1. Introduction
Historically, subtractive manufacturing, such as milling, has been heavily used in dentistry [
1]. In recent years, additive manufacturing (AM) has been dominant among the many fabrication processes [
1]. While milling involves a block of material being modeled with a controlled cutter, AM offers a more innovative approach. As its name implies, in this technique, material is added following a CAD model. This technique allows for the production of customizable and intricate structures with a reduced amount of waste, easier post-production processes, and shorter manufacture times [
2]. AM is able to deliver accurate, fast, and repeatable prints by crafting each product with progressive layering. These benefits have led to the wide-ranging integration of 3D printing across many fields. In particular, in dentistry, with its high demand for rapid patient-personalized models, AM has been utilized in various ways. Examples range anywhere from crowns and surgical guides to dental prostheses and aligners.
Four main 3D-printing techniques employed in dentistry can be highlighted [
3]: stereolithography (SLA), digital light processing (DLP), selective laser sintering (SLS), and selective laser melting (SLM). Other typologies of 3D printing such as fused deposition modelling (FDM) and polymer jetting (PJ) are not as prevalent as they have not been comprehensively investigated in the literature [
1]. Some fabrication methods, like FDM and SLS, implement materials that melt, sinter, or soften with heat from electromagnetic irradiation or an electron beam [
3]. In SLA, a UV-sensitive liquid resin that selectively hardens when a material surface is exposed to a UV laser beam is employed [
4], while in SLS, a laser is used as the energy source to selectively sinter a powdered material, solidifying it. FDM is a useful technique thanks to its geometric flexibility and low cost; with this technique, it is possible to produce polymer prostheses with hollow, semi-hollow, and solid structures, allowing the easy fabrication of various dental applications [
5]. The SLS technique, on the other hand, can also be used to cast various metal alloys, including for metal frameworks for dentures, crowns, and dental implants, while the polymers used in SLS are usually applied in the manufacture of surgical guides and dental models [
6]. Similar to SLA, PJ technology is based on the principle of photopolymerization. In PJ, a liquid resin is jetted drop by drop and subsequently cured using UV light [
7]. Polyjet printers can be used to produce items of varying density, hardness, and flexibility at high resolution, and commonly employed devices include customized dental models, surgical guides, and scaffolds [
6].
The introduction of these innovative techniques has led to the use of new polymeric materials for the fabrication of temporary crowns and bridges. These new biomaterials have been proposed as an alternative to ceramics and metals and include polypropylene (PP), vinyl and styrenic polymers, acrylonitrile–butadiene–styrene (ABS), acrylates, polylactic acid (PLA), nylon, polyamides (PA), polyetheretherketone (PEEK), and polymethylmethacrylate (PMMA) [
1]. Data from the literature on AM-printing polymers, although discordant on some aspects, have shown that their rigidity and fracture toughness are not sufficient to withstand complex chewing forces, making them not fully suitable for prosthesis use [
3]. Their flexural strength, hardness, and fracture load are statistically lower than those for prostheses made using the subtractive technique. Nevertheless, there were reports of an increase in flexural strength, even after aging, with a marginal discrepancy essentially comparable to MM machining [
8].
While there are several studies on the properties of AM materials, the properties selected by researchers are not always suitable [
9,
10,
11]. To gain a full picture of the mechanical performance of these viscoelastic materials, further investigation is needed.
Since dental prostheses are exposed to dynamic, rather than static, loading, dynamic mechanical tests are more suitable for this application. Dynamic mechanical analysis (DMA) is a technique used to characterize materials by applying sinusoidal stress and measuring the resulting deformation, allowing the complex modulus to be determined [
12,
13]. DMA can simulate a cyclic masticatory load, better anticipating a material’s clinical performance. Within DMA, there are several tests that one can perform. In this work, bending analysis was chosen because it mimics the flexural movement that occurs during mastication.
The present study aims to assess the dynamic mechanical properties as well as biological viability of 11 new dental polymeric biomaterials. The goal is to evaluate their potential clinical use in dentistry by mimicking the oral cavity and its physiological conditions considering chewing frequency.
The null hypotheses of this study are as follows:
- -
Dental resins show no mechanical differences in terms of elastic modulus, stiffness, and morphology.
- -
No differences can be detected in the in vitro biological behavior of oral mucosa cells cultured on dental resins.
2. Materials and Methods
After a preliminary literature review [
14], 11 new AM polymeric materials, designed for crowns and denture bases, were selected for mechanical and biological characterization: PC-10 (PC), ANTERO 800NA (AN), ABS-M30 (ABS), NYLON 12 (N12), NYLON 6 (N6), Gamma VERO (GV), FullCure 720 (FC), Endur RGD 450 (EN), PA 603-CF (CF), PA 620-MF (MF), and WhiteSinter (PA12) (WS) from Zare Srl–Proxera Srl, Reggio Emilia, Italy (
Table 1).
Since the specification sheets are covered by privacy or copyright, the datasheets of the best-known companies (Ultimaker 3 (Ultimaker B.V., New York, NY, USA) for FDM printing and Stratasys (Way Eden Prairie, MN, USA) for Polyjet) were taken as references.
Different specimens were used for the analyses:
- -
Six bar specimens for each material (N = 66), with dimensions of 10.5 × 86 × 2 mm for DMA;
- -
Six disks for each material (N = 66), with a radius of 20 mm and a 2 mm thickness, for MTT analysis at 3 h (N = 33) and 24h (N = 33).
All samples were measured using a 150 mm caliper (Mitutoyo, Kawasaky, Japan), which is accurate to 1/20 mm.
To determine the elastic modulus, 66 samples were subjected to isotherm mode at 37 °C and analyzed, varying the frequency from 1 to 101 Hz with a force amplitude of 1 N. Specimens were tested for 3 min with 10 repeated measurements for each sample, performing 60 tests for each material, for a total of 660 tests (during a 3-week period). Mettler Toledo DMA/SDTA861 (Mettler Toledo Inc., Columbus, OH, USA) equipped with “Three Points Bending” was used.
The Young’s modulus formula, , was used to quantify the deformation of the applied load, with σ = stress (MPa); ε = strain, a dimensionless quantity defined as length variation over initial length, often expressed as a percentage.
In viscoelastic materials, force reactions are both tangential and normal, and the Young’s modulus formula becomes E* = E′ + iE″, with E′ = storage module, which indicates stored material elasticity linked to reversible elastic energy; E’’ = loss module, which indicates the capacity for irreversible energy loss transformed into heat; and i = √(−1), an imaginary unit.
Considering elasticity, the prism deformation derives from the ı angle between applied and load forces. The ı phase angle quantifies delay in deformation with respect to load force, which in viscoelastic systems is 0 ≤ δ ≤ 90°. The loss factor,
, indicates viscoelasticity, i.e., deformation energy loss, and expresses the link between loss and storage modules. Young’s modulus does not provide evidence of a substantial effect on specimen size [
15].
Human oral keratinocytes (PCS-200-014, ATCC®, Manassas, VA, USA) were grown as monolayer cultures in sterile polystyrene T-75 flasks (Thermo Fisher Scientific, Waltham, MA, USA) containing Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Paisley, UK) supplemented with 7% foetal bovine serum (FBS), penicillin (10,000 U/mL), streptomycin (10,000 μg/mL), and 25 μg/mL of amphotericin B as anti-fungal agent (Gibco, Paisley, UK).
Cells were maintained in a humidified incubator at 37 °C with 5% CO
2 and monitored with a phase-contrast inverted microscope (Leitz, Germany), with twice weekly medium changes. Upon reaching 80% confluence (logarithmic growth phase), cells were detached with a mixture of 0.25% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA), counted in a Countess Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA, USA), and plated [
16].
Each specimen disk was sterilized after 30 min of exposure of each face to UV light and incubated in sterile serum-free medium at 37 °C for 30 min.
A total of 5 × 10
4 cells/mL were harvested and seeded in empty 1.9 cm
2 wells in an optical, clear 24-well flat bottom plate (control) (Thermo Fisher Scientific, Waltham, MA, USA) and in wells containing each type of sample. Cultures were maintained in a 5% CO
2 humidified atmosphere at 37 °C; after 3 h and 24 h, the culture medium was discarded, and the disks were rinsed with phosphate-buffered saline (PBS) and transferred to new wells. Cytotoxicity was analyzed using a yellow-to-purple colorimetric assay (MTT) for mitochondrial succinic dehydrogenase activity (SDH) [
17]. After 3 and 24 h of incubation, 50 μL of MTT solution (5 mg/mL) was added to each well, and plates were incubated in darkness for 4 h at 37 °C. According to ISO 10993-5 [
18], fewer living cells appear in media with less mitochondrial SDH activity, a phenomenon directly related to the blue-violet formazan produced by SDH activity and 3[4,5-dimethy1-2-thiazoly1]-2-5-dipheny1-2H tetrazolium bromide’s (MTT, Sigma Chemical Co., St. Louis, MO, USA) reduction to formazan precipitate. MTT-derived formazan crystals were dissolved by adding 300 μL/well of dimethyl sulphoxide (DMSO, Sigma Chemical Co., St. Louis, MO, USA) for 30 min with gentle shaking. Absorbance was measured at 570 nm with an automatic microplate spectrophotometer reader (Bio-Rad, Model 680 XR, Hercules, CA, USA). Control group absorbance values and percentages of viable cells were compared. Cell viability was calculated according to an optical density (OD)-related formula: % cell viability = OD of disk samples/OD of control × 100.
Results are presented as means ± SD (standard deviation) and using boxplots representing the distribution of data, outliers, and the uncertainty repeatability measurements of Young’s moduli. One-way analysis of variance (ANOVA) was performed using GraphPad Prism 5.01 software (Prism, Folsom, CA, USA). DMA data were analyzed via Microsoft Office Excel 2019 using the statistical toolbox. A p value < 0.05 was considered significant.
3. Results
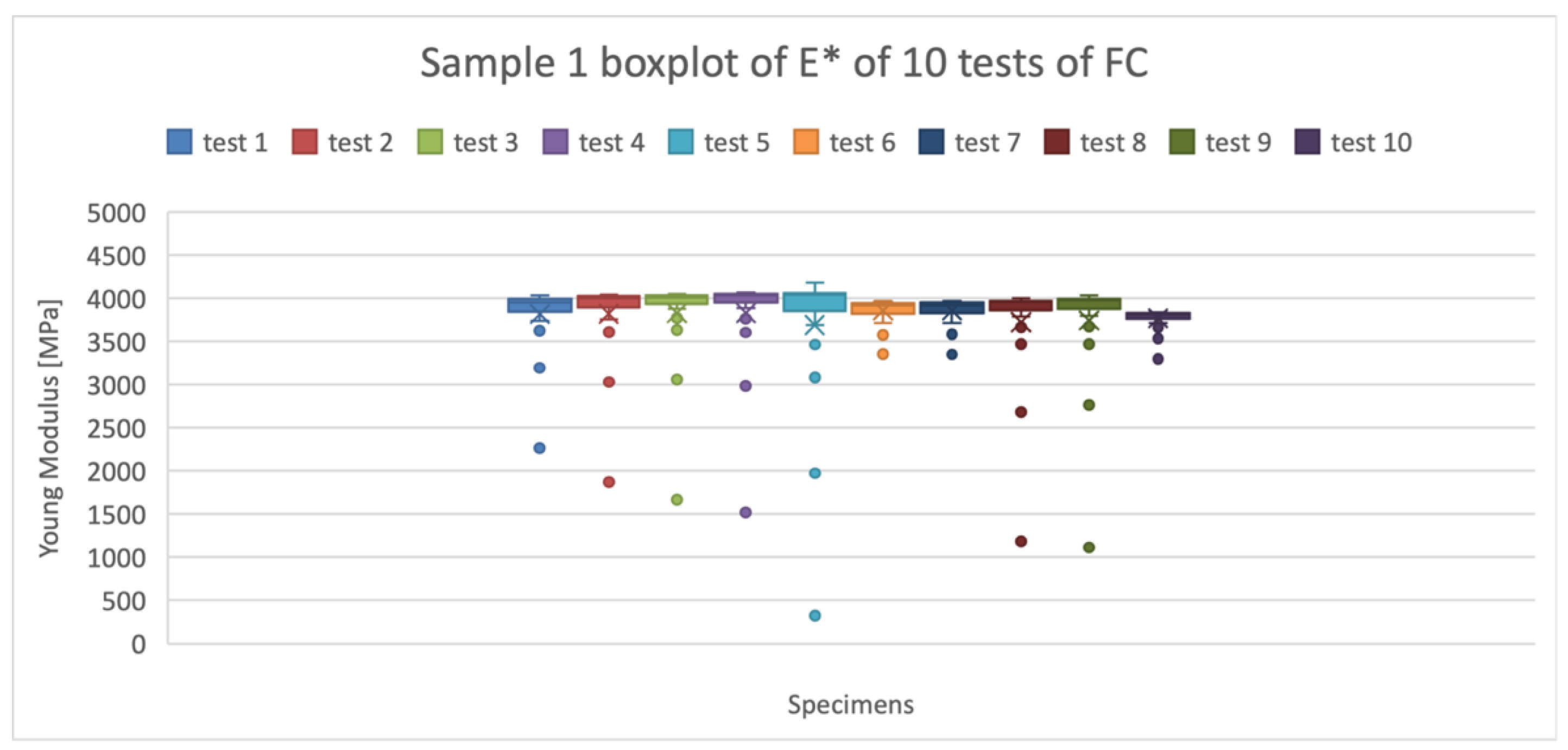
Figure 1 shows an example of the boxplots obtained for the mean values of Young Modulus for the material FC. The ten boxplots represent the 10 measurements for one of the six specimens of the material. The outliers (anomalous values) shown in the graph (
Figure 1), represent the values of the Young’s Modulus at low frequencies, ranging from 1 to 6 Hz. This can be related to the low-frequency (less than 5 Hz)-recording difficulty of the Mettler Toledo machine due to factors such as noise and the signal-to-noise ratio. The conclusive Young’s Modulus parameters were obtained from the five synthesis numbers [minimum, 1st quartile (Q1), median, 3rd quartile (Q3), maximum] that describe the distribution features of the ten tests for each sample.
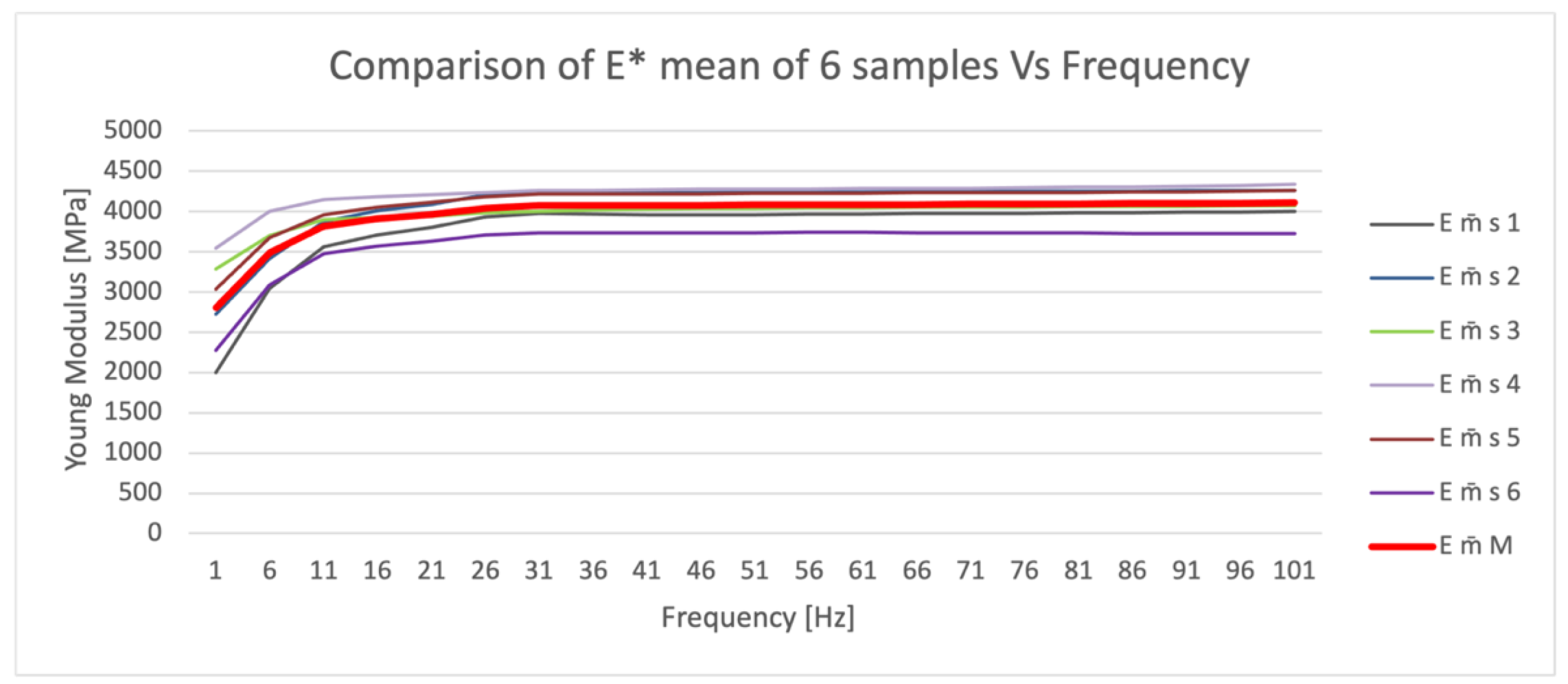
Figure 2 shows the means of the 10 tests performed for each of the six FC specimens (m̄ s 1;6), in which outliers at low frequencies are highlighted, and the final trend of the mean (m̄ M) (red line) of the Young’s modulus values.
The elastic modulus data manifested non-uniform, frequency-dependent results. High uncertainty was detected at low frequencies (1 to 11 Hz), and it may be acceptable to reject these results as they potentially constitute measurement uncertainty. At low frequencies, the material behaves less rigidly; therefore, it is less fragile but also less strong. The greater the stiffness, the higher the fragility and the greater fracturing of the material. The elastic modulus and uncertainty of measurement did not show significant changes at higher frequencies (between 11 and 101 Hz).
In
Table 2, the results of repeated measurements for elastic moduli, total uncertainty, and the percentage of uncertainty are reported. The uncertainty values range from 25.2% (EN printed with Polyjet, Zare Srl–Proxera Srl, Reggio Emilia, Italy) to 3.4% (MF printed with SLS). Materials printed with SLS show less uncertainty, while higher levels of uncertainty due to repeatability are related to the FDM and Polyjet printing techniques.
FC shows higher uncertainty than PC or AN, which have relatively low uncertainty. SLS yields more variable values than Polyjet. The SLS samples were generally stiffer than the Polyjet and FDM samples, causing an increase in Young’s modulus.
At higher frequencies, the elastic modulus of the different materials is not influenced by frequency as it is at lower frequencies. In fact, the anomalous curve between 1 and 11 Hz was confirmed in all materials.
The FDM-printed materials’ elastic modulus values ranged from 1647.23 MPa (N12) to 3180.52 Mpa (AN), with uncertainty estimated with SDs of ±96.28 and ±144.81 MPa, respectively. The Polyjet-printed materials’ elastic moduli ranged from 2561.86 MPa (EN) to 3969.60 MPa (FC), with standard deviations of ±646.02 and ±415.67 MPa, respectively. The SLS-printed materials’ elastic moduli ranged from 1998.15 (WS) to 5974.90 MPa (CF), with SDs of ±70.58 and ±440.31 MPa, respectively.
In general, stiffer materials turned out to be arranged in a descending order, with CF (SLS) yielding 5974.90 MPa, GV (Polyjet) yielding 3969.60 MPa, and AN (FDM) yielding 3180 MPa.
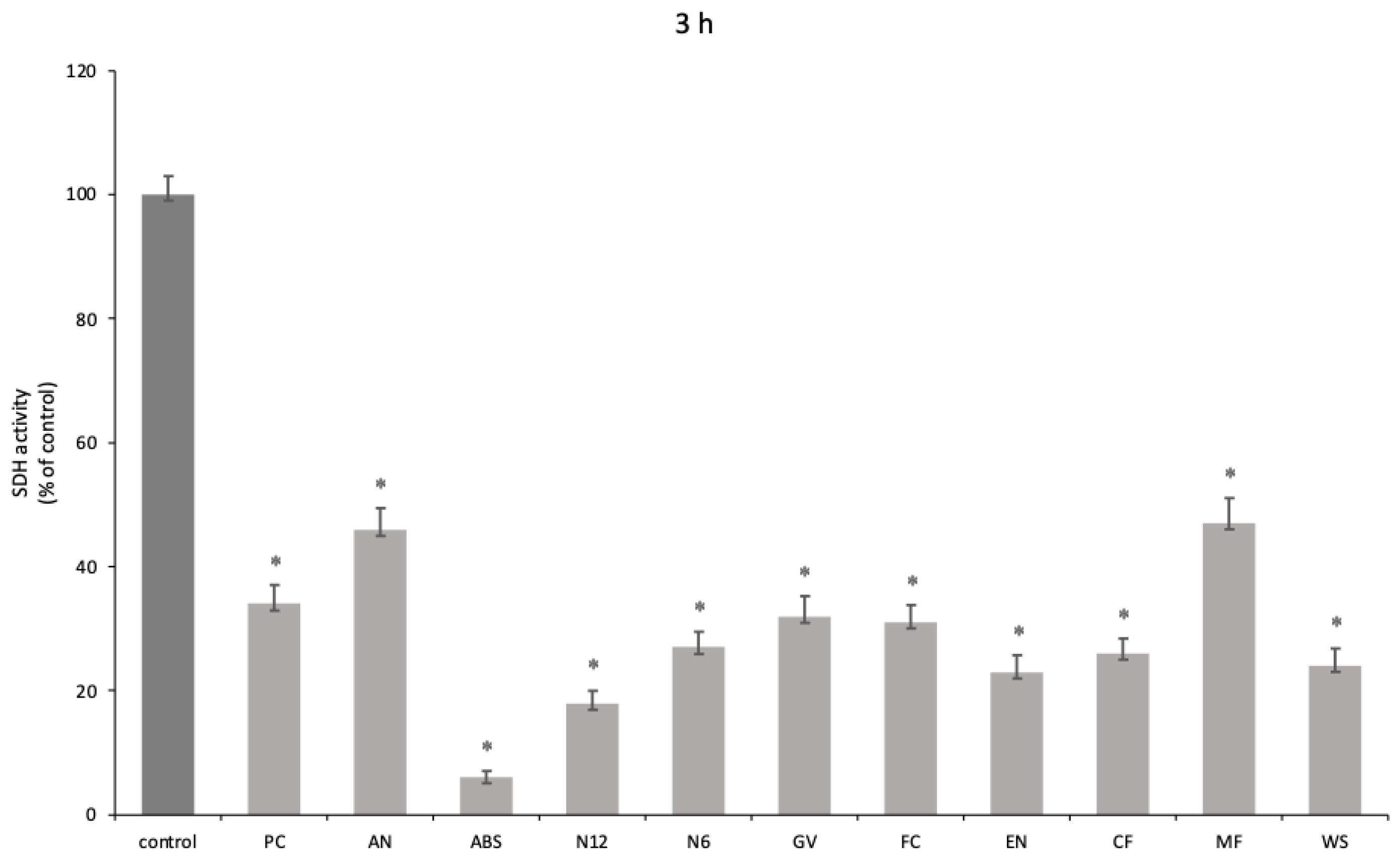
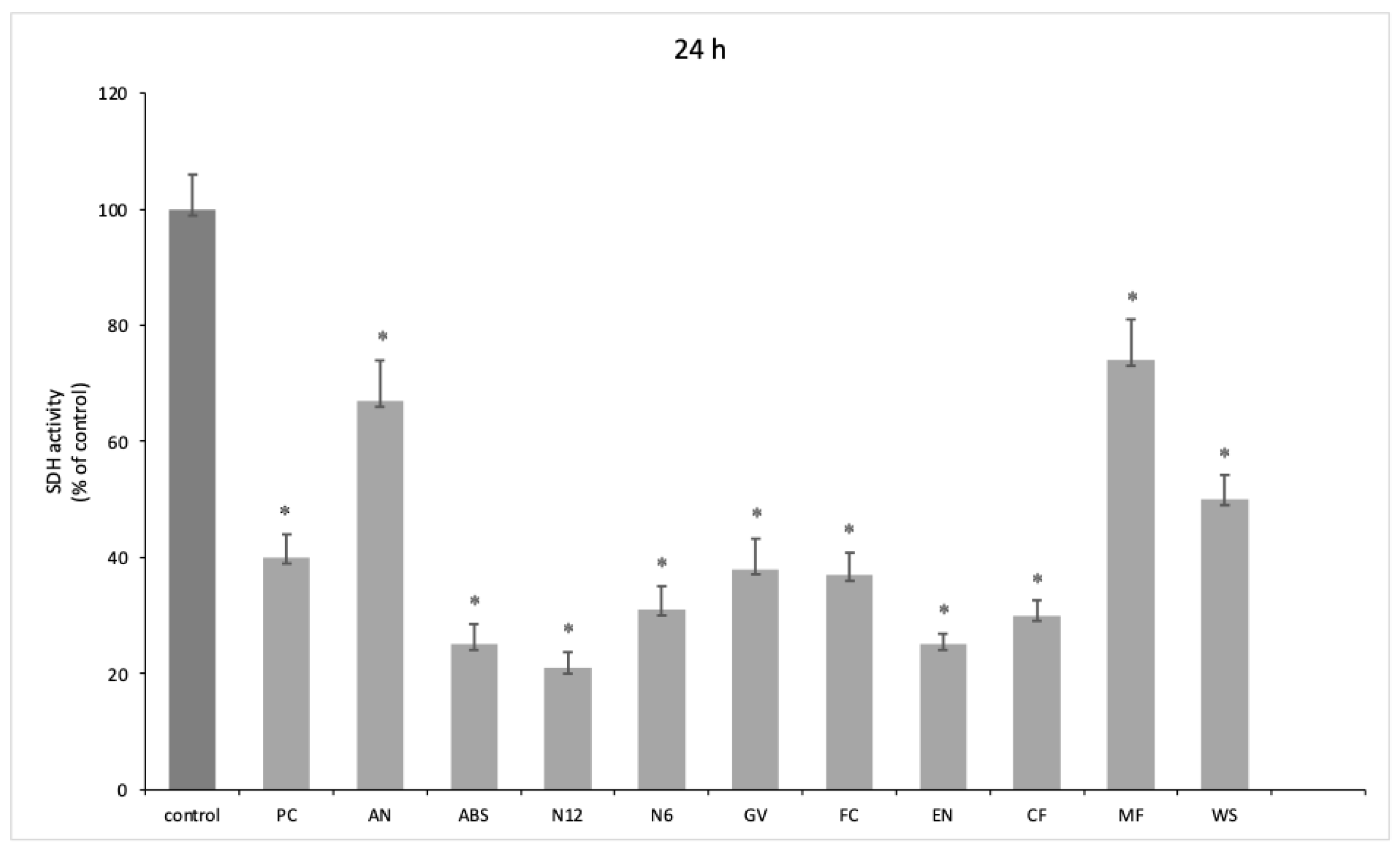
A significant reduction in oral human keratinocyte viability was highlighted at both 3 h (
p < 0.001) (
Figure 3) and 24 h of exposure (
p < 0.001) (
Figure 4) for all the tested materials. Furthermore, a degree of recovery of cell viability was observed at 24 h, in particular for AN and ABS, printed using FDM, and MF and WS, printed using SLS.
4. Discussion
Additive printing technology has certainly demonstrated promising applications, especially in the medical and dental fields. AM technology is based on 3D mathematical model data that are used to direct operations when using layered printing technology [
19]. The primary benefit of utilizing AM technology in dental applications lies in its effective support for treating patients in vulnerable or special needs categories. This technology has proven to be a more accessible technique, offering shorter completion times without compromising on performance.
On the other hand, the primary drawbacks involve the visible layering caused by the compound’s stratification and the technical challenges associated with printing certain materials commonly used in dentistry.
The purpose of this study was to assess the mechanical-dynamic and biological properties of key AM materials designed for dental applications. Specifically, our focus was on polymeric materials, commonly employed in the fabrication of temporary crowns and bridges and denture bases.
For repeated stresses or impacts at low frequencies, which simulate the loading of a chewing cycle, the materials tested were found to be more capable of absorbing the impacts, while at high frequencies, the materials could become more fragile and undergo cracking. The materials tested appeared to maintain similar Young’s moduli above 21 Hz and could therefore be used in the mouth without causing problems related to chewing load.
The selection of the best technique for dental applications ultimately depends on the specific requirements of the design to be printed and the specific application. SLA and Polyjet are usually considered the most advantageous techniques because of their high resolution, accuracy, and ability to produce models with smooth surface finishes. SLS is also useful because of its versatility and ability to produce models with high strength and durability. FDM, on the other hand, may be a cheaper option but may not be suitable for components that require a high level of precision and detail [
20].
The criteria for choosing a printing methodology not only concern technique but also the type of machine itself, considering printing in terms of time, cost, and production waste.
In terms of mechanical properties, the materials examined demonstrated a relatively uniform response, encompassing rigidity and strength. For a material to be deemed mechanically suitable, it is essential for it to possess properties akin to alloys for permanent constructions or resins for temporary restorations. Take zirconia, for example, which is extensively employed in contemporary prosthetic dentistry for crafting crowns and fixed partial dentures. Through integration with CAD/CAM design techniques, it has facilitated the transition from conventional metal–ceramic prostheses. This shift is attributable to its comparable mechanical and biological attributes arising from its microstructural composition, noteworthy compressive strength (2000 MPa), and well-established biocompatibility, as evidenced by both in vivo and in vitro studies [
21].
The results of our study show that the mechanical behavior observed was closely related to the printing technique employed. Among the various techniques used, PolyJet seems to be the one that might have the greatest potential for further application. Polyjet is a 3D technology that produces objects with microscopic-layer resolution and accuracy down to 0.014 mm. Recent studies have shown that PolyJet printing has higher accuracy and reproducibility than other 3D techniques [
22]. Materials printed with FDM technology, which have increased strength and a lower Young’s modulus, prove to be the best with respect to their use in prosthetics. Although PolyJet printing offers superior aesthetics in terms of final prosthesis design, featuring exceptional detail definition, it demonstrates greater variability in mechanical test results compared to the FDM technique.
The analysis performed shows that the FDM printing technique seems to provide better mechanical properties than Polyjet and even milling [
14,
23]. Compared to milling, FDM is associated with greater toughness and elasticity, as well as low uncertainty values. However, comparisons cannot be made for every material included in this study, as milling data were not available due to the newness of the material [
24,
25].
Hence, our recommendation leans towards the utilization of FDM due to the commendable mechanical properties and sample reliability it offers. However, the biological perspective yields less consistent results. While the mechanical properties show a correlation with the printing technique employed, no discernible pattern emerges within the groups of printing types with regard to biology. Rather, the properties are solely influenced by the specific type of material used.
An aspect to consider in our study is the apparent discrepancy between the physiological frequencies of the masticatory cycle and those used in the mechanical tests. The authors’ intention was to simulate and accelerate the aging process and assess how the materials would behave under more demanding conditions, considering that this approach provides valuable insights into the durability and reliability of denture base resins, especially in the context of long-term performance and clinical use. A more in-depth analysis of what happens at frequencies lower than 5 Hz will be the purpose of a future scientific paper.
Another aspect to consider is the possible change in the behavior of these resins under wet conditions. In a parallel study conducted by our working group evaluating the effect of saliva on mechanical performance by analyzing flexural modulus loss, it was observed that surface material properties and manufacturing techniques were the main factors influencing the properties of resins exposed to artificial saliva for 72 h [
26].
When characterizing dental materials, it is necessary to consider materials with reduced adverse biological effects on oral cells. Previous in vitro studies reported that the biological properties, particularly biocompatibility, are comparable between materials produced by additive, subtractive, and/or traditional techniques for both ceramics and resins [
27,
28]. However, our study showed large variability in terms of biological properties. As the biological mechanisms contributing to changes in the MTT test remain unclear, it is evident that differences in behavior may be predominantly associated with the duration of exposure. Consequently, it is advisable to scrutinize this aspect further and conduct experiments with extended time frames for a more comprehensive evaluation.
Our results show how for oral keratinocytes at both 3 h and 24 h, there is a decrease in viability, although at 24 h, the cell numbers seem to increase. The ABS materials showed the most cytotoxicity. In addition, these samples showed great alterations post experiment, with a turbid culture medium, visible disc erosion, and the absorption of enough medium to cause a substantial decrease in the volume measured. The Polyjet-printed material also appeared to have absorbed much of the volume and altered the color of the specimen-disc (
Figures S1–S4). MF showed promising biological results, inducing increased viability in oral keratinocytes.
Limitations
The significant challenge we faced is that certain suppliers provided a range for the elastic modulus rather than a more precise value for specific materials (GV and EN). None of these manufacturers gave an uncertainty value. Of the existing standards, the elastic modulus of bending tests derived from the American Society for Testing and Materials (ASTM) International standards was evaluated. In the literature, it is difficult to find the elastic moduli of the same materials made by milling for comparison with AM printing because they are materials that cannot be made without 3D printing. Moreover, they are new materials, poorly studied in dentistry, and applied in other contexts with different analyses. The main difference turns out to be that milled ones have lower values in terms of flexural modulus.
It was noted that the mean of the PC material fell within the interquartile range of the boxplot, and the representation of the standard deviation was symmetrical. However, for other materials, although the boxplot appears contained within a narrower limit, the mean and standard deviation are displaced, resulting in more outliers. These anomalies could be attributed to internal cracks in the specimens, the chosen printing technique, printing conditions, material cooling, or machinery factors, even though the uncertainty value is reliable. Additionally, the time factor should be considered, as the tests extended over a prolonged period. Despite the specimens being maintained in a stable condition as per the manufacturers’ requirements, the duration between tests likely influenced the materials. The specimens may have accumulated moisture via humidity or experienced alterations in their original mechanical properties [
29].
Polymeric materials supplied by a company are usually used for making semi-definitive prosthetic restorations, and these resin materials can be made in any color. However, the supplier has provided specimens in colors such as dark blue that are not usually employed in dentistry. Coloring could certainly be an influencing factor for both mechanical and biological properties.
An additional limitation present in the literature is the lack of studies on oral cell populations and thus of previous information on biological properties with which to refer to and use as a basis for our research.