Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Definitions and Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800, Erratum in Eur. J. Cardiothorac. Surg. 2022, 62, ezac209. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: A weighted meta-analysis of 3,519 patients from 16 studies. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Burrage, M.; Moore, P.; Cole, C.; Cox, S.; Lo, W.C.; Rafter, A.; Garlick, B.; Garrahy, P.; Mundy, J.; Camuglia, A. Transcatheter Aortic Valve Replacement is Associated with Comparable Clinical Outcomes to Open Aortic Valve Surgery but with a Reduced Length of In-Patient Hospital Stay: A Systematic Review and Meta-Analysis of Randomised Trials. Heart Lung Circ. 2017, 26, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Tokarek, T.; Dziewierz, A.; Dudek, D. MitraClip for mitral valve regurgitation and transcatheter aortic valve implantation for severe aortic valve stenosis: State-of-the-art. Adv. Interv. Cardiol./Postępy Kardiol. Interwencyjnej 2021, 17, 155–162. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Vahanian, A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Bekeredjian, R.; Szabo, G.; Balaban, Ü.; Bleiziffer, S.; Bauer, T.; Ensminger, S.; Frerker, C.; Herrmann, E.; Beyersdorf, F.; Hamm, C.; et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: In-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur. Heart J. 2019, 40, 1323–1330. [Google Scholar] [CrossRef]
- Dziewierz, A.; Tokarek, T.; Kleczynski, P.; Sorysz, D.; Bagienski, M.; Rzeszutko, L.; Dudek, D. Impact of chronic obstructive pulmonary disease and frailty on long-term outcomes and quality of life after transcatheter aortic valve implantation. Aging Clin. Exp. Res. 2018, 30, 1033–1040. [Google Scholar] [CrossRef]
- Poulsen, M.K.; Dahl, J.S.; Kjeldsen, B.J.; Nørregaard-Hansen, K.; Pedersen, K.E.; Mickley, H.; Nissen, H. Impact of chronic obstructive pulmonary disease on survival and symptoms of severe aortic valve stenosis. Scand. Cardiovasc. J. 2015, 49, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, M.; Knoop, H.; Ewers, A.; Mügge, A.; Walther, J.W. Impact of lung diseases on morbidity and mortality after transcatheter aortic valve implantation: Insights from spirometry and body plethysmography. Am. Heart J. 2015, 170, 837–842.e1. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Kolar, T.; Lakič, N.; Kotnik, A.; Štubljar, D.; Fras, Z.; Bunc, M. Similar clinical outcomes with transcatheter aortic valve implantation and surgical aortic valve replacement in octo-genarians with aortic stenosis. Front. Cardiovasc. Med. 2022, 9, 947197. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Waghefi, A.; Petrov, A.; Jatzke, P.; Wilbring, M.; Kappert, U.; Matschke, K.; Alexiou, K.; Arzt, S. Minimally Invasive Isolated Aortic Valve Replacement in a Potential TAVI Cohort of Patients Aged ≥ 75 Years: A Propensity-Matched Analysis. J. Clin. Med. 2023, 12, 4963. [Google Scholar] [CrossRef] [PubMed]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Ismayl, M.; Abbasi, M.A.; Al-Abcha, A.; Robertson, S.; El-Am, E.; Goldsweig, A.M.; Alkhouli, M.; Guerrero, M.; Anavekar, N.S. Outcomes of Transcatheter Aortic Valve Implantation in Nonagenarians and Octogenarians (Analysis from the National Inpatient Sample Database). Am. J. Cardiol. 2023, 199, 59–70. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Poletti, E.; Sticchi, A.; Crimi, G.; Giannini, F.; Latib, A.; Capodanno, D.; Colombo, A. Thrombotic Versus Bleeding Risk After Transcatheter Aortic Valve Replacement: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2088–2101. [Google Scholar] [CrossRef]
- Ferlini, M.; Mauri, S.; Rossini, R. Dual antiplatelet therapy after TAVR: A drop in the bucket? Int. J. Cardiol. 2019, 280, 46–48. [Google Scholar] [CrossRef]
- Jiritano, F.; Santarpino, G.; Serraino, G.F.; Ten Cate, H.; Matteucci, M.; Fina, D.; Mastroroberto, P.; Lorusso, R. Peri-procedural thrombocytopenia after aortic bioprosthesis implant: A systematic review and meta-analysis comparison among conventional, stentless, rapid-deployment, and transcatheter valves. Int. J. Cardiol. 2019, 296, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Jafar, M.; Zahid, S.; Ahmed, F.; Khan, M.Z.; Sattar, Y.; Fischman, D.L.; Virani, S.S.; Alam, M. Predictors of In-Hospital Mortality in Patients with End-Stage Renal Disease Undergoing Transcatheter Aortic Valve Replacement: A Nationwide Inpatient Sample Database Analysis. Cardiovasc. Revasc. Med. 2022, 34, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Adbul-Hamid, A.R.; Mulley, G.P. Why do so few older people with aortic stenosis have valve replacement surgery? Age Ageing 1999, 28, 261–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olszewska, K.; Tokarek, T.; Bętkowska-Korpała, B.; Dziewierz, A.; Kleczyński, P.; Sorysz, D.; Dudek, D. Assessment of cognitive functions and quality of life in patients scheduled for transcatheter aortic valve implantation: A pilot study. Adv. Interv. Cardiol./Postępy Kardiol. Interwencyjnej. 2017, 13, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef]
- Green, P.; Russo, M.; Arnold, S.; Doshi, D.; Pichard, A.; Szeto, W.; Leon, M. TCT-36 frailty in intermediate risk patients undergoing transcatheter or surgical aortic valve replacement, cut points and relationship with outcomes: An analysis of the placement of aortic transcatheter valves (PARTNER) 2 cohort a randomized trial. J. Am. Coll. Cardiol. 2016, 68, B15. [Google Scholar] [CrossRef]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefèvre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef]
- Bendayan, M.; Messas, N.; Perrault, L.P.; Asgar, A.W.; Lauck, S.; Kim, D.H.; Arora, R.C.; Langlois, Y.; Piazza, N.; Martucci, G.; et al. Frailty and Bleeding in Older Adults Undergoing TAVR or SAVR: Insights from the FRAILTY-AVR Study. JACC Cardiovasc. Interv. 2020, 13, 1058–1068. [Google Scholar] [CrossRef]
- Moss, S.; Doyle, M.; Hong, R.; Manganas, C.; Peeceeyen, S. Octogenarians and aortic valve surgery: Surgical outcomes in the geriatric population. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 134–141. [Google Scholar] [CrossRef]
- Gavalaki, A.; Roussakis, A.; Zoubourlis, P.; Contrafouris, C.; Zarkalis, D.; Perreas, K. Outcomes and quality of life after aortic valve surgery in octogenarians. J. Card. Surg. 2020, 35, 341–344. [Google Scholar] [CrossRef]
- Ay, K.N. Impact of age on long term survival following transcatheter aortic valve implantation. J. Geriatr. Cardiol. 2019, 16, 265–271. [Google Scholar] [CrossRef]
- van den Brink, F.S.; Wijtsma, I.; Amrane, H.; Vossenberg, T.N.E.; Haenen, J.; Porta, F.; Hofma, S.H. Outcome of transcatheter aortic valve replacement in patients over 85 years of age versus patients aged 85 and younger. Neth. Heart J. 2022, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, J.; van Mourik, M.S.; van Kesteren, F.; Henstra, M.J.; Piek, J.J.; Henriques, J.P.; Baan, J., Jr. Comparison of outcomes of Transfemoral aortic valve implantation in patients <90 with those >90 years of age. Am. J. Cardiol. 2018, 121, 1581–1586. [Google Scholar] [PubMed]
- Vlastra, W.; Chandrasekhar, J.; Vendrik, J.; Gutierrez-Ibanes, E.; Tchétché, D.; de Brito, F.S.; Delewi, R. Transfemoral TAVR in nonagenarians: From the CENTER collaboration. JACC Cardiovasc. Interv. 2019, 12, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Murthi, M.; Velagapudi, S.; Sharma, B.; Ezegwu, O.; Akuna, E.; Park, D.Y.; Vardar, U. Comparison of in-hospital mortality and clinical outcomes between patients aged more than and less than 80 years undergoing transcatheter aortic valve replacement. Cureus 2022, 14, e24534. [Google Scholar] [CrossRef]
- Kawada, T. Clinical Outcomes of Nonagenarians After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 124, 1486–1487. [Google Scholar] [CrossRef] [PubMed]
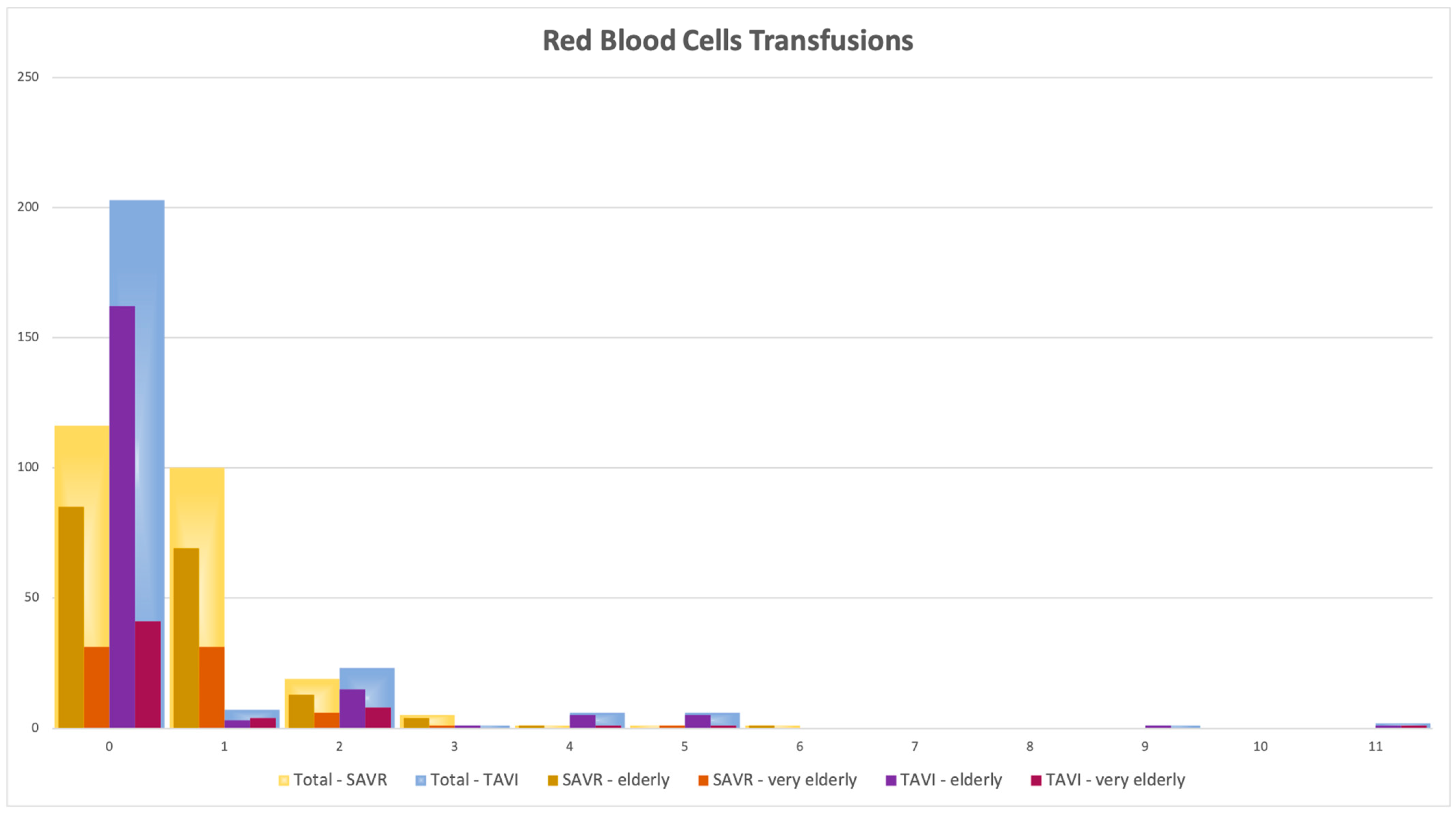
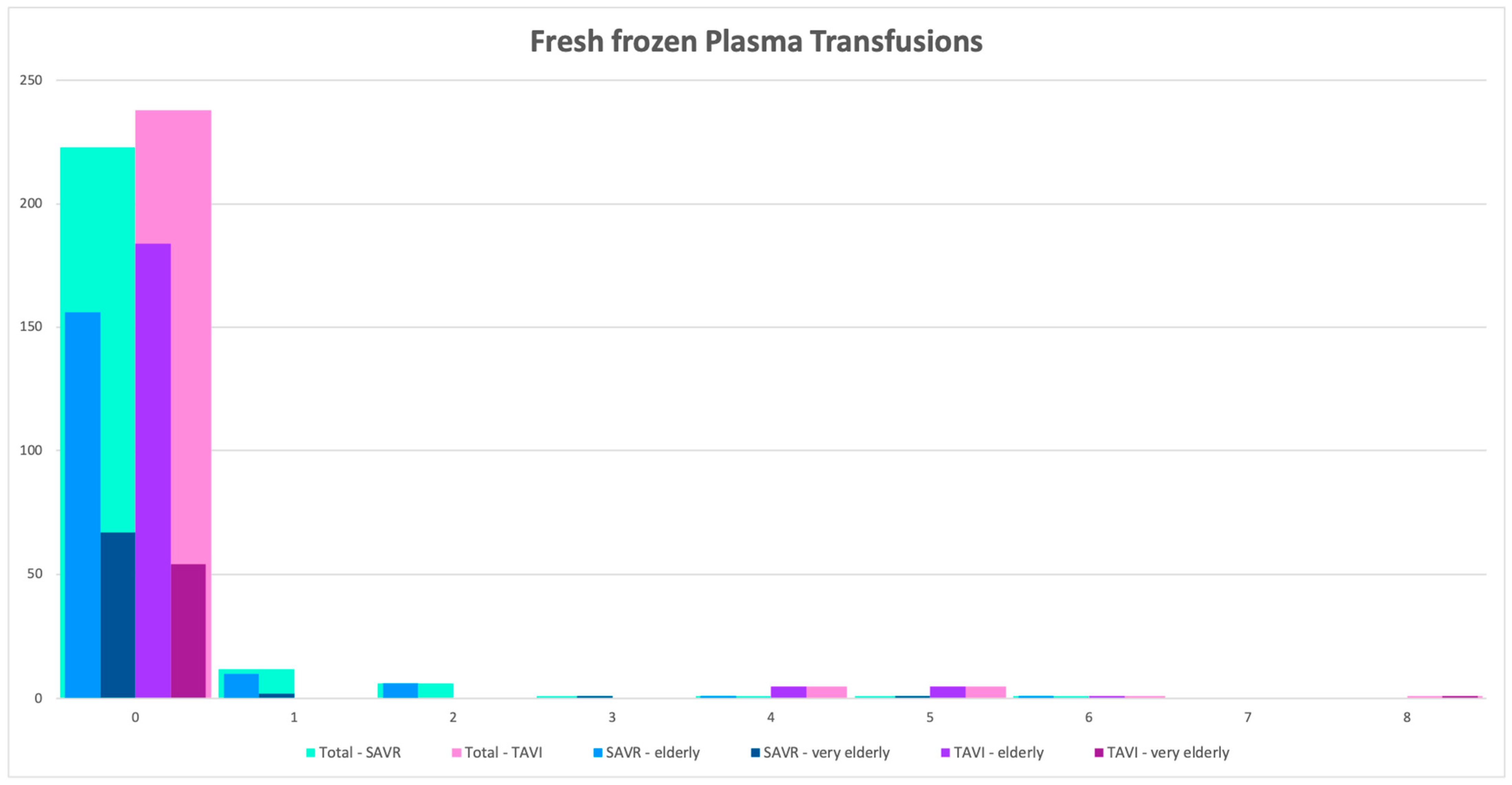
| Variable | Overall | Elderly (Age < 80) 375 Pts | Very Elderly (Age ≥ 80) 127 Pts | p Value |
|---|---|---|---|---|
| Age | 77.0 ± 3.5 | 75.5 ± 0.1 | 81.3 ± 0.1 | <0.001 |
| Male | 259 (51.59%) | 195 (52.0%) | 64 (50.4%) | 0.754 |
| BMI (kg/m2) | 27.8 ± 5.4 | 28.0 ± 5.9 | 27.2 ± 5.3 | 0.105 |
| EF (%) | 53.2 ± 20.1 | 52.9 ± 23.1 | 53.7 ± 10.4 | 0.369 |
| Hemoglobin (g/dL) | 12.6 ± 5.9 | 12.5 ± 1.8 | 12.3 ± 1.7 | 0.767 |
| DM type 2 | 149 (30.2%) | 112 (30.4%) | 37 (29.6%) | 0.861 |
| Dyslipidemia | 256 (59.5%) | 196 (61.2%) | 60 (54.5%) | 0.216 |
| Hypertension | 379 (87.1%) | 279 (86.6%) | 100 (88.5%) | 0.613 |
| COPD | 132 (26.3%) | 92 (25%) | 40 (32%) | 0.127 |
| NYHA class III or IV | 300 (70.1%) | 227 (70.7%) | 73 (68.2%) | 0.626 |
| Smoking | 124 (29.4%) | 99 (31.7%) | 25 (22.9%) | 0.083 |
| Liver cirrhosis | 8 (1.59%) | 7 (1.87%) | 1 (0.79%) | 0.401 |
| Dialysis | 18 (4.79%) | 12 (4.2%) | 6 (6.4%) | 0.386 |
| AF | 228 (46.6%) | 177 (48.2%) | 51 (41.8%) | 0.218 |
| Peripheral vascular disease | 49 (9.86%) | 38 (10.2%) | 11 (8.7%) | 0.623 |
| Prior MI | 80 (15.9%) | 64 (17.0%) | 16 (12.7%) | 0.247 |
| Permanent pace-maker | 49 (9.86%) | 30 (8.0%) | 19 (15.3%) | 0.018 |
| Cerebrovascular disease | 35 (8.29%) | 23 (7.3%) | 12 (11.0%) | 0.233 |
| EuroSCORE II | 7.7 (8.7) | 7.70 (0.5) | 7.51 (0.9) | 0.572 |
| Variable | Group SAVR 247 Pts | p Value | Group TAVI 255 Pts | p Value | ||
|---|---|---|---|---|---|---|
| Age < 80 173 Pts | Age ≥ 80 74 Pts | Age < 80 202 Pts | Age ≥ 80 53 Pts | |||
| Age | 74.9 (2.8) | 82.2 (1.7) | <0.001 | 76.0 (2.3) | 80.4 (1.2) | <0.001 |
| Male | 90 (50.8%) | 33 (47.1%) | 0.600 | 105 (53%) | 31 (54%) | 0.857 |
| BMI (kg/m2) | 27.1 (5.2) | 26.3 (4.5) | 0.144 | 28.6 (5.5) | 28.2 (5.9) | 0.299 |
| EF (%) | 53.5 ± 9.7 | 52.8 ± 6.8 | 0.595 | 52.5 ± 30.7 | 54.9 ± 13.9 | 0.586 |
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 12.3 ± 1.8 | 0.012 | 12.0 ± 1.9 | 12.3 ± 1.5 | 0.329 |
| DM type 2 | 31 (17.5%) | 16 (22.8%) | 0.335 | 81 (42%) | 21 (38%) | 0.575 |
| Dyslipidemia | 83 (46.9%) | 30 (42.8%) | 0.566 | 113 (79%) | 30 (75%) | 0.586 |
| Hypertension | 142 (80.2%) | 59 (84.3%) | 0.460 | 137 (94%) | 41 (95%) | 0.824 |
| COPD | 42 (24.3%) | 20 (29.4%) | 0.412 | 50 (25.6%) | 20 (35%) | 0.161 |
| NYHA class III or IV | 56 (42%) | 24 (45%) | 0.693 | 171 (90%) | 49 (90%) | 0.961 |
| Smoking | 40 (22.7%) | 11 (15.7%) | 0.221 | 59 (43%) | 14 (36%) | 0.403 |
| Liver cirrhosis | 0 (0%) | 1 (1.43%) | 0.111 | 7 (3.54%) | 0 (0%) | 0.150 |
| Dialysis | 3 (3.4%) | 4 (11.1%) | 0.09 | 9 (4.6%) | 2 (3.5%) | 0.719 |
| AF | 93 (52.8%) | 33 (48.5%) | 0.546 | 84 (44%) | 18 (33%) | 0.161 |
| Peripheral vascular disease | 12 (6.8%) | 4 (5.8%) | 0.779 | 26 (13.4%) | 7 (12%) | 0.826 |
| Prior MI | 39 (22%) | 10 (14.5%) | 0.183 | 25 (12.6%) | 6 (10%) | 0.669 |
| Permanent pace-maker | 12 (6.8%) | 12 (17.6%) | 0.011 | 18 (9.1%) | 7 (12%) | 0.457 |
| Cerebrovascular disease | 5 (2.8%) | 4 (5.7%) | 0.275 | 18 (13.2%) | 8 (20%) | 0.260 |
| EuroScore II | 2.3 ± 2.0 | 3.8 ± 3.1 | <0.001 | 10.1 ± 9.4 | 10.2 ± 9.8 | 0.861 |
| Variable | Group SAVR 247 Pts | p Value | Group TAVI 255 Pts | p Value | ||
|---|---|---|---|---|---|---|
| Age < 80 | Age ≥ 80 | Age < 80 | Age ≥ 80 | |||
| Stroke | 0 (0%) | 1 (1.4%) | 0.144 | 5 (2.9%) | 1 (1.9%) | 0.706 |
| Bleeding | 18 (10.2%) | 4 (5.7%) | 0.268 | 20 (10.8%) | 10 (18.2%) | 0.147 |
| Re-operation for bleeding | 13 (7.3%) | 2 (2.8%) | 0.183 | 0 (0%) | 0 (0%) | - |
| Intracranial bleeding | 1 (0.6%) | 1 (1.4%) | 0.495 | 0 (0.0%) | 1 (1.8%) | 0.006 |
| Atrial fibrillation | 75 (42.4%) | 32 (45.7%) | 0.633 | 9 (4.9%) | 3 (5.5%) | 0.860 |
| Vascular complications | 2 (1.1%) | 0 (0.0%) | 0.372 | 21 (11.4%) | 8 (14.6%) | 0.523 |
| ICU stay | 2.5 (2.3) | 3.4 (3.2) | 0.006 | 2.42 (3.77) | 3.32 (7.60) | 0.116 |
| Hospital LOS | 12.46 (13.27) | 13.10 (6.49) | 0.220 | 11.96 (9.74) | 11.84 (11.26) | 0.468 |
| Re-operation for prosthesis dysfunction | 0 (0%) | 0 (0%) | - | 0 (0%) | 1 (1.8%) | 0.066 |
| Post-op endocarditis | 0 (0%) | 2 (2.8%) | 0.024 | 0 (0%) | 0 (0%) | - |
| In-hospital mortality | 0 (0%) | 2 (2.8%) | 0.024 | 8 (4.8%) | 4 (8%) | 0.389 |
| MACE | 14 (7.9%) | 6 (8.6%) | 0.864 | 11 (5.5%) | 5 (8.7%) | 0.378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiritano, F.; Serraino, G.F.; Sorrentino, S.; Napolitano, D.; Costa, D.; Ielapi, N.; Bracale, U.M.; Mastroroberto, P.; Andreucci, M.; Serra, R. Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement. Prosthesis 2024, 6, 175-185. https://doi.org/10.3390/prosthesis6010014
Jiritano F, Serraino GF, Sorrentino S, Napolitano D, Costa D, Ielapi N, Bracale UM, Mastroroberto P, Andreucci M, Serra R. Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement. Prosthesis. 2024; 6(1):175-185. https://doi.org/10.3390/prosthesis6010014
Chicago/Turabian StyleJiritano, Federica, Giuseppe Filiberto Serraino, Sabato Sorrentino, Desirèe Napolitano, Davide Costa, Nicola Ielapi, Umberto Marcello Bracale, Pasquale Mastroroberto, Michele Andreucci, and Raffaele Serra. 2024. "Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement" Prosthesis 6, no. 1: 175-185. https://doi.org/10.3390/prosthesis6010014
APA StyleJiritano, F., Serraino, G. F., Sorrentino, S., Napolitano, D., Costa, D., Ielapi, N., Bracale, U. M., Mastroroberto, P., Andreucci, M., & Serra, R. (2024). Risk of Bleeding in Elderly Patients Undergoing Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement. Prosthesis, 6(1), 175-185. https://doi.org/10.3390/prosthesis6010014










