Abstract
Is it possible that two different transitions in the non-relativistic quantum mechanical model of the hydrogen atom give the same frequency of radiation? That is, can different energy level transitions in a hydrogen atom have the same photon radiation frequency? This question, which was asked during a Ph.D. oral exam in 1997 at the University of Colorado Boulder, is well-known among physics graduate students. We show a general solution to this question, in which all equifrequency transition pairs can be obtained from the set of solutions of a Diophantine equation. This fun puzzle is a simple yet concrete example of how number theory can be relevant to quantum systems, a curious theme that emerges in theoretical physics but is usually inaccessible to a general audience.
1. Introduction
For more than a century, quantum mechanics has been the fundamental theory that guides our understanding of how nature works at the scale of atoms and subatomic particles. The hydrogen atom is the simplest possible atom for theoretical investigation, consisting of only a single proton and a single electron orbiting around [1]. While the hydrogen atom has been intensively studied since the dawn of quantum mechanics (Figure 1), as a demonstration of what measurements can reveal about atoms, there are still surprises and hidden structures [2]. One example is the emergence of equifrequency transitions, in which many distinctive jumps between atomic levels can radiate identical photon energy. This question was raised during a Ph.D. oral exam in 1997 at the University of Colorado Boulder [3] and soon became well-known in the physics community, especially among graduate students. The answer is definitely yes, and an infinite number of transitions have been found [4]; however, to the best of our knowledge, a generalization is still lacking.
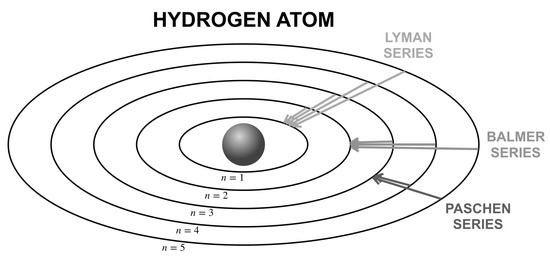
Figure 1.
Energy-level transitions in the non-relativisitic quantum mechanical model of the hydrogen atom. An electron jumps from an outer ring -th to an inner ring -th, emits a photon with radiation energy .
Here, we show the connection between the above question and a Diophantine equation [5], and present a general solution, i.e., how all equifrequency transition pairs can be obtained. While this finding might not address any foundational issue or important problem in quantum mechanics, it definitely provides us with a more complete understanding of the most popular atom in all quantum mechanics textbooks, and the relationship between atomic levels (disregarding degeneracies due to angular momentum and spin). It is also a simple illustration of how number theory can be of relevance to physics [6], in a way that is accessible to non-experts.
2. A General Solution for All Equifrequency Transitions
In quantum mechanics, the n-th energy level of a hydrogen atom is given by , where is a positive integer and eV is the Rydberg energy [7]. For simplicity, we will not consider any relativistic effects [8] or other corrections (such as fine structure [9,10]) to this equation. The challenge is to find all transition pairs (, ) with an equal radiation energy, which means:
Here, we will find a general solution to this equation, including trivial solutions where and .
Consider the Diophantine equation [5] with a parameter and unknowns ,
With any two solutions and to this equation, for any positive integer pair that satisfies
a solution to Equation (1) can be obtained:
which can be checked by direct substitution. To generate all solutions to Equation (3), we use any and ,
where the operation determines the greatest common divisor of [11].
We can prove that the above procedure comprises all solutions of Equation (1). Starting from this equation, denote and . Write . Note that and i.e., and . Then, we rewrite (1) as,
and put the fraction into irreducible form where and both are non-zero,
Thus,
and hence there exists such that,
Note here that condition (7) is exactly Equation (3) and condition (9) provides us two solutions (2). Combined with the above paragraph, we see that these two conditions (7) and (9) are both necessary and sufficient. This completes the proof.
To generate the set of all non-zero integer solutions to Equation (2), we need the set of all non-zero rational solutions into their dehomogenized version (by dividing both sides of (2) by ):
This equation is very similar to the Pell equation [12], but can be solved using a much simpler method. By taking any that satisfies (10) and any , we obtain all triples,
of (2) where .
The geometric way [13] of dealing with Equation (10) is to draw a line in the -plane passing through with a rational slope ; for example, the line (see Figure 2). For , this line will cut the curve (10) at another point,
and, more importantly, all solutions of Equation (10) can be attained in this way by varying q. Note that gives , and changing the sign of q changes the sign of . Hence, if we let where , ; then, , , where
The positive triple can be obtained from (11) with the correct sign choice.
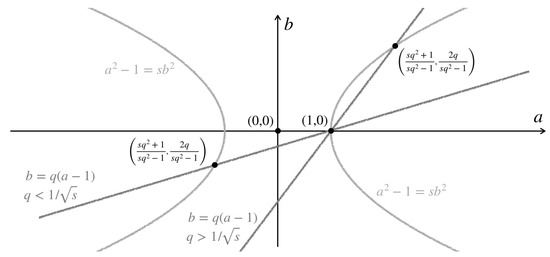
In summary, we can generate a solution to Equation (2) with parameter from any number . Given the pair, we go through Equations (13) and (), pick a value and use Equation (11) to arrive at . Then, with two such solutions, say, and , we choose a value and use Equation (5) to obtain before plugging in Equation (4) to obtain a pair . See Figure 3 for a demonstration. The key difference in our approach compared to previous ones is using (2), where we can generate all possible rational solutions, which enables us to find all possible solutions to the puzzle (1).
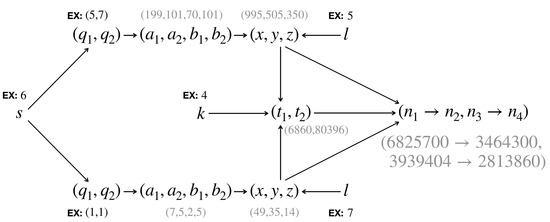
Figure 3.
A demonstration for the procedure to obtain an equifrequency transition pair. Here we start by selecting , then from and we get , from and we get . Then, with , we arrive at , which can be checked as satisfying Equation (1).
3. Families of Equifrequency Transitions
Perondi [4] found an infinite number of solutions to the generalization of (1):
for any . His approach is to start with a set of k primes , for some , and then try to find an integer , for which
has a solution. By partitioning [14], the set of indices into two sets and denoting , he found that if and is divisible by and , then is a solution to
which, again, using the identity . Now, by enlarging (or shrinking) if necessary, and splitting the set of indices differently, he found n distinct pairs . Then, he chose a positive integer , which is divisible by n ’s, and found n pairs solution to (16).
Similar to the above, we can find all solutions to the generalized Equation (15) of Perondi by simply solving the first equation, which is equal to the ith equation, for all i, using the method we found in the previous section. First, choose and n distinct triple , satisfying:
Then, we want to find , such that
It suffices that is divisible by
and the remaining are deduced from (19). The final solution to the generalized Equation (15) is:
Author Contributions
Conceptualization, T.V.P.; investigation, T.K.D. and T.V.P.; validation, T.V.P.; writing—original draft preparation, T.K.D. and T.V.P.; writing—review and editing, T.K.D. and T.V.P.; visualization, T.V.P.; supervision, T.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Duy V. Nguyen and the xPhO journal club for their support to share this finding with a wider audience. We also thank Kirk McDonald for making clear the assumptions made in the problem statement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lakhtakia, A. Models and Modelers of Hydrogen: Thales, Thomson, Rutherford, Bohr, Sommerfeld, Goudsmit, Heisenberg, Schr Dinger, Dirac, Sallhofer; World Scientific: Singapore, 1996. [Google Scholar]
- Mills, R.L. The hydrogen atom revisited. Int. J. Hydrogen Energy 2000, 25, 1171–1183. [Google Scholar] [CrossRef]
- Kantor, Y. Question 06/00: Hydrogen Atom, 2000. Available online: https://www.tau.ac.il/~kantor/QUIZ/00/Q06.00.html (accessed on 11 July 2022).
- Kantor, Y. Answer to the Question 06/00: Hydrogen Atom, 2000. See the Method Given by L.F. Perondi. Available online: https://www.tau.ac.il/~kantor/QUIZ/00/Q06.00.html (accessed on 11 July 2022).
- Mordell, L.J. Diophantine Equations; Academic Press: Cambridge, MA, USA, 1969. [Google Scholar]
- Aoki, T.; Kanemitsu, S.; Nakahara, M.; Ohno, Y. Zeta Functions, Topology and Quantum Physics; Springer Science & Business Media: Berlin, Germany, 2008; Volume 14. [Google Scholar]
- Griffiths, D.J.; Schroeter, D.F. Introduction to Quantum Mechanics; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Auvil, P.R.; Brown, L.M. The relativistic hydrogen atom: A simple solution. Am. J. Phys. 1978, 46, 679–681. [Google Scholar] [CrossRef]
- Lamb, W.E., Jr.; Retherford, R.C. Fine structure of the hydrogen atom. Part I. Phys. Rev. 1950, 79, 549. [Google Scholar] [CrossRef]
- Lamb, W.E., Jr.; Retherford, R.C. Fine structure of the hydrogen atom. Part II. Phys. Rev. 1951, 81, 222. [Google Scholar] [CrossRef]
- Long, C.T. Elementary Introduction to Number Theory; Prentice Hall: Hoboken, NJ, USA, 1987. [Google Scholar]
- Jacobson, M.J.; Williams, H.C. Solving the Pell Equation; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Hindry, M.; Silverman, J.H. Diophantine Geometry: An Introduction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 201. [Google Scholar]
- Halmos, P.R. Naive Set Theory; van Nostrand: Princeton, NJ, USA, 1960; p. 28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).