Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
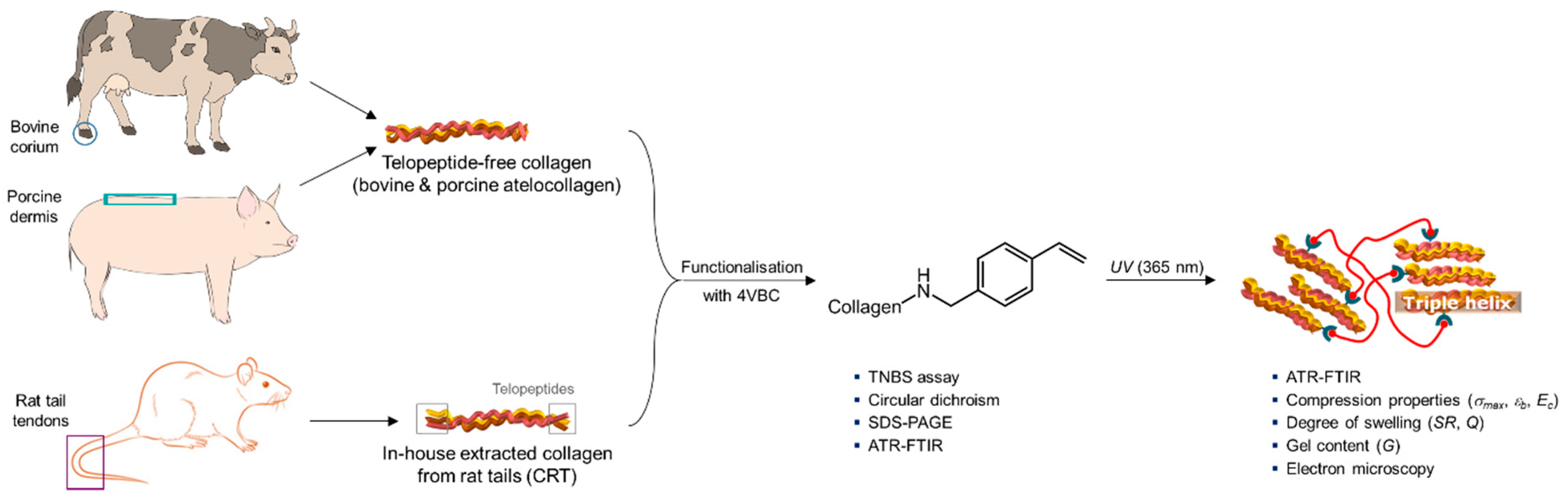
2.2. Synthesis of 4VBC-Functionalised Collagen Products
2.3. 2,4,6-Trinitrobenzenesulfonic Acid (TNBS) Assay
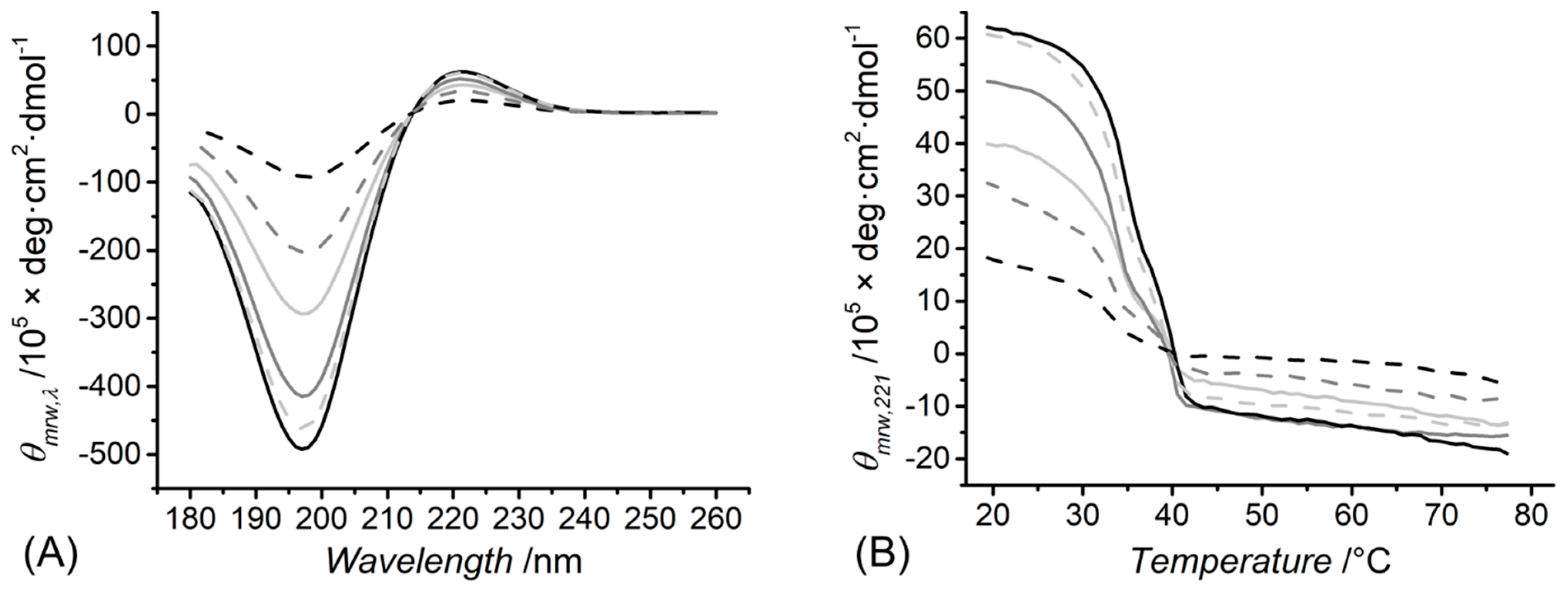
2.4. Circular Dichroism
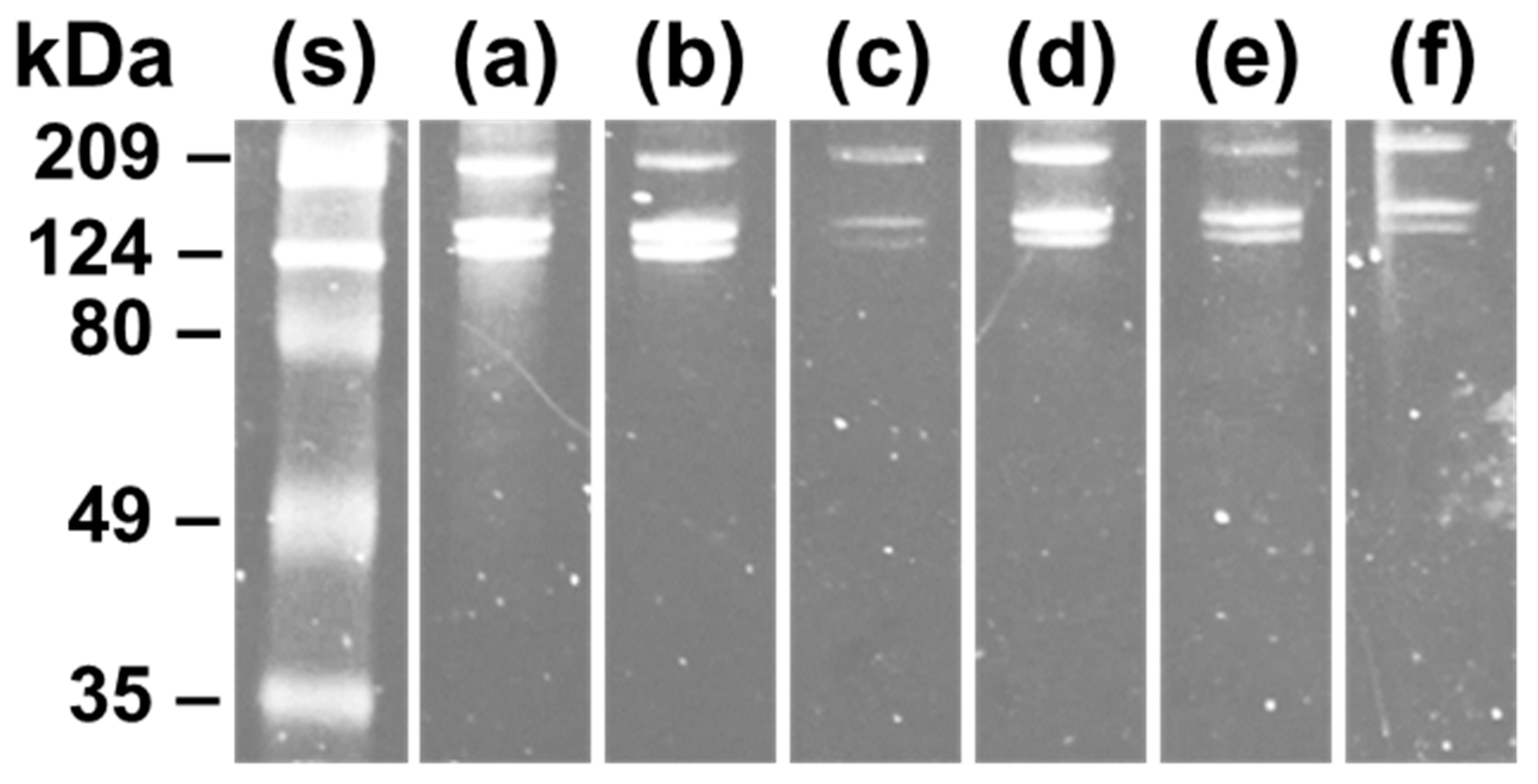
2.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
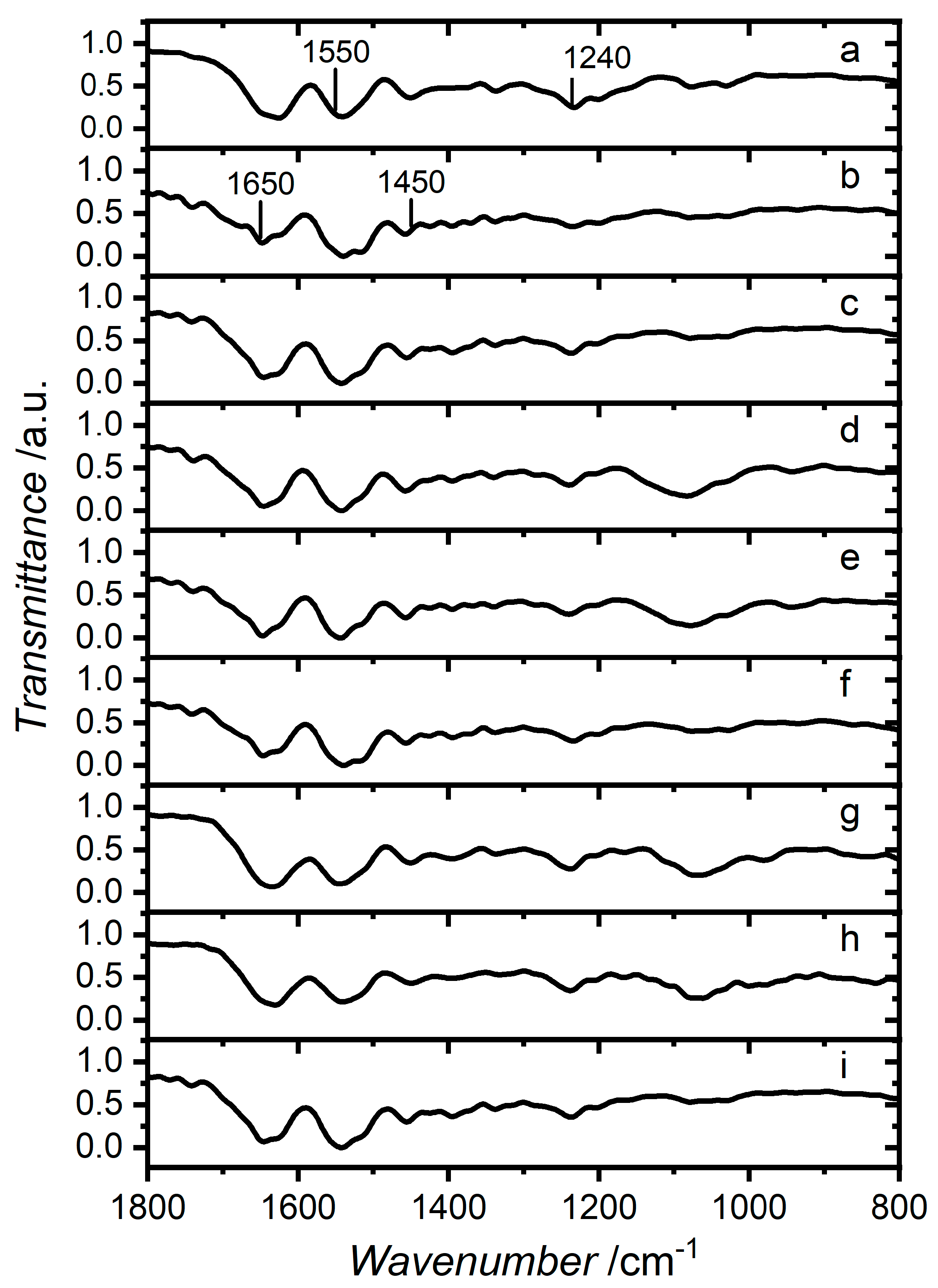
2.6. Attenuated Total Reflection Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.7. Synthesis of UV-Cured Hydrogel Networks
2.8. Swelling Tests
2.9. Gel Content
2.10. Compression Tests
2.11. Scanning Electron Microscopy
2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puetzer, J.L.; Ma, T.; Sallent, I.; Gelmi, A.; Stevens, M.M. Driving hierarchical collagen fiber formation for functional tendon, ligament, and meniscus replacement. Biomaterials 2021, 269, 120527. [Google Scholar] [CrossRef] [PubMed]
- Gachon, E.; Mesquida, P. Mechanical strain alters the surface charge of collagen fibrils. ACS Nano 2021, 15, 9820–9826. [Google Scholar] [CrossRef]
- Das, A.; Abas, M.; Biswas, N.; Banerjee, P.; Ghosh, N.; Rawat, A.; Khanna, S.; Roy, S.; Sen, C.K. A modified collagen dressing induces transition of inflammatory to reparative phenotype of wound macrophages. Sci. Rep. 2019, 9, 14293. [Google Scholar] [CrossRef] [Green Version]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for guided bone regeneration: A road from bench to bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef]
- Maghdouri-White, Y.; Sori, N.; Petrova, S.; Wriggers, H.; Kemper, N.; Dasgupta, A.; Coughenour, K.; Polk, S.; Thayer, N.; Rodriguez, M.C.; et al. Biomanufacturing organized collagen-based microfibers as a tissue engineered device (TEND) for tendon regeneration. Biomed. Mater. 2021, 16, 025025. [Google Scholar] [CrossRef]
- Trabuco, E.C.; Klingele, C.J.; Gebhart, J.B. Xenograt use in reconstructive pelvic surgery: A review of the literature. Int. Urogynecol. J. 2007, 18, 555–563. [Google Scholar] [CrossRef]
- Whooley, J.; Cunnane, E.M.; Amaral, R.D.; Joyce, M.; MacCraith, E.; Flood, H.D.; O’Brien, F.J.; Davis, N.F. Stress urinary incontinence and pelvic organ prolapse: Biologic graft materials revisited. Tissue Eng. Part B Rev. 2020, 26, 475–483. [Google Scholar] [CrossRef]
- Suzuki, R.; Nakamura, R.; Nakaegawa, Y.; Nomoto, Y.; Fujimoto, I.; Semura, K.; Hazama, A.; Omori, K. Optimal bovine collagen concentration to achieve tracheal epithelial coverage of collagen sponges. Laryngoscope 2016, 126, E396–E403. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Dai, J.; Zhu, X.; Cao, R.; Song, N.; Liu, M.; Liu, X.; Zhu, J.; Pan, F.; Qin, L.; et al. Biomimetic trachea engineering via a modular ring strategy based on bone-marrow stem cells and atelocollagen for use in extensive tracheal reconstruction. Adv. Mater. 2021, in press. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ronsin, O.; Gravez, B.; Farman, N.; Baumberger, T.; Jaisser, F.; Coradin, T.; Hélary, C. Nanostructured dense collagen-polyester composite hydrogels as amphiphilic platforms for drug delivery. Adv. Sci. 2021, 8, 2004213. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, Y.; Nishimoto, S.; Suye, S.I.; Fujita, S. Native collagen hydrogel nanofibres with anisotropic structure using core-shell electrospinning. Sci. Rep. 2018, 8, 6248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Xie, X.; Lei, J.; Ge, L.; Yuan, L.; Li, D.; Mu, C. Controlling the pore structure of collagen sponge by adjusting the cross-linking degree for construction of heterogeneous double-layer bone barrier membranes. ACS Appl. Bio Mater. 2020, 3, 2058–2067. [Google Scholar] [CrossRef]
- Kwong, M.T.; Stell, D.; Akinluyi, E. Medical device regulation from a health service provider’s perspective. Prosthesis 2021, 3, 261–266. [Google Scholar] [CrossRef]
- Kaule, S.; Bock, A.; Dierke, A.; Siewert, S.; Schmitz, K.; Stiehm, M.; Klar, E.; Leuchter, M.; Lenarz, T.; Zygmunt, M.; et al. Medical device regulation and current challenges for the implementation of new technologies. Curr. Dir. Biomed. 2020, 6, 334–337. [Google Scholar] [CrossRef]
- Letourneur, D.; Joyce, K.; Chauvierre, C.; Bayon, Y.; Pandit, A. Enabling MedTech translation in academia: Redefining value proposition with updated regulations. Adv. Healthc. Mater. 2021, 10, 2001237. [Google Scholar] [CrossRef]
- Engel, J.; Bächinger, H.P. 2005. Structure, stability and folding of the collagen triple helix. In Collagen: Primer in Structure, Processing and Assembly, 1st ed.; Brinckmann, J., Notbohm, H., Bachinger, H.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 7–33. ISBN 9783642062322. [Google Scholar]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Aragona, F.; D’Urso, L.; Marcolongo, R. Immunologic aspects of bovine injectable collagen in humans. Eur. Urol. 1998, 33, 129–133. [Google Scholar] [CrossRef]
- Delgado, L.M.; Shologu, N.; Fuller, K.; Zeugolis, D.I. Acetic acid and pepsin result in high yield, high purity and low macrophage response collagen for biomedical applications. Biomed. Mater. 2017, 12, 065009. [Google Scholar] [CrossRef] [Green Version]
- Tronci, G. The application of collagen in advanced wound dressings. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Elsevier Ltd.: London, UK, 2019; pp. 363–389. ISBN 9780081021927. [Google Scholar]
- Andrée, B.; Ichanti, H.; Kalies, S.; Heisterkamp, A.; Strauß, S.; Vogt, P.M.; Haverich, A.; Hilfiker, A. Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci. Rep. 2019, 9, 5437. [Google Scholar] [CrossRef]
- Gabler, C.; Spohn, J.; Tischer, T.; Bader, R. Biomechanical, biochemical and cell biological evaluation of different collagen scaffolds for tendon augmentation. BioMed. Res. Int. 2018, 2018, 7246716. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Tables on Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies. Available online: https://www.who.int/bloodproducts/tablestissueinfectivity.pdf (accessed on 19 December 2021).
- Lambert, Z.J.; Greenlee, J.J.; Cassmann, E.D.; West Greenlee, M.H. Differential accumulation of misfolded prion strains in natural hosts of prion diseases. Viruses 2021, 13, 2453. [Google Scholar] [CrossRef]
- Marín, B.; Otero, A.; Lugan, S.; Espinosa, J.C.; Marín-Moreno, A.; Vidal, E.; Hedman, C.; Romero, A.; Pumarola, M.; Badiola, J.J.; et al. Classical BSE prions emerge from asymptomatic pigs challenged with atypical/Nor98 scrapie. Sci. Rep. 2021, 11, 17428. [Google Scholar] [CrossRef]
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J. Mater. Sci. Mater. Med. 2021, 32, 12. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Cliche, S.; Gariépy, C.; Germain, L.; Berthod, F. Comparative study of bovine, porcine and avian collagens for the production of a tissue engineered dermis. Acta Biomater. 2011, 7, 3757–3765. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Russell, S.J.; Wood, D.J.; Tronci, G. Monomer-induced customization of UV-cured atelocollagen hydrogel networks. Front. Chem. 2018, 6, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronci, G.; Russell, S.J.; Wood, D.J. Photo-active collagen systems with controlled triple helix architecture. J. Mater. Chem. B 2013, 1, 3705–3715. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Russell, S.J.; Wood, D.J.; Tronci, G. A hydroxamic acid–methacrylated collagen conjugate for the modulation of inflammation-related MMP upregulation. J. Mater. Chem B 2018, 6, 3703–3715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Liang, H.; Yin, J.; Man, K.; Yang, X.B.; Calciolari, E.; Donos, N.; Russell, S.J.; Wood, D.; Tronci, G. A long-lasting guided bone regeneration membrane from sequentially functionalised photoactive atelocollagen. Acta Biomater. 2021, in press. [Google Scholar] [CrossRef]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Oechsle, A.M.; Akgün, D.; Krause, F.; Maier, C.; Gibis, M.; Kohlus, R.; Weiss, J. Microstructure and physical–chemical properties of chicken collagen. Food Struct. 2016, 7, 29–37. [Google Scholar] [CrossRef]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tronci, G.; Doyle, A.; Russell, S.J.; Wood, D.J. Structure-property-function relationships in triple-helical collagen hydrogels. Mater. Res. Soc. Symp. Proc. 2012, 1498, 145–150. [Google Scholar] [CrossRef]
- Yasunaga, H.; Ando, I. Dynamic behavior of water in hydro-swollen crosslinked polymer gel as studied by PGSE 1H NMR and pulse 1H NMR. Polym. Gels Netw. 1993, 1, 83–92. [Google Scholar] [CrossRef]
- Tanaka, N.; Matsukawa, S.; Kurosu, H.; Ando, I. A study on dynamics of water in crosslinked poly (N-isopropylacrylamide) gel by nmr spectroscopy. Polymer 1998, 39, 4703–4706. [Google Scholar] [CrossRef]
- Gemeinhart, R.A.; Park, H.; Park, K. Pore structure of superporous hydrogels. Polym. Adv. Technol. 2000, 11, 617–625. [Google Scholar] [CrossRef]
- Yacob, N.; Hashim, K. Morphological effect on swelling behaviour of hydrogel. In Proceedings of the AIP, Kuala Lupmur, Malaysia, 30 September–2 October 2014; Volume 1584, pp. 153–159. [Google Scholar] [CrossRef] [Green Version]

| Sample ID | [Lys]/10−4 × mol∙g−1 | F/mol.% | RPN | Td/°C |
|---|---|---|---|---|
| CRT | 2.65 ± 0.05 (a) | N/A | 0.12 | 35 |
| BA | 2.60 ± 0.19 | N/A | 0.15 | 34 |
| PA | 2.32 ± 0.04 (a) | N/A | 0.13 | 36 |
| R-4VBC | 1.96 ± 0.19 | 26 ± 7 (a) | 0.12 | 32 |
| B-4VBC | 2.06 ± 0.07 | 21 ± 3 | 0.13 | 35 |
| P-4VBC | 2.06 ± 0.02 | 11 ± 1 (a) | 0.22 | 32 |
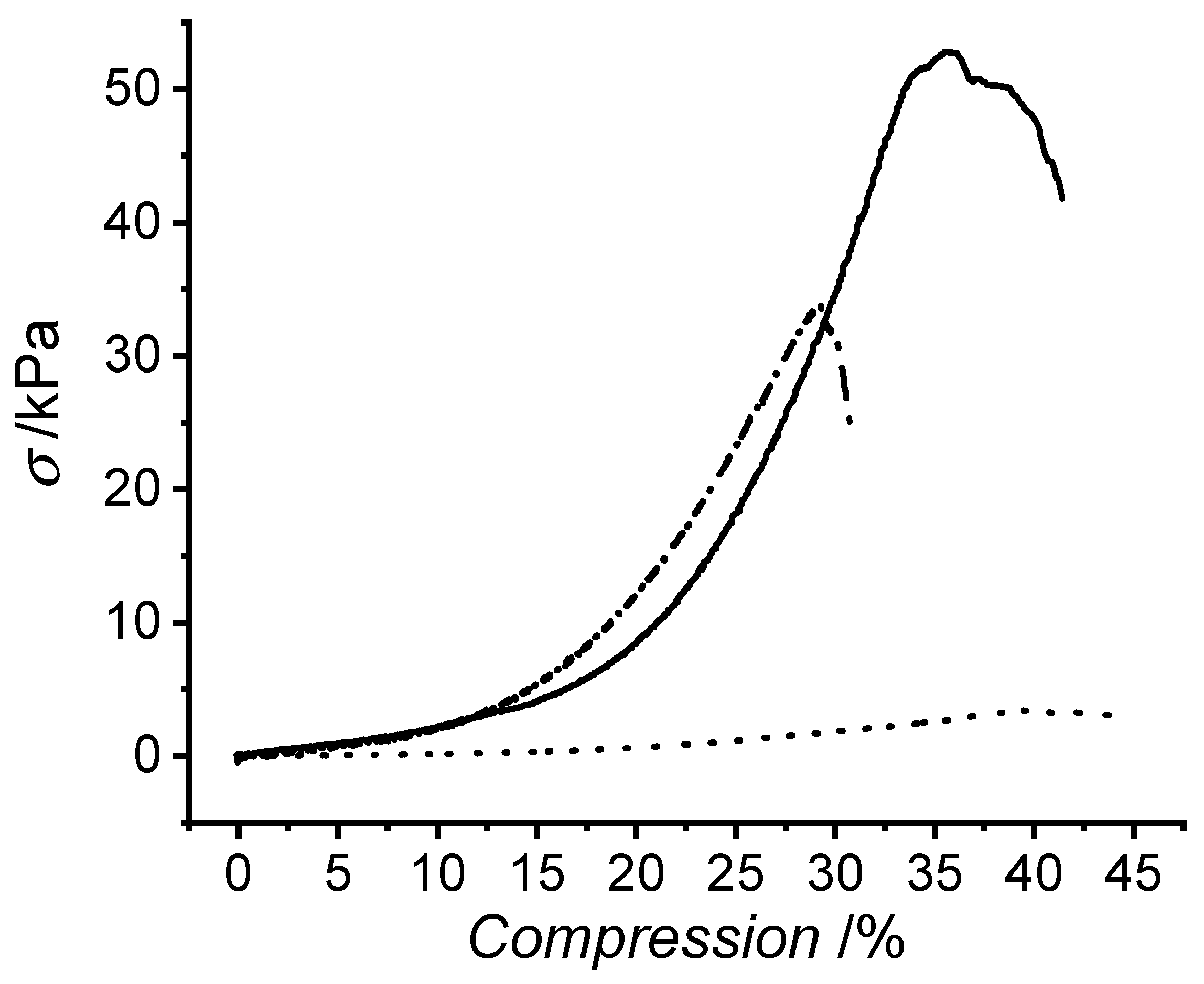
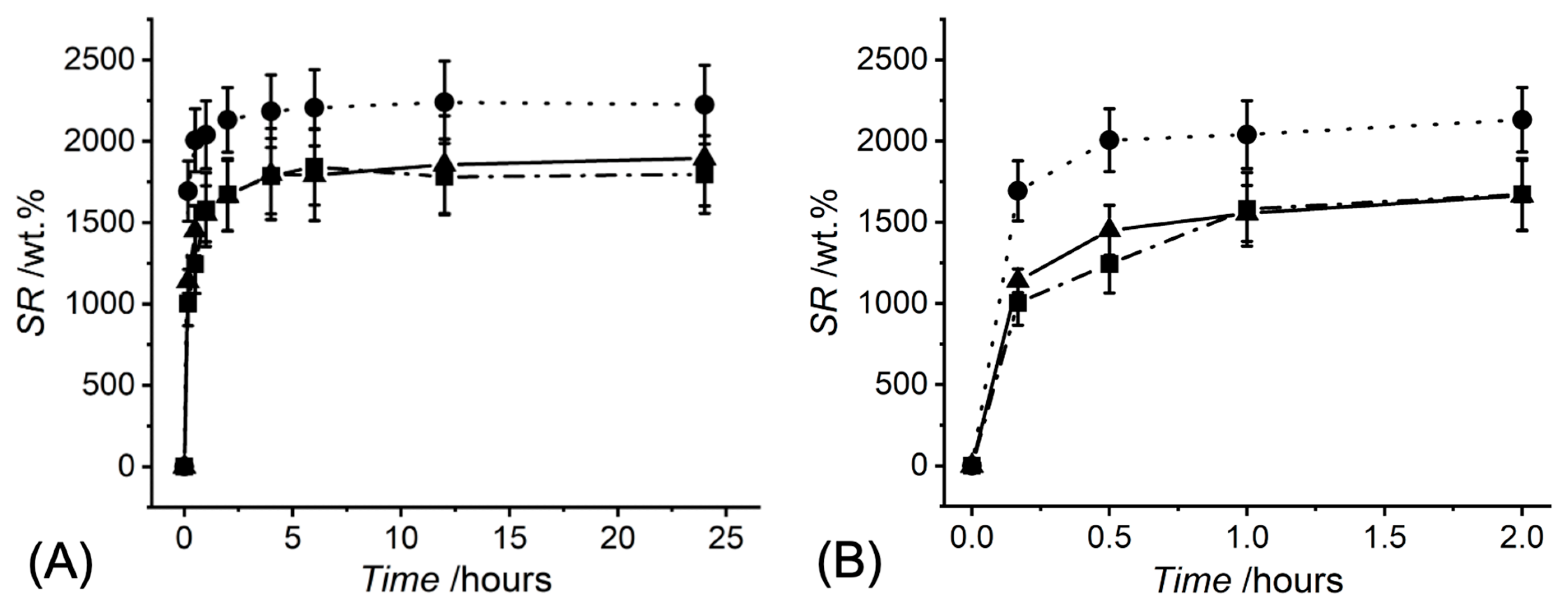
| Sample ID | σmax/kPa | εb/% | Ec/kPa | SR/wt.% | Q/vol.% | G/wt.% |
|---|---|---|---|---|---|---|
| R-4VBC* | 40 ± 14 | 44 ± 7 (b) | 67 ± 30 | 1895 ± 292 (a) | 1165 ± 179 | 98 ± 1 |
| B-4VBC* | 36 ± 8 | 37 ± 4 (c) | 79 ± 25 | 1796 ± 239 (b) | 1296 ± 172 | 97 ± 1 |
| P-4VBC* | 4 ± 1 (c) | 54 ± 11 (b)(c) | 6 ± 2 (c) | 2224 ± 242 (a)(b) | 2106 ± 229 (c) | 62 ± 5 (c) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooker, C.; Tronci, G. Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks. Prosthesis 2022, 4, 1-14. https://doi.org/10.3390/prosthesis4010001
Brooker C, Tronci G. Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks. Prosthesis. 2022; 4(1):1-14. https://doi.org/10.3390/prosthesis4010001
Chicago/Turabian StyleBrooker, Charles, and Giuseppe Tronci. 2022. "Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks" Prosthesis 4, no. 1: 1-14. https://doi.org/10.3390/prosthesis4010001
APA StyleBrooker, C., & Tronci, G. (2022). Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks. Prosthesis, 4(1), 1-14. https://doi.org/10.3390/prosthesis4010001







