation of patients with incomplete spinal cord injury-observed benefits during gait studies. Spinal Cord 1993, 31, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef]
- Bekhet, A.H.; Bochkezanian, V.; Saab, I.M.; Gorgey, A.S. The effects of electrical stimulation parameters in managing spasticity after spinal cord injury: A systematic review. Am. J. Phys. Med. Rehabil. 2019, 98, 484–499. [Google Scholar] [CrossRef]
- Martino Cinnera, A.; Bonnì, S.; Pellicciari, M.C.; Giorgi, F.; Caltagirone, C.; Koch, G. Health-related quality of life (HRQoL) after stroke: Positive relationship between lower extremity and balance recovery. Top. Stroke Rehabil. 2020, 27, 534–540. [Google Scholar] [CrossRef]
- Meadows, C.C.; Gable, P.A.; Lohse, K.R.; Miller, M.W. Motivation and motor cortical activity can independently affect motor performance. Neuroscience 2016, 339, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Fumagali, G.L.C.; Krueger, E. Efeito modulador da interface EMG-FES na abertura de mão em indivíduos após AVC. Rev. Neurociênc. 2021, 29, 1–23. [Google Scholar] [CrossRef]
- Zhuang, M.; Wu, Q.; Wan, F.; Hu, Y. State-of-the-art non-invasive brain–computer interface for neural rehabilitation: A review. J. Neurorestoratology 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Marquis, A.; Popovic, M.R. EEG-triggered functional electrical stimulation therapy for restoring upper limb function in chronic stroke with severe hemiplegia. Case Rep. Neurol. Med. 2016, 2016, 9146213. [Google Scholar] [CrossRef]
- Dutta, A.; Kobetic, R.; Triolo, R.J. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Trans. Biomed. Eng. 2008, 55, 791–794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).