Contemporary Tools for the Cure against Pernicious Microorganisms: Micro-/Nanorobots
Abstract
:1. Introduction
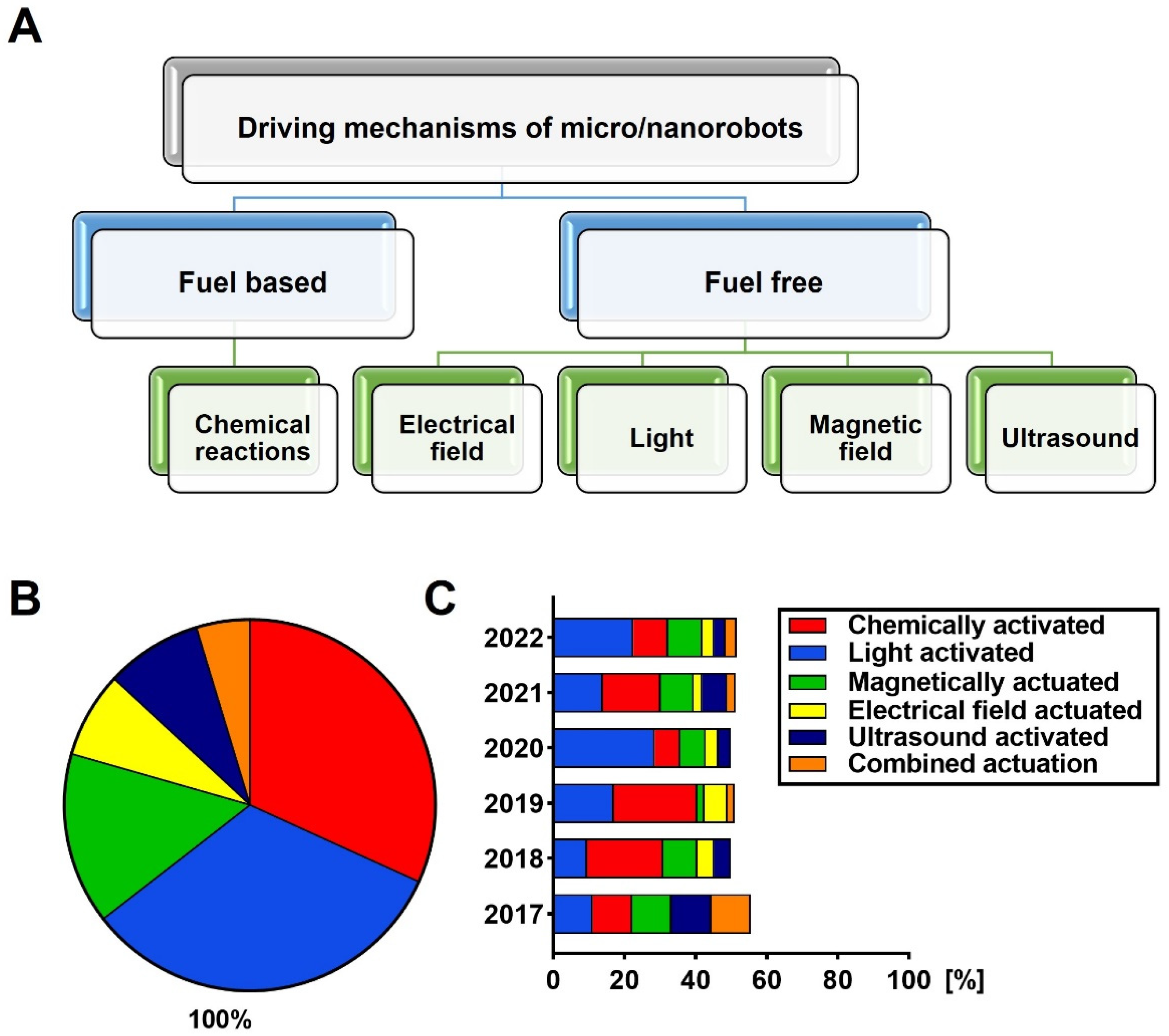
2. Overview of Micro-/Nanorobots
Interactions of Micro-/Nanorobots with Microorganisms
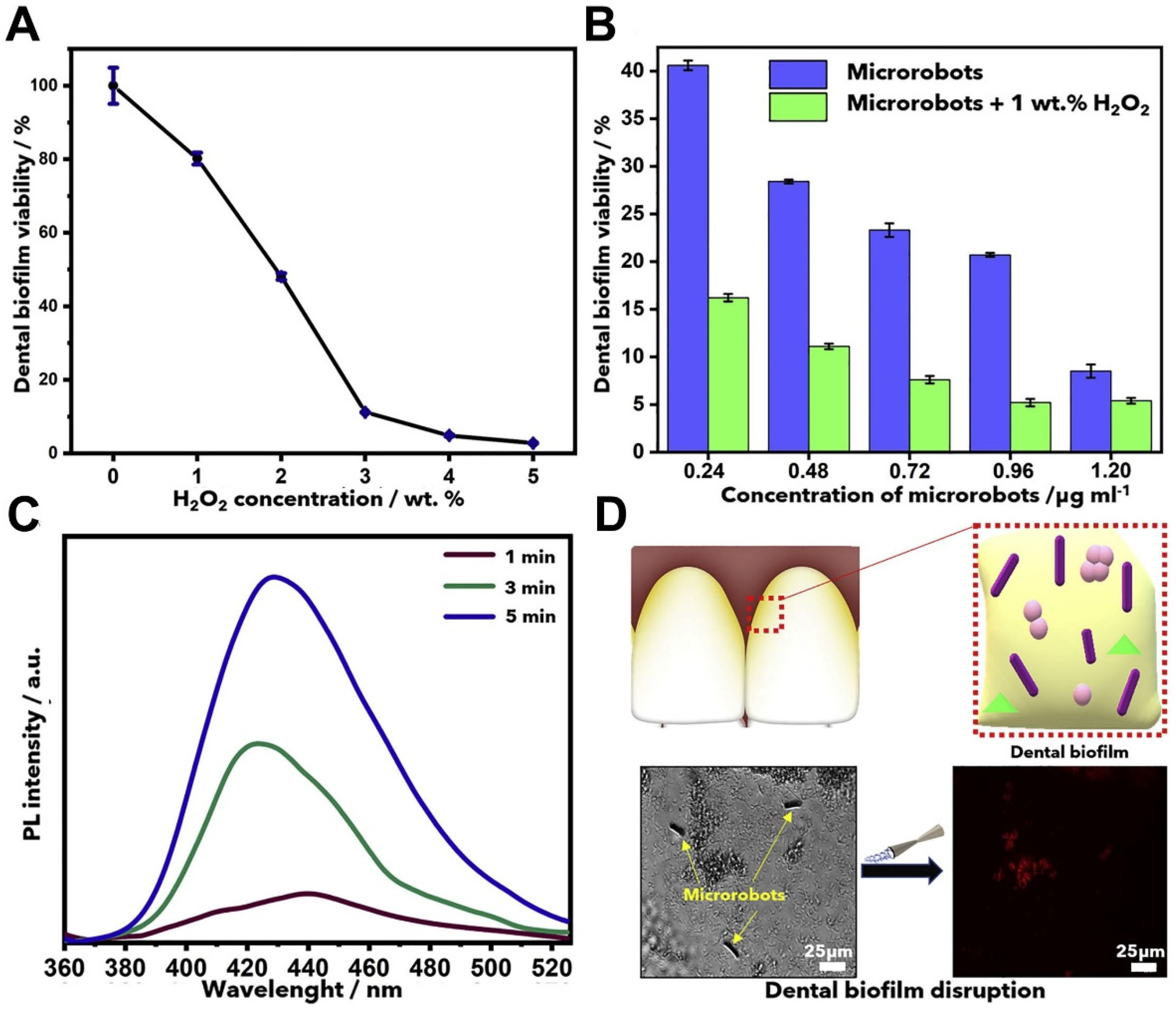
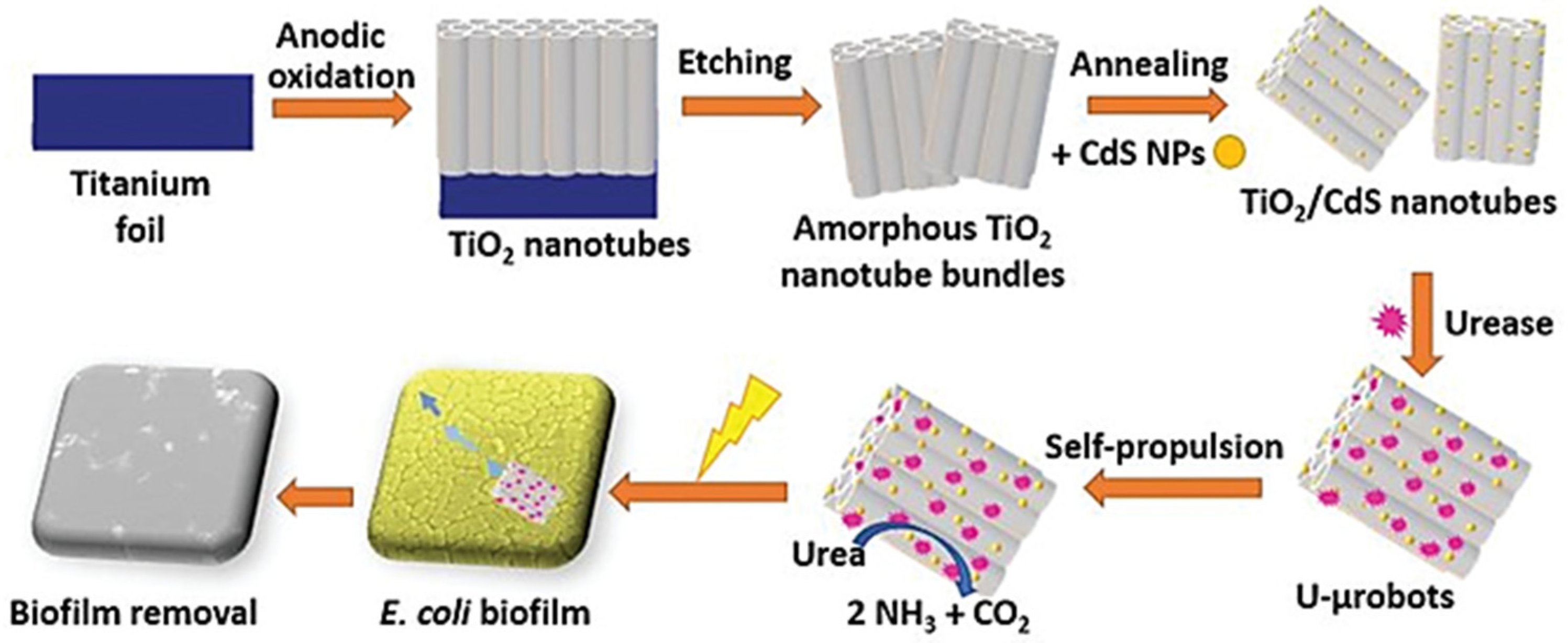
3. Removal of Biofilms
4. Effects of Micro-/Nanorobots on Various Microbial Species
4.1. Escherichia coli
4.2. Staphylococcus aureus
4.3. Other Microorganisms
5. Discussion
5.1. Advantages and Disadvantages of Current Micro-/Nanorobots
5.2. Recent Situation of In Vivo Antimicrobial Studies
5.3. Application Routes, Limitations, and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahady, G. Medicinal Plants for the Prevention and Treatment of Bacterial Infections. Curr. Pharm. Des. 2005, 11, 2405–2427. [Google Scholar] [CrossRef] [PubMed]
- 33,000 People Die Every Year Due to Infections with Antibiotic-Resistant Bacteria. Available online: https://www.ecdc.europa.eu/en/news-events/33000-people-die-every-year-due-infections-antibiotic-resistant-bacteria (accessed on 10 August 2021).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.W.; Stapleton, P.D.; Paul Luzio, J. New ways to treat bacterial infections. Drug Discov. Today 2002, 7, 1086–1091. [Google Scholar] [CrossRef]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H. Inadequate Antimicrobial Treatment: An Important Determinant of Outcome for Hospitalized Patients. Clin. Infect. Dis. 2000, 31, S131–S138. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, T.H.; Xu, Y.; Bay, L.; Schønheyder, H.C.; Jakobsen, T.; Bjarnsholt, T.; Thomsen, T.R. Sampling challenges in diagnosis of chronic bacterial infections. J. Med. Microbiol. 2021, 70, 001302. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.M.; Chen, H.; Da Wang, Z.; Liu, Z.Y.; Yu, Y.Q.; Li, L.; Miao, Z.Y.; Wang, X.W.; Wang, Q.; Mao, C.; et al. Nitric Oxide-Driven Nanomotor for Deep Tissue Penetration and Multidrug Resistance Reversal in Cancer Therapy. Adv. Sci. 2021, 8, 2002525. [Google Scholar] [CrossRef]
- Wan, M.; Chen, H.; Wang, Q.; Niu, Q.; Xu, P.; Yu, Y.; Shen, J. Bio-inspired nitric-oxide-driven nanomotor. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Sonntag, L.; Simmchen, J.; Magdanz, V. Nano-and Micromotors Designed for Cancer Therapy. Molecules 2019, 24, 3410. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Azzopardi, E.A.; Ferguson, E.L.; Thomas, D.W. The enhanced permeability retention effect: A new paradigm for drug targeting in infection. J. Antimicrob. Chemother. 2013, 68, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in Micro-/Nanorobotics: Materials Development, Actuation, Localization, and System Integration for Biomedical Applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Taboryski, R. Recent Advances in Microswimmers for Biomedical Applications. Micromachines 2020, 11, 1048. [Google Scholar] [CrossRef]
- Taylor, E.; Webster, T.J. Reducing infections through nanotechnology and nanoparticles. Int. J. Nanomed. 2011, 6, 1463–1473. [Google Scholar]
- Zhang, Z.; Wang, L.; Chan, T.K.; Chen, Z.; Ip, M.; Chan, P.K.; Zhang, L. Micro-/Nanorobots in Antimicrobial Applications: Recent Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2022, 11, 2101991. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W. Nano/Microscale Motors: Biomedical Opportunities and Challenges. ACS Nano 2012, 6, 5745–5751. [Google Scholar] [CrossRef]
- Giri, G.; Maddahi, Y.; Zareinia, K. A Brief Review on Challenges in Design and Development of Nanorobots for Medical Applications. Appl. Sci. 2021, 11, 10385. [Google Scholar] [CrossRef]
- Mathesh, M.; Sun, J.; Wilson, D.A. Enzyme catalysis powered micro/nanomotors for biomedical applications. J. Mater. Chem. B 2020, 8, 7319–7334. [Google Scholar] [CrossRef]
- Naeem, S.; Naeem, F.; Mujtaba, J.; Shukla, A.K.; Mitra, S.; Huang, G.; Gulina, L.; Rudakovskaya, P.; Cui, J.; Tolstoy, V.; et al. Oxygen generation using catalytic nano/micromotors. Micromachines 2021, 12, 1251. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, J.; Wang, D.-W.; Xu, J.; Liang, K. Biofriendly micro/nanomotors operating on biocatalysis: From natural to biological environments. Biophys. Rep. 2020, 6, 179–192. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, L.; Zhong, W.; Yan, Q.; Gao, Y.; Hong, W.; She, Y.; Yang, G. Recent Advances in Motion Control of Micro/Nanomotors. Adv. Intell. Syst. 2020, 2, 2000049. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Mostaghaci, B.; Yasa, O.; Park, B.W.; Singh, A.V.; Sitti, M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 27–44. [Google Scholar] [CrossRef]
- Ding, S.; O’banion, C.P.; Welfare, J.G.; Lawrence, D.S. Cell Chemical Biology Review Cellular Cyborgs: On the Precipice of a Drug Delivery Revolution. Cell Chem. Biol. 2018, 25, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Timin, A.S.; Litvak, M.M.; Gorin, D.A.; Atochina-Vasserman, E.N.; Atochin, D.N.; Sukhorukov, G.B. Cell-Based Drug Delivery and Use of Nano-and Microcarriers for Cell Functionalization. Adv. Healthc. Mater. 2018, 7, 1700818. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Wang, J.; Soto, F.; Ma, P.; Ahmed, R.; Yang, H.; Chen, S.; Wang, J.; Liu, C.; Akin, D.; Fu, K.; et al. Acoustic Fabrication of Living Cardiomyocyte-based Hybrid Biorobots. ACS Nano 2022, 16, 10219–10230. [Google Scholar] [CrossRef]
- Furusawa, K.; Teramae, R.; Ohashi, H.; Shimizu, M. Development of Living “Bio-Robots” for Autonomous Actuations. J. Robot Mechatron. 2022, 34, 279–284. [Google Scholar] [CrossRef]
- Ramanujam, E.; Rasikannan, L.; Anandhalakshmi, P.A.; Kamal, N.A. Xenobots. Int. J. Sociotechnol. Knowl. Dev. 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Y.; Chen, Y.; Peng, F. Emerging Micro/Nanomotor-Based Platforms for Biomedical Therapy. Adv. Intell. Syst. 2020, 2, 1900081. [Google Scholar] [CrossRef]
- Xu, T.; Gao, W.; Xu, L.-P.; Zhang, X.; Wang, S. Fuel-Free Synthetic Micro-/Nanomachines. Adv. Mater. 2017, 29, 1603250. [Google Scholar] [CrossRef]
- Guo, J.; Gallegos, J.J.; Tom, A.R.; Fan, D. Electric-Field-Guided Precision Manipulation of Catalytic Nanomotors for Cargo Delivery and Powering Nanoelectromechanical Devices. ACS Nano 2018, 12, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef]
- Villa, K.; Pumera, M. Fuel-free light-driven micro/nanomachines: Artificial active matter mimicking nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef]
- Safdar, M.; Simmchen, J.; Jänis, J. Light-driven micro- and nanomotors for environmental remediation. Environ. Sci. Nano 2017, 4, 1602–1616. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Y.; Peng, F. The Energy Conversion behind Micro-and Nanomotors. Micromachines 2021, 12, 222. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef]
- Aghakhani, A.; Cetin, H.; Erkoc, P.; Tombak, G.I.; Sitti, M. Flexural wave-based soft attractor walls for trapping microparticles and cells. Lab Chip 2021, 21, 582–596. [Google Scholar] [CrossRef]
- Wrede, P.; Degtyaruk, O.; Kalva, S.K.; Deán-Ben, X.L.; Bozuyuk, U.; Aghakhani, A.; Akolpoglu, B.; Sitti, M.; Razansky, D. Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature. Sci. Adv. 2022, 8, 9132. [Google Scholar] [CrossRef]
- Wan, M.; Li, T.; Chen, H.; Mao, C.; Shen, J. Biosafety, Functionalities, and Applications of Biomedical Micro/nanomotors. Angew. Chem. Int. Ed. 2021, 60, 13158–13176. [Google Scholar] [CrossRef]
- Choi, H.; Yi, J.; Cho, S.H.; Hahn, S.K. Multifunctional micro/nanomotors as an emerging platform for smart healthcare applications. Biomaterials 2021, 279, 121201. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Wang, F.; Peng, F.; Tu, Y. The Application of Micro- and Nanomotors in Classified Drug Delivery. Chem. Asian J. 2019, 14, 2336–2347. [Google Scholar] [CrossRef]
- Alós, J.I. Antibiotic resistance: A global crisis. Enferm. Infecc. Microbiol. Clin. 2015, 33, 692–699. [Google Scholar] [CrossRef]
- Paterson, I.K.; Hoyle, A.; Ochoa, G.; Baker-Austin, C.; Taylor, N.G.H. Optimising Antibiotic Usage to Treat Bacterial Infections. Sci. Rep. 2016, 6, 37853. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-propelled micromotors for cleaning polluted water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; St Angelo, S.K.; Cao, Y.; Crespi, V.H. Catalytic Nanomotors: Autonomous Movement of Striped Nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Jurado-Sánchez, B.; Escarpa, A. Dual-Propelled Lanbiotic Based Janus Micromotors for Selective Inactivation of Bacterial Biofilms. Angew. Chem. Int. Ed. 2021, 60, 4915–4924. [Google Scholar] [CrossRef] [PubMed]
- Kiristi, M.; Singh, V.V.; de Ávila, B.E.-F.; Uygun, M.; Soto, F.; Uygun, D.A.; Wang, J. Lysozyme-Based Antibacterial Nanomotors. ACS Nano 2015, 9, 9252–9259. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, C.; Zhan, C.; Wang, Y.; You, Y.; Pan, X.; Jiao, J.; Zhang, R.; Dong, Z.; Wang, W.; et al. Enzymatic Micromotors as a Mobile Photosensitizer Platform for Highly Efficient On-Chip Targeted Antibacteria Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1807727. [Google Scholar] [CrossRef]
- Andeventer, P.E.; Weigel, K.M.; Salazar, J.; Erwin, B.; Irvine, B.; Doebler, R.; Nadim, A.; Cangelosi, G.A.; Niemz, A. Mechanical disruption of lysis-resistant bacterial cells by use of a miniature, low-power, disposable device. J. Clin. Microbiol. 2011, 49, 2533–2539. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Nanomachines: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-3-527-65147-4. [Google Scholar]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Peterson, B.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; Van Winkelhoff, A.-J.; Neut, D.; Stoodley, P.; Van Der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, P.; McLaren, A.; Tavaziva, G.; Overstreet, D. Biofilm Antimicrobial Susceptibility Increases With Antimicrobial Exposure Time. Clin. Orthop. Relat. Res. 2016, 474, 1659–1664. [Google Scholar] [CrossRef]
- Li, J.; de Ávila, B.E.-F.; Gao, W.; Zhang, L.; Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Villa, K.; Viktorova, J.; Plutnar, J.; Ruml, T.; Hoang, L.; Pumera, M. Chemical Microrobots as Self-Propelled Microbrushes against Dental Biofilm. Cell Rep. Phys. Sci. 2020, 1, 100181. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Yuan, K.; Ji, F.; Gao, J.; Zhang, Z.; Du, X.; Tian, Y.; Wang, Q.; Zhang, L. Magnetic Microswarm Composed of Porous Nanocatalysts for Targeted Elimination of Biofilm Occlusion. ACS Nano 2021, 15, 5056–5067. [Google Scholar] [CrossRef] [PubMed]
- Villa, K.; Sopha, H.; Zelenka, J.; Motola, M.; Dekanovsky, L.; Beketova, D.C.; Macak, J.M.; Ruml, T.; Pumera, M. Enzyme—Photocatalyst Tandem Microrobot Powered by Urea for Escherichia coli Biofilm Eradication. Small 2022, 2106612. Early view. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wu, S.; Sun, Y.; Ren, J.; Qu, X. Self-Propelled Active Photothermal Nanoswimmer for Deep-Layered Elimination of Biofilm in Vivo. Nano Lett. 2020, 20, 7350–7358. [Google Scholar] [CrossRef]
- Hwang, G.; Paula, A.J.; Hunter, E.E.; Liu, Y.; Babeer, A.; Karabucak, B.; Stebe, K.; Kumar, V.; Steager, E.; Koo, H. Catalytic antimicrobial robots for biofilm eradication. Sci. Robot. 2019, 4, eaaw2388. [Google Scholar] [CrossRef]
- Ussia, M.; Urso, M.; Dolezelikova, K.; Michalkova, H.; Adam, V.; Pumera, M. Active Light-Powered Antibiofilm ZnO Micromotors with Chemically Programmable Properties. Adv. Funct. Mater. 2021, 31, 2101178. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef]
- Tezel, G.; Timur, S.S.; Kuralay, F.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Current status of micro/nanomotors in drug delivery. J. Drug Target. 2020, 29, 29–45. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235. [Google Scholar] [CrossRef]
- Mellata, M. Human and Avian Extraintestinal Pathogenic Escherichia coli: Infections, Zoonotic Risks, and Antibiotic Resistance Trends. Foodborne Pathog. Dis. 2013, 10, 916. [Google Scholar] [CrossRef] [Green Version]
- Delezuk, J.A.M.; Ramírez-Herrera, D.E.; de Ávila, B.E.-F.; Wang, J. Chitosan-based water-propelled micromotors with strong antibacterial activity. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; Stanton, M.M.; Parmar, J.; Sánchez, S. Microbots Decorated with Silver Nanoparticles Kill Bacteria in Aqueous Media. ACS Appl. Mater. Interfaces 2017, 9, 22093–22100. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Orozco, J.; Kagan, D.; Guix, M.; Gao, W.; Sattayasamitsathit, S.; Wang, J. Bacterial Isolation by Lectin-Modified Microengines. Nano Lett. 2012, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized ultrasound-propelled magnetically guided nanomotors: Toward practical biomedical applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; Blanco-Cabra, N.; Eguskiza, A.; Hortelao, A.C.; Torrents, E.; Sanchez, S. Drug-Free Enzyme-Based Bactericidal Nanomotors against Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 14964–14973. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; Deleo, F.R. Pathogenesis of Staphylococcus aureus Abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [Green Version]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Chen, C.F.; Yi, Y.; Chen, L.J.; Wu, L.F.; Song, T. Construction of a microrobot system using magnetotactic bacteria for the separation of Staphylococcus aureus. Biomed. Microdevices 2014, 16, 761–770. [Google Scholar] [CrossRef]
- Blakemore, R.P. Magnetotactic Bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Wang, P.; Wu, L.F.; Song, T. Magnetically-induced elimination of Staphylococcus aureus by magnetotactic bacteria under a swing magnetic field. Nanomedicine 2017, 13, 363–370. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Yi, Y.; Chen, C.; Wu, L.F.; Song, T. Killing of Staphylococcus aureus via Magnetic Hyperthermia Mediated by Magnetotactic Bacteria. Appl. Environ. Microbiol. 2016, 82, 2219–2226. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Angsantikul, P.; Liu, W.; de Ávila, B.E.-F.; Chang, X.; Sandraz, E.; Wang, J. Biomimetic Platelet-Camouflaged Nanorobots for Binding and Isolation of Biological Threats. Adv. Mater. 2018, 30, 1704800. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Pan, G.; Sattayasamitsathit, S.; Galarnyk, M.; Wang, J. Micromotors to capture and destroy anthrax simulant spores. Analyst 2015, 140, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Vading, M.; Nauclér, P.; Kalin, M.; Giske, C.G. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS ONE 2018, 13, e0195258. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.L.; Da Silva, B.C.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A.; Campanini, E.B.; Pranchevicius, M.C.D.S. High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a brazilian intensive care unit. Front. Microbiol. 2019, 9, 3198. [Google Scholar] [CrossRef] [Green Version]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 20. [Google Scholar] [CrossRef]
- Xie, L.; Pang, X.; Yan, X.; Dai, Q.; Lin, H.; Ye, J.; Cheng, Y.; Zhao, Q.; Ma, X.; Zhang, X.; et al. Photoacoustic Imaging-Trackable Magnetic Microswimmers for Pathogenic Bacterial Infection Treatment. ACS Nano 2020, 14, 2880–2893. [Google Scholar] [CrossRef]
- Krakowka, S.; Eaton, K.A.; Leunk, R.D. Antimicrobial Therapies for Helicobacter pylori Infection in Gnotobiotic Piglets. Antimicrob. Agents Chemother. 1998, 42, 1549. [Google Scholar] [CrossRef] [Green Version]
- de Ávila BE, F.; Angsantikul, P.; Li, J.; Angel Lopez-Ramirez, M.; Ramírez-Herrera, D.E.; Thamphiwatana, S.; Wang, J. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Paryab, A.; Madaah Hosseini, H.R.; Abedini, F.; Dabbagh, A. Synthesis of magnesium-based Janus micromotors capable of magnetic navigation and antibiotic drug incorporation. New J. Chem. 2020, 44, 6947–6957. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De Lanauze, D.; Xu, Y.Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; LaFleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Bennet, M.; Landau, L.; Vach, P.; Pignol, D.; Bazylinski, D.A.; Frankel, R.B.; Klumpp, S.; Faivre, D. Diversity of Magneto-Aerotactic Behaviors and Oxygen Sensing Mechanisms in Cultured Magnetotactic Bacteria. Biophys. J. 2014, 107, 527. [Google Scholar] [CrossRef] [Green Version]
- Bennet, M.; McCarthy, A.; Fix, D.; Edwards, M.R.; Repp, F.; Vach, P.; Dunlop, J.; Sitti, M.; Buller, G.; Klumpp, S.; et al. Influence of Magnetic Fields on Magneto-Aerotaxis. PLoS ONE 2014, 9, e101150. [Google Scholar]
- Stanton, M.M.; Park, B.W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- Yasa, O.; Erkoc, P.; Alapan, Y.; Sitti, M. Microalga-Powered Microswimmers toward Active Cargo Delivery. Adv. Mater. 2018, 30, 1804130. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Yin, L.; Zhang, Q.; Askarinam, N.; Mundaca-Uribe, R.; Wang, J. ACE2 Receptor-Modified Algae-Based Microrobot for Removal of SARS-CoV-2 in Wastewater. J. Am. Chem. Soc. 2021, 143, 12194–12201. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Sieber, S.; Gademann, K. Green Algae as a Drug Delivery System for the Controlled Release of Antibiotics. Chem A Eur. J. 2020, 26, 16644–16648. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Han, Y.; Gong, X. Micro/Nanorobots for Medical Diagnosis and Disease Treatment. Micromachines 2022, 13, 648. [Google Scholar] [CrossRef]
- Choi, J.; Hwang, J.; Kim, J.-Y.; Choi, H. Recent Progress in Magnetically Actuated Microrobots for Targeted Delivery of Therapeutic Agents. Adv. Healthc. Mater. 2021, 10, 2001596. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, C.; Dong, L.; Zhao, J. A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo. Micromachines 2021, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics 2020, 12, 665. [Google Scholar] [CrossRef]
- Dong, X.; Sitti, M. Controlling two-dimensional collective formation and cooperative behavior of magnetic microrobot swarms. Int. J. Robot. Res. 2020, 39, 5, 617–638. [Google Scholar] [CrossRef]
- Halder, A.; Sun, Y. Biocompatible propulsion for biomedical micro/nano robotics. Biosens. Bioelectron. 2019, 139, 111334. [Google Scholar] [CrossRef]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/Nanorobots at Work in Active Drug Delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Erkoc, P.; Yasa, I.C.; Ceylan, H.; Yasa, O.; Alapan, Y.; Sitti, M. Mobile Microrobots for Active Therapeutic Delivery. Adv. Ther. 2019, 2, 1800064. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Yang, H.; Liu, W.; Ma, Y.; Wu, J.; Zong, X.; Yuan, P.; Chen, X.; Yang, C.; Li, X.; et al. Multifunctional Parachute-like Nanomotors for Enhanced Skin Penetration and Synergistic Antifungal Therapy. ACS Nano 2021, 15, 14218–14228. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Laux, P.; Luch, A. Micro-nanorobots: Important considerations when developing novel drug delivery platforms. Expert Opin. Drug Deliv. 2019, 16, 1259–1275. [Google Scholar] [CrossRef]
- Kumar Daima, H.; Xia, Z.; Ying, Y.; Yang, X.; Ye, W.; Qi, Y. Overcoming Multidrug Resistance in Bacteria Through Antibiotics Delivery in Surface-Engineered Nano-Cargos: Recent Developments for Future Nano-Antibiotics. Futur. Nano-Antibiot. Front. Bioeng. Biotechnol. 2021, 9, 696514. [Google Scholar]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020, 11, 748–787. [Google Scholar] [CrossRef] [PubMed]

| Micro-/Nanorobot Types | Targeted Bacteria | Outcomes |
|---|---|---|
| Pt/TiO2 | Dental biofilm | The effect of TiO2 microrobots of up to 1% H2O2 in the in vitro biofilm model system was investigated. In addition, its toxic effect on HEK293T and HaCaT cell lines was investigated [63]. |
| p-Fe3O4 MPs | E. coli and B. cereus biofilms | Firstly, the effect of p-Fe3O4 MPs microrobots on biofilm in PBS and LCM solutions has been demonstrated. In addition, its toxic effect on 3T3 cells was investigated [64]. |
| Enzyme-bound Fe3O4 NPs | Streptococcus mutans biofilms | It has been reported that Fe3O4 nanorobots formulated with mutase dextranase enzymes have a high biofilm effect in vitro [67]. |
| ZnO: Ag | S. aureus and P. aeruginosa biofilms | More than 80% killing was observed in the in vitro biofilm environment of ZnO: Ag nanorobots, together with the photocatalytic effect [68]. |
| NIR-Mesoporous Silica Half-Shell | S. aureus biofilms | The toxic effect of NIR-guided HSMV on 3T3 fibroblasts was investigated. In addition, its effect on the biofilm in mice was studied and high efficiency was obtained [66]. |
| Mg/Au/PLGA/Alg/Chi | E. coli | The Mg/Au/PLGA/Alg/Chi nanorobot has been designed to kill E.coli bacteria by 90% in seawater and drinking water [73]. |
| Au/Ni/PANI/Pt microtubular | E. coli | It was observed that E.coli bacteria were cleared in vitro with lectin-modified Au/Ni/PANI/Pt microtubular [75]. |
| Au/Ni/Au Nanowire | E. coli | E.coli was removed with lectin-modified Au/Ni/Au nanowire in vitro [76]. |
| MRS-1-MSM | E. coli | MRS-1 bacteria and MSM were combined, and efficient results were obtained on E. coli in vitro by loading ciprofloxacin antibiotics [98]. |
| Ag/Mg Janus | E. coli | The effect of Ag/Mg Janus microrobots on E. coli bacteria was investigated on water and PBS and high efficiency was obtained [74]. |
| U-MNSP | E. coli | Mesoporous silica nanoparticles urease, lysozyme, and the combination of urease and lysozyme were modified in 3 different ways and their effect on E. coli was investigated. Up to 80% kill has been reported with the urease-functionalized MNSP [77]. |
| (COOH-PPy): PEDOT/Ni/Pt | Bacillus globigii | PEDOT/Ni/Pt microrobots were modified with an anti-B antibody that has been shown to kill B. globigii spores in high-efficiency in vitro environments such as lake water [85]. |
| PDA-MSP | Klebsiella pneumoniae | The photothermal effect of PDA-MSP magnetic microrobots resulted in high activity on Klebsiella pneumoniae bacteria in vitro [90]. |
| Mg/TiO2 Janus | Helicobacter pylori | When the efficiency of CLR antibiotic-loaded Mg-TiO2 Janus microrobots on Helicobacter pylori-infected mice was examined, an efficiency close to 99% was obtained [92]. |
| Mg/Fe3O4 Janus | Pseudomonas aeruginosa | When the effects of Janus nanorobots, consisting of magnesium and superparamagnetic iron oxide, with and without antibiotics were examined, efficiencies of 80% and 100% were obtained in vitro, respectively [94]. |
| sMF attached to MO-1 cells | S. aureus | Magnetotactic bacteria designed with a low frequency and low heat generating oscillating magnetic field were coated with rabbit anti-MO-1 polyclonal antibody and its effect on S. aureus bacteria in vitro was investigated [82]. |
| Magnetotactic bacteria MO-1 | S. aureus | Magnetotactic bacterial nanorobots rabbit anti-MO-1 cell polyclonal antibodies have been modified and its effect on S.auerus has been observed in vitro [80]. |
| Alternating Magnetic Field (AMF)-MO-1 | S. aureus | Effective results have been reported in mice at 43.0 °C by hyperthermia by modifying magnetotactic bacteria with rabbit anti-MO-1 polyclonal antibodies [83]. |
| Platelet-Membrane Cloaked Nanorobots | S. aureus | The effect of platelet-membrane cloaked nanorobots on S. aureus was investigated in vitro [84]. |
| ACE2-Algae | SARS-CoV-2 | SARS-CoV-2 was removed from the water by using C. reinhardtii algae [100]. |
| Antibiotic-loaded algae | Bacillus subtilis | High efficiency as a result of photo light triggering of vancomycin-loaded algae B. subtilis bacteria was inhibited [101]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaydin, M.S.; Doganturk, L.; Ulucan-Karnak, F.; Akdogan, O.; Erkoc, P. Contemporary Tools for the Cure against Pernicious Microorganisms: Micro-/Nanorobots. Prosthesis 2022, 4, 424-443. https://doi.org/10.3390/prosthesis4030034
Ozaydin MS, Doganturk L, Ulucan-Karnak F, Akdogan O, Erkoc P. Contemporary Tools for the Cure against Pernicious Microorganisms: Micro-/Nanorobots. Prosthesis. 2022; 4(3):424-443. https://doi.org/10.3390/prosthesis4030034
Chicago/Turabian StyleOzaydin, Mustafa Sami, Lorin Doganturk, Fulden Ulucan-Karnak, Ozan Akdogan, and Pelin Erkoc. 2022. "Contemporary Tools for the Cure against Pernicious Microorganisms: Micro-/Nanorobots" Prosthesis 4, no. 3: 424-443. https://doi.org/10.3390/prosthesis4030034
APA StyleOzaydin, M. S., Doganturk, L., Ulucan-Karnak, F., Akdogan, O., & Erkoc, P. (2022). Contemporary Tools for the Cure against Pernicious Microorganisms: Micro-/Nanorobots. Prosthesis, 4(3), 424-443. https://doi.org/10.3390/prosthesis4030034









