Metabolic Disorders in Transition Dairy Cows in a 500-Cow Herd—Analysis, Prevention and Follow-Up
Abstract
1. Introduction
2. Case Presentation
2.1. Background
2.2. Herd Assessment
2.2.1. Housing and Cow Behaviour
2.2.2. Scoring of Cows
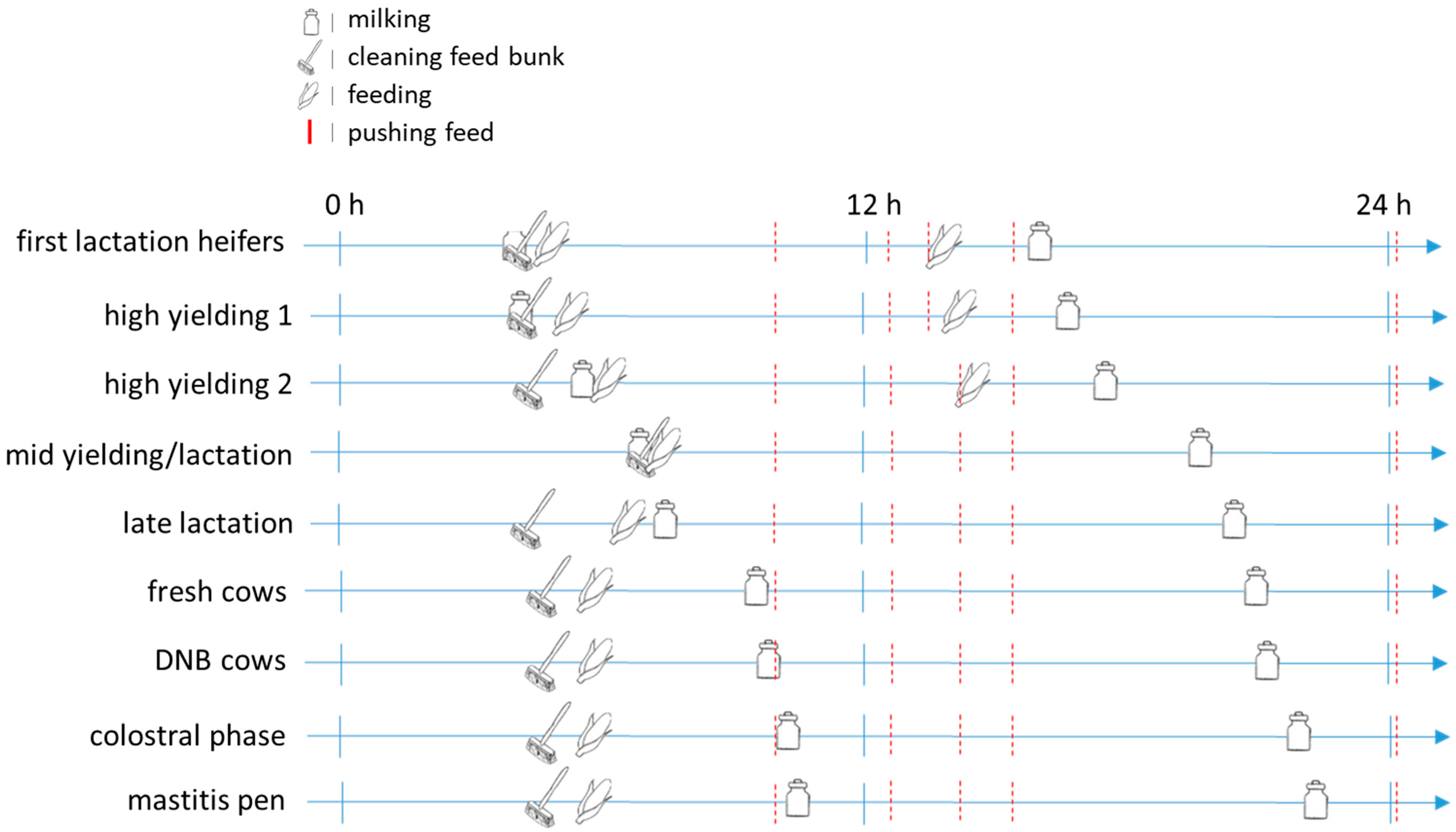
2.2.3. Husbandry and Management Routines
2.2.4. Feeding
2.2.5. Disease Incidence
2.2.6. Metabolic Profile
- The level of postpartum lipomobilisation was too high. There were high BHB (1.15 mmol/L in fresh cow group) and FFS (635 and 1000 µmol/L in colostral and fresh cow group) concentrations, resulting in increased metabolic strain on the liver and, consequently, liver damage, reflected by increases in AST (171 and 93 U/L in the colostral-phase and fresh cow groups) and bilirubin (7.0 µmol/L in the colostral phase).
- Postpartum subclinical hypocalcaemia was observed in the colostral group (2.17 mmol/L).
- The antepartum period shown increased amounts of creatine kinase (slightly, possibly due to overcrowding and lameness).
- The postpartum period exhibited an increased amount of potassium (possibly due to the high potassium concentrations in the rations).
- During the antepartum period, there was an increase in calcium, chloride, and potassium, reflecting the high mineral load in the rations fed, as well as antepartum calcium excretion
- The antepartum period also saw high levels of creatinine, possibly indicating an insufficient water supply.
- The close-up group exhibited a relatively high net acid–base excretion and acid–base quotient, reflecting the relatively high DCAD diets fed to this group. The lowest base-acid–ratio (BAR) was observed in the dry cow group.
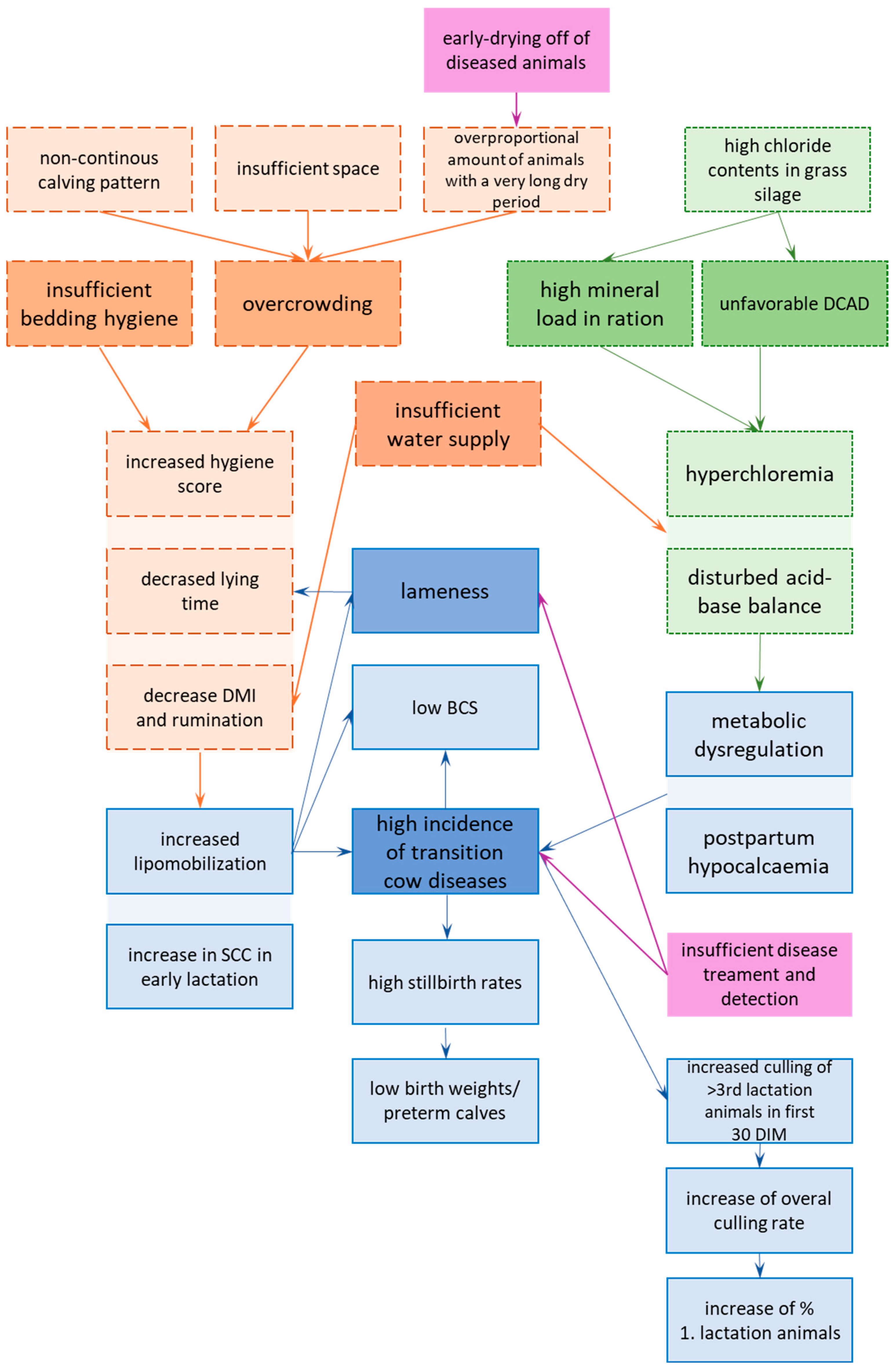
2.3. List of Initial Problems
- Housing: Overcrowding and insufficient bedding hygiene in the transition cow facility [64], due to the following:
- -
- Insufficient facility design;
- -
- Non-continuous calving patterns;
- -
- A high number of preterm dried-off cows;
- -
- Insufficient implementation of the cleaning routines.
- -
- Low DCAD in grass silage.
- -
- Ration composition.
- -
- Insufficient water supply [65], due to the following:
- ○
- A lack of sufficient water supply (volume).
- ○
- The high mineral loads in the rations.
- Animal health: High lameness prevalence [66].
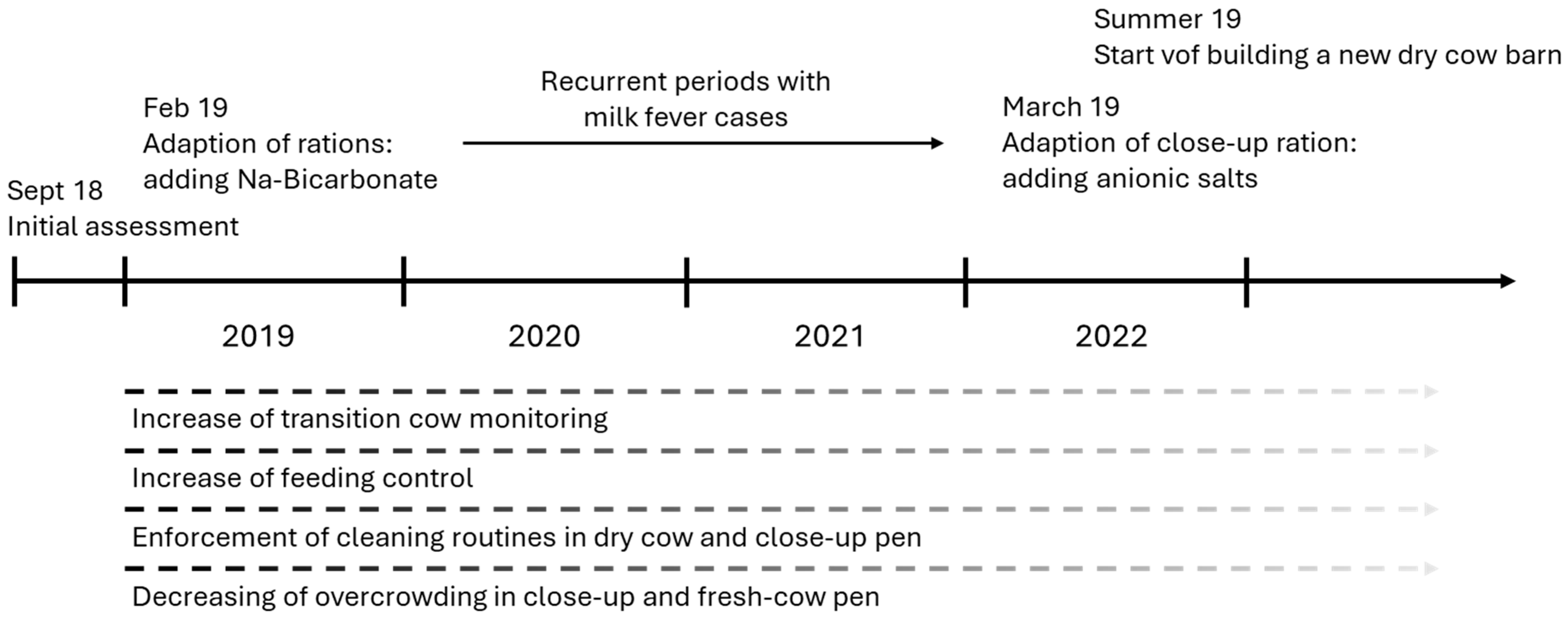
2.4. Problem Solution and Follow-Up
2.4.1. Feeding
2.4.2. Disease Monitoring and Animal Health Management Protocols
2.4.3. Housing and Management
3. Discussion
4. Conclusions
5. Practical Suggestions for Practice
- Implementation of structured fresh cow checks to ensure early detection and treatment.
- Thorough evaluation of DCAD and calcium levels in dry cow diets, with consistent ration analysis to prevent hypocalcaemia.
- Monitoring of dry matter intake (DMI) and adjusting feeding strategies to encourage adequate intake.
- Adequate clinical examination and blood analysis in downer cows to verify the cause of disease.
- Metabolic profiling to monitor subclinical imbalances, particularly around calving, especially in herds with an increased risk of experiencing transition cow problems.
- Avoidance of overcrowding in close-up and fresh cow pens by managing calving distribution and dry-off timing.
- Improvements in water access and cubicle comfort, especially in high-risk pens.
- Engagement of all stakeholders—including veterinarians, nutritionists, and farm staff—in regular reviews of herd health data and housing conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pen/Group | Number of Animals | Number of Cubicles | Animals/Cubicles | Paved Area/Animal (m2) 1 | Feed Bunk Space/Animal (cm) | Number of Troughs | Animals/ Trough | Trough Space/Animal (cm) |
|---|---|---|---|---|---|---|---|---|
| barn1: dry cows | 50 | * | * | 5.7 | 42 | 3 | 17 | 7 |
| barn1: springing heifers | 27 | * | * | 6.3 | 46 | 3 | 9 | 6 |
| barn1: close-up cows | 3 | * | * | 51.1 | 373 | 3 | 1 | 119 |
| barn1: calving cows | 5 | * | * | 16.7 | 122 | 2 | 3 | 27 |
| barn1: calving heifers | 4 | * | * | 23.3 | 170 | 1 | 4 | 8 |
| barn2: DNB cows | 8 | 12 | 0.7 | 5.4 | 185 | 2 | 4 | 5 |
| barn2: mastitis pen | 8 | 12 | 0.7 | 4.4 | 189 | 2 | 4 | 8 |
| barn2: colostral phase | 12 | 19 | 0.6 | 4.9 | 187 | 4 | 3 | 11 |
| barn2: fresh cows | 22 | 38 | 0.6 | 4.8 | 214 | 4 | 6 | 6 |
| barn3: first lactation heifers | 80 | 83 | 1.0 | 6.1 | 53 | 5 | 16 | 8 |
| barn3: high yielding 1 | 99 | 100 | 1.0 | 6.5 | 67 | 4 | 25 | 8 |
| barn4: high yielding 2 | 75 | 77 | 1.0 | 6.2 | 53 | 5 | 15 | 9 |
| barn4: mid yielding/lactation | 81 | 84 | 1.0 | 4.5 | 45 | 4 | 20 | 7 |
| barn4: late lactation | 50 | 70 | 0.7 | 5.9 | 60 | 4 | 13 | 8 |
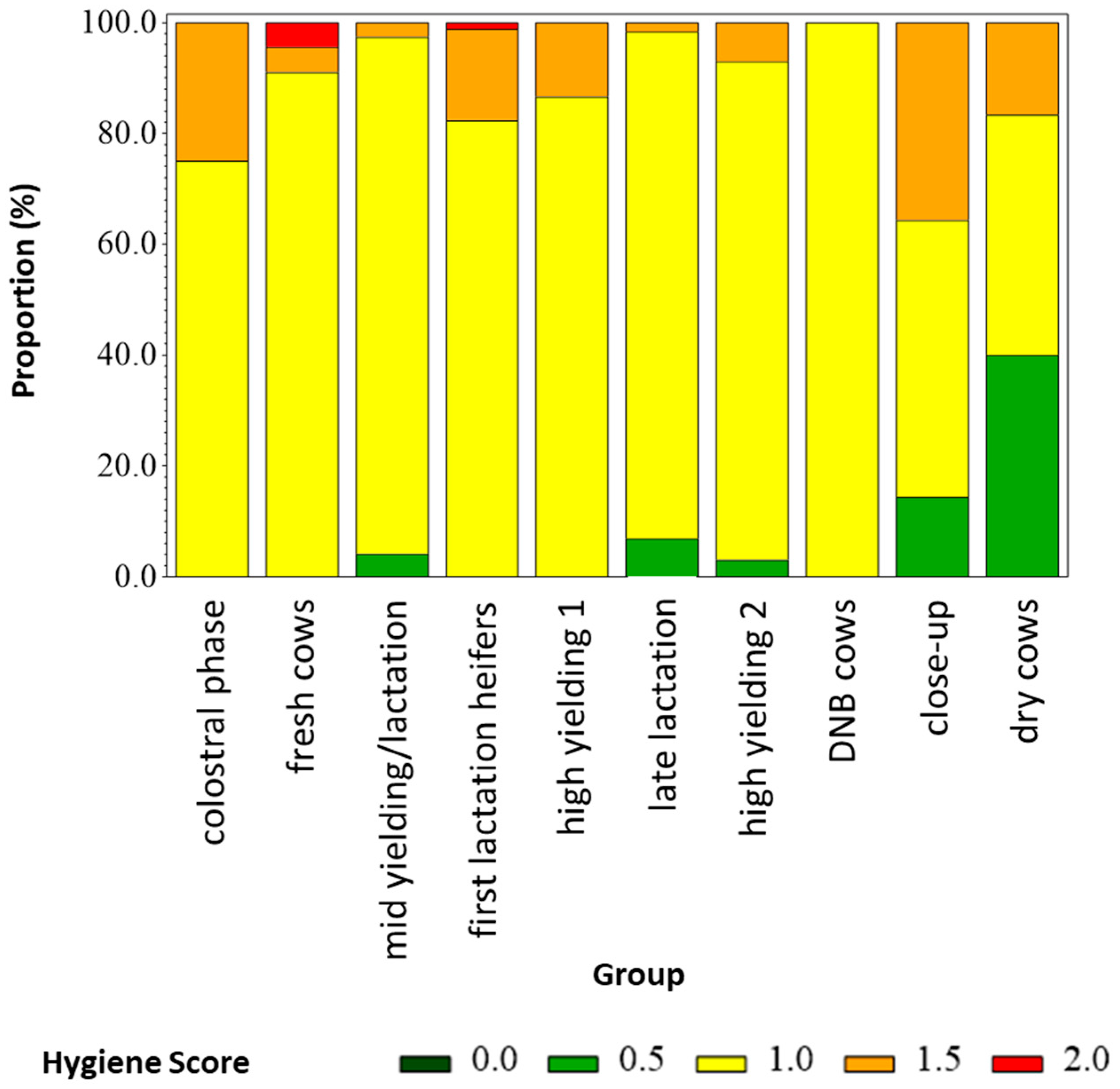
| Group | N Scored | Dry and Clean | Moderatly Dirty and/or Wet | Highly Dirty and/or Wet | Plentiful and Even | Moderatly Covered and/or Uneven | Ground Visible/Highly Uneven |
|---|---|---|---|---|---|---|---|
| barn2: DNB cows | 12 | 0 | 33 | 67 | 42 | 42 | 17 |
| barn2: mastitis pen and colostral phase * | 29 | 0 | 31 | 69 | 21 | 52 | 28 |
| barn2: fresh cows | 38 | 87 | 13 | 0 | 87 | 13 | 0 |
| barn3: first lactation heifers | 80 | 3 | 63 | 35 | 9 | 45 | 46 |
| barn3: high yielding 1 | 69 | 10 | 58 | 32 | 23 | 46 | 30 |
| barn4: high yielding 2 | 97 | 0 | 71 | 29 | 0 | 73 | 27 |
| barn4: mid yielding/lactation | 79 | 0 | 59 | 41 | 0 | 68 | 32 |
| barn4: late lactation | 70 | 0 | 64 | 36 | 0 | 73 | 27 |
| Summary | 474 | 9 | 57 | 34 | 14 | 57 | 29 |
| Group | CCQ >85% | PEL >75% | CCI >50% |
|---|---|---|---|
| barn1: dry cows | - | 33 | 31 |
| barn1: close-up cows | - | 21 | 18 |
| barn1: springing heifers | - | 25 | 37 |
| barn1: calving heifers | - | 33 | 33 |
| barn2: DNB cows | 75 | 38 | 25 |
| barn2: mastitis pen and colostral phase * | 56 | 23 | 56 |
| barn2: fresh cows | 50 | 18 | 63 |
| barn3: first lactation heifers | 78 | 49 | 42 |
| barn3: high yielding 1 | 92 | 60 | 47 |
| barn4: high yielding 2 | 88 | 72 | 41 |
| barn4: mid yielding/lactation | 95 | 75 | 48 |
| barn4: late lactation | 94 | 57 | 46 |


| Total | 1st Lactation | 2nd Lactation | 3rd Lactation | |||||
|---|---|---|---|---|---|---|---|---|
| Lameness-Grade | N | BCS ↓ (%) | N | BCS ↓ (%) | N | BCS ↓ (%) | N | BCS ↓ (%) |
| 1 (not lame) | 220 | 47.9 | 104 | 54.5 | 61 | 46.4 | 55 | 37.0 |
| 2 (uneven gait) | 144 | 46.2 | 42 | 43.9 | 36 | 50.0 | 66 | 45.5 |
| 3+ (mild to severe) | 80 | 56.4 | 16 | 75.0 | 15 | 57.1 | 49 | 50.0 |
| Ingredient (in kg Fresh Matter) | Fresh Cows | First Lactation Heifers | High Yielding | Mid-Yielding/Lactation | Late Lactation | Dry Cows | Close-Up |
|---|---|---|---|---|---|---|---|
| Corn silage | 17.50 | 21.00 | 22.00 | 19.50 | 16.50 | 12.00 | 14.00 |
| Grass silage no. 1 | 12.00 | 14.00 | 15.00 | 7.50 | - | - | 6.00 |
| Grass silage no. 2 | - | - | - | 7.50 | 15.00 | 15.00 | - |
| Carrot pomache | 4.00 | 4.00 | 6.00 | - | - | - | - |
| Peas | - | - | - | - | - | - | 0.50 |
| Corn | 1.50 | 1.70 | |||||
| Barley | - | - | - | 4.80 | 4.60 | - | 1.30 |
| Rapeseed extraction meal | - | - | - | - | - | - | 0.50 |
| Concentrate and mineral mix no. 1 * | 6.50 | 7.70 | - | - | - | - | - |
| Concentrate and mineral mix no. 2 * | - | - | 11.20 | - | - | - | - |
| Concentrate and mineral mix no. 3 * | - | - | - | 3.00 | 3.00 | - | - |
| Barley straw | 0.30 | 0.30 | 0.30 | - | - | - | 1.30 |
| Molasses | 0.70 | 0.80 | 1.00 | 0.50 | - | - | - |
| Propyleneglycol | 0.25 | - | - | - | - | - | - |
| Glycerin | - | - | - | - | - | - | 0.30 |
| Feed lime | - | - | - | - | - | 0.10 | 0.15 |
| Mineral feed dry cows * | - | - | - | - | - | 0.13 | 0.13 |
| Concentrate no. 1 * | - | - | - | - | - | - | 0.40 |
| Salt | - | - | - | - | - | - | 0.03 |
| Sodiumbicarbonate | - | - | - | - | - | 0.10 | - |
| Sum kg fresh matter | 42.75 | 49.50 | 55.50 | 42.80 | 39.10 | 27.33 | 24.61 |
| Ingredient (DM Basis) | Unit | Fresh Cows | First Lactation Heifers | High Yielding | Mid Yielding/Lactation | Late Lactation | Dry Cows | Close-Up |
|---|---|---|---|---|---|---|---|---|
| Dry matter | G | 19,365 | 22,418 | 25,172 | 20,214 | 19,184 | 11,185 | 11,231 |
| Dry matter from roughages | g | 10,829 | 12,802 | 13,517 | 12,969 | 12,498 | 10,869 | 8304 |
| % roughages | % | 56 | 57 | 54 | 64 | 65 | - | - |
| % dry matter | % | 45.3 | 45.3 | 45.4 | 47.2 | 49.1 | 40.9 | 45.6 |
| Crude fibre | g | 2492 | 2934 | 3096 | 3123 | 3224 | 2868 | 2095 |
| NEL/kg DM | MJ | 7.18 | 7.14 | 7.21 | 6.67 | 6.44 | 5.55 | 6.45 |
| Predicted milk from NEL | L | 31.5 | 37.9 | 46.6 | 30.2 | 24.2 | - | - |
| Predicted milk from protein | L | 30.8 | 37.2 | 43.4 | 29.7 | 25.9 | - | - |
| Predicted milk from nXP | L | 31.9 | 38.4 | 42.3 | 29 | 23.8 | - | - |
| Crude protein (CP) in DM | % | 16.5 | 16.7 | 16.5 | 15.2 | 14.7 | 12.7 | 13.3 |
| nXP in DM | % | 15.7 | 15.9 | 15.9 | 14.5 | 14.1 | 12.3 | 13.7 |
| % UDP per kg CP | % | 27 | 27 | 26.2 | 18.6 | 18.4 | - | - |
| Ruminal Nitrogen Balance (RNB) | g | 24 | 30 | 27 | 21 | 20 | 8 | -6 |
| % sugar per kg DM | % | 6.2 | 6.3 | 6.4 | 5.4 | 4.5 | 5 | 3.9 |
| % starch per kg DM | % | 16.1 | 16.3 | 17.6 | 20 | 19.8 | 5.5 | 14.7 |
| % starch and sugar per kg DM | % | 22.4 | 22.6 | 24 | 25.5 | 24.3 | 10.5 | 18.6 |
| % crude fibre in DM | % | 16.2 | 16.4 | 15.9 | 17.9 | 19.4 | - | - |
| % fat in DM | % | 4.4 | 4.5 | 4.4 | 2.7 | 2.6 | 2.6 | 2.8 |
| Calcium | g | 134.6 | 159 | 175 | 123.2 | 128.5 | 99 | 100.9 |
| Phosphorous | g | 75.7 | 89.1 | 94.2 | 61.7 | 55.9 | 27 | 36.8 |
| Sodium | g | 69.1 | 81.4 | 76.3 | 49 | 56.1 | 71.7 | 32.8 |
| Magnesium | g | 47.1 | 55.5 | 59.4 | 47.8 | 47.2 | 33.2 | 31.3 |
| Potassium per kg DM | g | 13.3 | 13.4 | 13.2 | 11.7 | 10 | 12.2 | 11.8 |
| Chloride per kg DM | g | 7.2 | 7.3 | 6.9 | 9 | 9.7 | 14.1 | 8.6 |
| Sulphur per kg DM | g | 3 | 3 | 3 | 2.4 | 2.4 | 2 | 2.4 |
| DCAD per kg DM | meq | 108 | 108 | 89 | 1 | -41 | 66 | 37 |
| Calcium:Phosphorous | 1.78 | 1.78 | 1.86 | 2 | 2.3 | 3.67 | 2.74 | |
| Potassium:Sodium | 3.72 | 3.69 | 4.36 | 4.82 | 3.41 | 1.91 | 4.04 | |
| Vitamine A | I.E. | 128,700 | 152,460 | 151,200 | 148,500 | 148,500 | 100,000 | 100,000 |
| Vitamine D | I.E. | 18,590 | 22,022 | 21,840 | 21,450 | 21,450 | 25,000 | 25,000 |
| Vitamine E | mg | 501 | 593 | 588 | 578 | 578 | 625 | 625 |
| Copper per kg DM | mg | 16 | 16 | 15 | 18 | 18 | 18 | 17 |
| Magnesium per kg DM | mg | 68 | 70 | 65 | 78 | 85 | 93 | 87 |
| Iodine per kg DM | mg | 0.84 | 0.86 | 0.77 | 0.92 | 0.98 | 0.99 | 0.98 |
| Selenium per kg DM | mg | 0.45 | 0.46 | 0.42 | 0.49 | 0.52 | 0.49 | 0.51 |
| Zinc per kg DM | mg | - | - | - | - | - | 115 | 111 |
| Cobalt per kg DM | mg | 0.29 | 0.3 | 0.28 | 0.33 | 0.36 | 0.32 | 0.29 |
- Metabolic Profile
| Group | No. Animals | Lameness (No. of Animals Per Grade) | No. Scored for Lameness * | Hock Lesions (Mean) | BCS | Rumen | Rectal T° (Mean) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | Min | Max | Filling (Mean) | Layering (Mean) | |||||
| dry cows (dry) | 10 | 0 | 5 | 4 | 1 | 0 | 0 | 10 | 2.4 | 3.38 | 2.25 | 5.00 | 2.5 | 2.6 | 38.5 |
| close-up (cl-up) | 10 | 3 | 3 | 3 | 1 | 0 | 0 | 10 | 2.9 | 3.33 | 2.25 | 4.75 | 2.4 | 2.2 | 38.8 |
| colostral phase (col) | 7 | 1 | 2 | 1 | 2 | 0 | 0 | 6 | 3.0 | 3.20 | 2.00 | 4.00 | 1.6 | 1.4 | 38.8 |
| fresh cows (fresh) | 10 | 2 | 8 | 0 | 0 | 0 | 0 | 10 | 2.9 | 2.70 | 2.00 | 3.50 | 1.7 | 2.0 | 38.9 |
| high yielding (high) | 10 | 2 | 6 | 2 | 0 | 0 | 0 | 10 | 2.7 | 2.67 | 2.25 | 3.50 | 2.4 | 2.3 | 38.6 |
| Group | No. AnimaLs | AST (U/L) | BHB (mmoL/L) | BiLi (µmoL/L) | Ca (mmoL/L) | CK (U/L) | CL (mmoL/L) | Crea (µmoL/L) | Fe (µmoL/L) | FFS (µmoL/L) | GGT (U/L) |
| <80 | <0.6–0.7 | <5.3 | 2.3–2.8 | <200 | 95–110 | 55–155 | 13–33 | * | <50 | ||
| dry | 10 | 62.3 | 0.64 | 1.2 | 2.42 | 215 | 101.3 | 69 | 26.0 | 75 | 22.4 |
| cL-up | 10 | 70.7 | 0.67 | 0.9 | 2.40 | 254 | 103.2 | 80 | 31.7 | 59 | 26.4 |
| coL | 7 | 171.1 | 0.89 | 7.0 | 2.17 | 543 | 99.1 | 74 | 18.1 | 635 | 24.1 |
| fresh | 10 | 93.2 | 1.15 | 3.4 | 2.42 | 129 | 97.1 | 70 | 20.5 | 1000 | 25.9 |
| high | 10 | 75.4 | 0.73 | 0.8 | 2.49 | 152 | 96.8 | 56 | 26.1 | 114 | 31.8 |
| Group | No. AnimaLs | K (mmoL/L) | Mg (mmoL/L) | Na (mmoL/L) | Phos (mmoL/L) | TP (g/L) | Urea (mmoL/L) | Se (µg/L) | Cu (µmoL/L) | Vit. A (mg/L) | Vit. E (mg/L) |
| 3.5–4.5 | 0.9–1.32 | 135–157 | 1.6–2.3 | 60–80 | 3.3–5.0 | 31.6–69.5 | 8.0–32.5 | 0.20–0.40 | 3.0–10.0 | ||
| dry | 10 | 4.50 | 0.90 | 135 | 2.03 | 74.7 | 3.01 | 49.2 | 11.6 | 0.23 | 5.7 |
| cL-up | 10 | 4.59 | 0.89 | 142 | 2.05 | 73.1 | 3.81 | 60.9 | 14.3 | 0.26 | 4.4 |
| coL | 7 | 4.73 | 0.85 | 137 | 1.71 | 67.4 | 3.93 | 75.4 | 18.3 | 0.15 | 3.2 |
| fresh | 10 | 4.73 | 1.05 | 135 | 1.73 | 76.0 | 2.13 | 63.9 | 13.1 | 0.2 | 5 |
| high | 10 | 4.66 | 1.02 | 133 | 1.67 | 78.7 | 3.06 | 63.2 | 15.4 | 0.29 | 8.1 |
| Group | No. AnimaLs | Ca (mmoL/L) | CL (mmoL/L) | Crea (mmoL/L) | K (mmoL/L) | Mg (mmoL/L) | Na (mmoL/L) | Phos (mmol/L) |
| <2.5 | 40–160 | 2.2–7 | 150–300 | 3.7–16 | >8.2 | 0.1–3.3 | ||
| dry | 10 | 3.80 | 242.1 | 10.13 | 330.6 | 15.0 | 60 | 0.24 |
| cL-up | 10 | 2.78 | 144.9 | 10.31 | 286.8 | 12.9 | 58 | 0.24 |
| coL | 7 | 1.60 | 59.2 | 7.73 | 196.2 | 7.0 | 55 | 1.40 |
| fresh | 10 | 0.06 | 59.2 | 4.49 | 179.6 | 4.6 | 103 | n.n. |
| high | 10 | 0.76 | 111.3 | 6.01 | 266.0 | 15.8 | 116 | 1.22 |
| Group | No. AnimaLs | pH | Bases (mmoL/L) | Acids (mmoL/L) | NH4+(mmoL/L) | Fract. NABE (mmoL/L) | BAQ | |
| 7.0–8.4 | 150–250 | 50–100 | <10 | 80–220 | 1.5–4.5 | |||
| dry | 10 | 8.11 | 143 | 75 | 5.8 | 62.2 | 1.8 | |
| cL-up | 10 | 8.40 | 187 | 80 | 5.6 | 101.4 | 2.2 | |
| coL | 7 | 8.46 | 191 | 59 | 6.3 | 125.7 | 2.9 | |
| fresh | 10 | 8.50 | 272 | 75 | 6.8 | 190.2 | 3.3 | |
| high | 10 | 8.53 | 235 | 46 | 4.3 | 184.7 | 4.7 | |
| Group * | No. Animals | Lameness (No. of Animals Per Grade) | No. Scored for Lameness | Hock Lesions (Mean) | BCS | Rumen | Rectal T (Mean) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | Min | Max | Filling (Mean) | Layering (Mean) | |||||
| late (1) | 10 | 4 | 4 | 1 | 1 | 0 | 0 | 10 | 2.7 | 3.06 | 2.25 | 4.50 | 2.6 | 2.8 | 38.4 |
| late (2) | 10 | 1 | 5 | 4 | 0 | 0 | 0 | 10 | 3.1 | 3.75 | 3.25 | 4.50 | 2.7 | 2.7 | 39.3 |
| dry (1) | 10 | 2 | 8 | 2 | 1 | 0 | 0 | 10 | 2.6 | 3.53 | 2.00 | 5.00 | 2.9 | 2.8 | 38.5 |
| dry (2) | 10 | 2 | 7 | 1 | 0 | 0 | 0 | 10 | 2.6 | 3.50 | 2.50 | 4.50 | 2.4 | 2.6 | 39.1 |
| Group * | No. Animals | AST (U/L) | BHB (mmoL/L) | BiLi (µmoL/L) | Ca (mmoL/L) | CK (U/L) | CL (mmoL/L) | Crea (µmoL/L) | Fe (µmoL/L) | FFS (µmoL/L) | GGT (U/L) |
| <80 | <0.6–0.7 | <5.3 | 2.3–2.8 | <200 | 95–110 | 55–155 | 13–33 | * | <50 | ||
| late (1) | 10 | 122.7 | 0.89 | 0.2 | 2.46 | 320 | 95.5 | 71 | 35.6 | 130 | 24.3 |
| late (2) | 10 | 74.3 | 1.19 | 0.4 | 2.45 | 118 | 94.8 | 84 | 31.7 | 90 | 29.0 |
| dry (1) | 10 | 83.5 | 0.91 | 0.3 | 2.30 | 420 | 95.8 | 74 | 29.7 | 106 | 18.4 |
| dry (2) | 10 | 71.3 | 0.62 | 1.1 | 2.37 | 223 | 96.7 | 95 | 29.1 | 186 | 25.2 |
| Group * | No. Animals | K (mmoL/L) | Mg (mmoL/L) | Na (mmoL/L) | Phos (mmoL/L) | TP (g/L) | Urea (mmoL/L) | Se (µg/L) | Cu (µmoL/L) | Vit. A (mg/L) | Vit. E (mg/L) |
| 3.5–4.5 | 0.9–1.32 | 135–157 | 1.6–2.3 | 60–80 | 3.3–5.0 | 31.6–69.5 | 8.0 –32.5 | 0.20–0.40 | 3.0–10.0 | ||
| late (1) | 10 | 4.21 | 0.94 | 136 | 1.68 | 73.8 | 3.65 | 64.4 | 10.4 | 0.27 | 6.8 |
| late (2) | 10 | 4.04 | 0.95 | 139 | 1.81 | 77.3 | 4.19 | 59.8 | 11.2 | 0.25 | 5.3 |
| dry (1) | 10 | 4.30 | 0.85 | 136 | 1.85 | 72.4 | 2.88 | 51 | 8.5 | 0.22 | 5.9 |
| dry (2) | 10 | 4.52 | 0.98 | 135 | 1.85 | 75.4 | 3.44 | 54.1 | 9.3 | 0.22 | 5.2 |
| Group * | No. Animals | Ca (mmoL/L) | CL (mmoL/L) | Crea (mmoL/L) | K (mmoL/L) | Mg (mmoL/L) | Na (mmoL/L) | Phos (mmol/L) |
| <2.5 | 40–160 | 2.2–7 | 150–300 | 3.7–16 | > 8.2 | 0.1-3.3 | ||
| late (1) | 7 | 26.0 | 209 | 5.9 | 162 | 11.0 | 78 | 0.8 |
| late (2) | 10 | 37.2 | 99 | 10.0 | 128 | 0.60 | 107 | 2.4 |
| dry (1) | 10 | 2.8 | 205 | 7.7 | 132 | 11.5 | 112 | 0.1 |
| dry (2) | 10 | 6.8 | 142 | 10.2 | 137 | 18.5 | 57 | 2.4 |
| Group * | No. Animals | pH | bases (mmoL/L) | acids (mmoL/L) | NH4+ (mmoL/L) | fract. NABE (mmoL/L) | BAR | |
| 7.0–8.4 | 150–250 | 50–100 | <10 | 80–220 | 1.5–4.5 | |||
| late (1) | 7 | 8.52 | 142 | 60 | 6.7 | 75.3 | 2.1 | |
| late (2) | 10 | 8.58 | 234 | 100 | 10.6 | 123.4 | 2.1 | |
| dry (1) | 10 | 8.47 | 132 | 65 | 4.4 | 62.6 | 1.9 | |
| dry (2) | 10 | 8.70 | 194 | 70 | 8.0 | 116.0 | 2.5 | |
References
- Goff, J.P. Transition cow immune function and interaction with metabolic diseases. In Proceedings of the Tri-state Dairy Nutrition Conference, Dubuque, IA, USA, 22–23 April 2008; pp. 45–57. [Google Scholar]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.P.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367. [Google Scholar] [CrossRef]
- Smith, B.I.; Risco, C.A. Management of periparturient disorders in dairy cattle. Vet. Clin. Food Anim. Pract. 2005, 21, 503–521. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Melendez, P. The Transition Dairy Cow; Cambridge Scholars Publishing: Newcastle upon Tyne, England, 2025. [Google Scholar]
- Hendriks, R.; Brand, A. Pathways to Health and Disease for Dairy Cows; Brill/Wageningen Academics: Wageningen, The Netherlands, 2023. [Google Scholar]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.L.; Opsomer, G. Maladaptation to the transition period and consequences on fertility of dairy cows. Rep. Dom. Anim. 2022, 57, 21–32. [Google Scholar] [CrossRef]
- Mulligan, F.; O’Grady, L.; Rice, D.; Doherty, M. Production diseases of the transition cow: Milk fever and subclinical hypocalcaemia. Iran. Vet. J. 2006, 59, 697. [Google Scholar]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Melendez, P.; Risco, C.A. Management of transition cows to optimize reproductive efficiency in dairy herds. Vet. Clin. Food Anim. Pract. 2005, 21, 485–501. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; LeBlanc, S.J. Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows. Animals 2025, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Puvača, N.; Glamočić, D.; Pugliese, G.; Colonna, M.A. The most important metabolic diseases in dairy cattle during the transition period. Animals 2024, 14, 816. [Google Scholar] [CrossRef]
- Kang, D.; Lungu, S.E.; Danso, F.; Dzou, C.F.; Chen, Y.; Zheng, X.; Nie, F.; Lin, H.; Chen, J.; Zhou, G. Animal health and nutrition: Metabolic disorders in cattle and improvement strategies. Front. Vet. Sci. 2025, 12, 1470391. [Google Scholar] [CrossRef]
- Deniz, A.; Aksoy, K.; Metin, M. Transition period and subclinical ketosis in dairy cattle: Association with milk production, metabolic and reproductive disorders and economic aspects. Med. Weter. 2020, 76, 495–502. [Google Scholar] [CrossRef]
- Eicker, S.; Stewart, S.; Fetrow, J.; Rapnicki, P. Monitoring transition cow programs. In Proceedings of the Mid-South Ruminant Nutrition Conference, Quebec City, QC, Canada, 24–25 April 2002; pp. 21–29. [Google Scholar]
- Liang, D.; Arnold, L.; Stowe, C.; Harmon, R.; Bewley, J. Estimating US dairy clinical disease costs with a stochastic simulation model. J. Dairy Sci. 2017, 100, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Tucho, T.T.; Ahmed, W.M. Economic and reproductive impacts of retained placenta in dairy cows. J. Reprod. Infertil. 2017, 8, 18–27. [Google Scholar]
- Peeler, E.J.; Otte, M.J.; Esslemont, R.J. Recurrence odds ratios for periparturient diseases and reproductive traits of dairy cows. Br. Vet. J. 1994, 150, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.; Green, M.; Green, L.E.; Chagunda, M.; Mason, C.; Archer, S.; Huxley, J. The contribution of previous lameness events and body condition score to the occurrence of lameness in dairy herds: A study of 2 herds. J. Dairy Sci. 2018, 101, 1311–1324. [Google Scholar] [CrossRef]
- Mulligan, F.J.; O’grady, L.; Rice, D.A.; Doherty, M.L. A herd health approach to dairy cow nutrition and production diseases of the transition cow. Anim. Reprod. Sci. 2006, 96, 331–353. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding-and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Tech. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Redfern, E.A.; Sinclair, L.A.; Robinson, P.A. Dairy cow health and management in the transition period: The need to understand the human dimension. Res. Vet. Sci. 2021, 137, 94–101. [Google Scholar] [CrossRef]
- Van Saun, R.J.; Sniffen, C.J. Transition cow nutrition and feeding management for disease prevention. Vet. Clin. Food Anim. Pr. 2014, 30, 689–719. [Google Scholar] [CrossRef]
- Cook, J.; Pepler, P.; Viora, L.; Hill, D. Assessing transition cow management in dairy cows for improved health, milk production, pregnancy, and culling outcomes. J. Dairy Sci. 2024, 107, 11381–11397. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, L.S.; Omontese, B.O. Monitoring and Improving the Metabolic Health of Dairy Cows during the Transition Period. Animals 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.; Kvidera, S.; Baumgard, L. Invited review: The influence of immune activation on transition cow health and performance—A critical evaluation of traditional dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Roche, J.R.; Bell, A.W.; Overton, T.R.; Loor, J.J. Nutritional management of the transition cow in the 21st century—A paradigm shift in thinking. Anim. Prod. Sci. 2013, 53, 1000–1023. [Google Scholar] [CrossRef]
- Ametaj, B.N. A systems veterinary approach in understanding transition cow diseases: Metabolomics. In Proceedings of the 4th International Symposium on Dairy Cow Nutrition and Milk Quality, Beijing, China, 8–10 May 2015; pp. 78–84. [Google Scholar]
- Block, E. Transition cow research—What makes sense today. In Proceedings of the High Plains Dairy Conference, Amarillo, TX, USA, 5–7 March 2010; pp. 75–98. [Google Scholar]
- Ametaj, B.N. Periparturient Diseases of Dairy Cows—A Systems Biology Approach; Springer: Cham, Switzerland, 2017. [Google Scholar]
- de Oliveira, F.M.; Ferraz, G.A.S.; André, A.L.G.; Santana, L.S.; Norton, T.; Ferraz, P.F.P. Digital and precision technologies in dairy cattle farming: A bibliometric analysis. Animals 2024, 14, 1832. [Google Scholar] [CrossRef]
- Rutten, C.; Velthuis, A.; Steeneveld, W.; Hogeveen, H. Invited review: Sensors to support health management on dairy farms. J. Dairy Sci. 2013, 96, 1928–1952. [Google Scholar] [CrossRef]
- Mottram, T.T.F.; den Uijl, I. Health and welfare monitoring of dairy cows. Digit. Agritechnol. 2022, 113–142. [Google Scholar]
- Neethirajan, S. Artificial intelligence and sensor technologies in dairy livestock export: Charting a digital transformation. Sensors 2023, 23, 7045. [Google Scholar] [CrossRef]
- Nordlund, K.V.; Cook, N.B. Using herd records to monitor transition cow survival, productivity, and health. Vet. Clin. Food Anim. Pract. 2004, 20, 627–649. [Google Scholar] [CrossRef]
- Redfern, E.A.; Sinclair, L.A.; Robinson, P.A. Why isn’t the transition period getting the attention it deserves? Farm advisors’ opinions and experiences of managing dairy cow health in the transition period. Prev. Vet. Med. 2021, 194, 105424. [Google Scholar] [CrossRef] [PubMed]
- Schären-Bannert, M.; Bittner-Schwerda, L.; Rachidi, F.; Starke, A. Case report: Complications after using the “blind-stitch” method in a dairy cow with a left displaced abomasum: Treatment, outcome, and economic evaluation. Front. Vet. Sci. 2024, 11, 1470190. [Google Scholar] [CrossRef]
- Schären, M.; Waurich, B.; Ebert, F.; Wöckel, A.; Wippermann, W.; Özcan, A.; Hermenau, G.; Wittich, J.; Dänicke, S.; Swalve, H.; et al. Risk factor analysis for dairy cow production diseases by a system analysis—First results from the EIP-project “Die Entwicklung des KUH-mehr-WERT Navigators”. In Proceedings of the International Congress on Production Diseases in Farm Animals (ICPD), Berne, Switzerland, 27–29 June 2019. [Google Scholar]
- Bewley, J.M.; Robertson, L.M.; Eckelkamp, E.A. A 100-Year Review: Lactating dairy cattle housing management. J. Dairy Sci. 2017, 100, 10418–10431. [Google Scholar] [CrossRef]
- Cook, N.B.; Smith, R.A. Housing to Optimize Comfort, Health and Productivity of Dairy Cattles; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; Volume 35. [Google Scholar]
- Thompson, J.S.; Huxley, J.N.; Hudson, C.D.; Kaler, J.; Gibbons, J.; Green, M.J. Field survey to evaluate space allowances for dairy cows in Great Britain. J. Dairy Sci. 2020, 103, 3745–3759. [Google Scholar] [CrossRef]
- DLG-Merkblatt 399. Wasserversorgung für Rinder. 2014. Available online: https://www.dlg.org/fileadmin/downloads/Merkblaetter/dlg-merkblatt_399.pdf (accessed on 19 August 2025).
- de Kruif, A.; Mansfeld, R.; Hoedemaker, M. Tierärztliche Bestandsbetreuung Beim Milchrind; Georg Thieme Verlag: Stuttgart, Germany, 2006. [Google Scholar]
- Cook, N.B.; Hess, J.P.; Foy, M.R.; Bennett, T.B.; Brotzman, R.L. Management characteristics, lameness, and body injuries of dairy cattle housed in high-performance dairy herds in Wisconsin. J. Dairy Sci. 2016, 99, 5879–5891. [Google Scholar] [CrossRef] [PubMed]
- Faull, W.B.; Hughes, J.W.; Clarkson, M.J.; Downham, D.Y.; Manson, F.J.; Merritt, J.B.; Murray, R.D.; Russell, W.B.; Sutherst, J.E.; Ward, W.R. Epidemiology of lameness in dairy cattle: The influence of cubicles and indoor and outdoor walking surfaces. Vet. Rec. 1996, 139, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, A.; van Winden, S.; Fishwick, J.C.; Bell, N.J. Monitoring cow comfort and rumen health indices in a cubicle-housed herd with an automatic milking system: A repeated measures approach. Iran. Vet. J. 2015, 68, 12. [Google Scholar] [CrossRef]
- Cook, N.; Bennett, T.; Nordlund, K. Monitoring indices of cow comfort in free-stall-housed dairy herds. J. Dairy Sci. 2005, 88, 3876–3885. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy-Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Reneau, J.K.; Seykora, A.J.; Heins, B.J.; Endres, M.I.; Farnsworth, R.J.; Bey, R.F. Association between hygiene scores and somatic cell scores in dairy cattle. J. Am. Vet. Med. Assoc. 2005, 227, 1297–1301. [Google Scholar] [CrossRef]
- Zaaijer, D.; Noordhuizen, J.P.T.M. A novel scoring system for monitoring the relationship between nutritional efficiency and fertility in dairy cows. Iran. Vet. J. 2003, 56, 145–152. [Google Scholar]
- PraeRi. Animal Health, Hygiene and Biosecurity in German Dairy Cow Operations—A Prevalence Study (PraeRi). Final Report. 2020. Available online: https://ibei.tiho-hannover.de/praeri/pages/1 (accessed on 19 August 2025).
- Garvey, M. Lameness in Dairy Cow Herds: Disease Aetiology, Prevention and Management. Dairy 2022, 3, 199–210. [Google Scholar] [CrossRef]
- Rachidi, F.; Černá, A.; Zenker, M.; Ullrich, E.; Starke, A. Untersuchung und Bewertung der Haupteinflussfaktoren auf die Entstehung von Infektiösen Klauenerkrankungen des Dermatitis—Digitalis—Komplexes; Landesamt für Umwelt, Landwirtschaft und Geologie: Dresden, Germany, 2021. [Google Scholar]
- Sanftleben, P.; Knierim, U.; Herrmann, H.-J.; Mueller, C.; Von Borell, E. Critical Control Points (CCP) in dairy housing and management. Zuchtungskunde 2007, 79, 339–362. [Google Scholar]
- Hu, W.; Murphy, M.R. Dietary cation-anion difference effects on performance and acid-base status of lactating dairy cows: A meta-analysis. J. Dairy Sci. 2004, 87, 2222–2229. [Google Scholar] [CrossRef]
- Charbonneau, E.; Pellerin, D.; Oetzel, G.R. Impact of lowering dietary cation-anion difference in nonlactating dairy cows: A meta-analysis. J. Dairy Sci. 2006, 89, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Beede, D.K. Formulating diets with optimum cation-anion difference for lactating dairy cows. In Proceedings of the Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 1–2 February 2005. [Google Scholar]
- de Kruif, A.; Mansfeld, R.; Hoedemaker, M. Tierärztliche Bestandsbetreuung Beim Milchrind, 3th überarbeitete Auflage ed.; Georg Thieme Verlag: Stuttgart, Germany, 2014. [Google Scholar]
- Van Saun, R. Indicators of dairy cow transition risks: Metabolic profiling revisited. Tierärztliche Praxis. Ausg. G Grosstiere/Nutztiere 2016, 44, 118–126. [Google Scholar]
- Schmitt, R.; Staufenbiel, R. 25 Jahre Stoffwechseluntersuchungen in Milchkuhherden—Aussgekräftige und obsolete Laborparameter. In Proceedings of the 11. Leipziger Tierärztekongress, Leipzig, Germany, 7–9 July 2022; pp. 196–200. [Google Scholar]
- Rossow, N.; Jacobi, U.; Schäfer, M.; Lippmann, R.; Furcht, G. Stoffwechselüberwachung bei Haustieren—Probleme, Hinweise und Referenzwerte; Institut für angewandte Tierhygiene Eberswalde: Eberswalde, Germany, 1987. [Google Scholar]
- Kraft, W.; Dürr, U.M. Klinische Labordiagnostik in der Tiermedizin; Schattauer Verlag: Stuttgart, Germany, 2013. [Google Scholar]
- Allen, M.S.; Piantoni, P. Metabolic control of feed intake: Implications for metabolic disease of fresh cows. Vet. Clin. Food Anim. Pract. 2013, 29, 279–297. [Google Scholar] [CrossRef]
- Jensen, M.B.; Vestergaard, M. Invited review: Freedom from thirst—Do dairy cows and calves have sufficient access to drinking water? J. Dairy Sci. 2021, 104, 11368–11385. [Google Scholar] [CrossRef]
- Calderon, D.F.; Cook, N.B. The effect of lameness on the resting behavior and metabolic status of dairy cattle during the transition period in a freestall-housed dairy herd. J. Dairy Sci. 2011, 94, 2883–2894. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Herman, J.A.; Johnson, G.W.; McArt, J.A.A. Herd-level monitoring and prevention of displaced abomasum in dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Schären-Bannert, M.; Wippermann, W.; Wöckel, A.; Vogel, L.; Waurich, B.; Rachidi, F.; Fröhlich, F.; Felgentreu, C.; Wittich, J.; Bannert, E.; et al. Evaluation of multifactorial digestive disorders in a dairy herd at different stages of lactation. Tierärztliche Praxis. Ausg. G Grosstiere/Nutztiere 2023, 51, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B.; Engelhard, T. Monitoring zur Kationen-Anionen-Bilanz (DCAB) im Erntegut von Grasaufwüchsen an unterschiedlichen Standorten. In Proceedings of the 62. Jahrestagung der Arbeitsgemeinschaft Grünland und Futterbau der Gesellschaft für Pflanzenbauwissenschaften e.V. (AGGF), Kiel, Germany, 30 August–1 September 2018; p. 155. [Google Scholar]
- Greiner, B. Untersuchungen zur DCAB von Grassilagen und deren Einflussfaktoren—Monitoring zur Kationen-Anionen-Bilanz (DCAB) im Erntegut von Grasaufwüchsen in Sachsen-Anhalt. In Proceedings of the 19. Dummerstorfer Seminar Futter und Fütterung, Dummerstorf, Germany, 4 December 2018. [Google Scholar]
- Shire, J.A.; Beede, D.K. DCAD revisited: Prepartum use to optimize health and lactational performance. In Proceedings of the Southwest Dairy Nutrition and Management Conference, Tucson, AZ, USA, 21–22 February 2013; pp. 1–11. [Google Scholar]
- Staufenbiel, R.; Löptien, A.; Montag, N.; Passfeld, M.; Goebbels, M. Aktualisierte Empfehlungen zur Anwendung von Anionenrationen (sauren Salzen) zur Prophylaxe der Hypokalzämie und Gebärparese der Milchkuh. In Proceedings of the Vortragsband: Dr. Pieper Tagungsbericht 2004 8. Symposium Fütterung und Management von Kühen mit Hohen Leistungen, Neuruppin, Germany, 28 October 2004; pp. 121–169. [Google Scholar]
- Gruber, L.; Pries, M.; Schwarz, F.J.; Spiekers, H.; Staudacher, W. Schätzung der Futteraufnahme bei der Milchkuh. DLG-Inf. 2006, 1, 1–29. [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; National Research Council—National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- De Souza, R.A.d.; Tempelman, R.J.; Allen, M.S.; VandeHaar, M.J. Updating predictions of dry matter intake of lactating dairy cows. J. Dairy Sci. 2019, 102, 7948–7960. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Rep. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- DWD. Klimareport Brandenburg, 1. Auflage.; Deutscher Wetterdienst: Offenbach am Main, Deutschland, Germany, 2019. [Google Scholar]
- DWD. Klimatologischer Rückblick Sommer. 2022. Available online: https://www.dwd.de/DE/leistungen/besondereereignisse/temperatur/20220921_bericht_sommer2022.pdf?__blob=publicationFile&v=6 (accessed on 19 August 2025).
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Phys. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Lean, I.; DeGaris, P. Transition Cow Management; Dairy Australia: Melbourne, Australia, 2010. [Google Scholar]
- Mills, K.E.; Weary, D.M.; von Keyserlingk, M.A.G. Identifying barriers to successful dairy cow transition management. J. Dairy Sci. 2020, 103, 1749–1758. [Google Scholar] [CrossRef]
- Noordhuizen, J.P.T.M. Dairy Herd Health and Management; Context: Warwickshire, UK, 2012. [Google Scholar]
- Rossow, N.; Teickner, R.; Wolter, F. Sicherung der Tiergesundheit in der Industriemäßigen Milchproduktion; VEB Gustav Fischer Verlag: Jena, Germany, 1975. [Google Scholar]
- Hutt, G. Total quality and the dairy farm business organisation. In Proceedings of the Western Large Herd Management Conference, Las Vegas, NV, USA, 22–24 April 1993. [Google Scholar]
- Hufe, P.; Felgentreu, C.; Wöckel, A.; Wippermann, W.; Waurich, B.; Schneider, F.; Wittich, J.; May, D.; Dänicke, S.; Swalve, H.; et al. Analysis of daily activities of herd managers and interrelations with the animal production and health situation on dairy farms in eastern Germany. In Proceedings of the 31st World Buiatric Congress 2022, Madrid, Spain, 4–8 September 2022. [Google Scholar]
- Hansen, B.G.; Greve, A. The role of human and social capital in dairy farming. Rural Soc. 2015, 24, 154–176. [Google Scholar] [CrossRef]
- Ji, B.; Banhazi, T.; Perano, K.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. A review of measuring, assessing and mitigating heat stress in dairy cattle. Biosyst. Eng. 2020, 199, 4–26. [Google Scholar] [CrossRef]
- Hampel, G.; Putzing, M.; Schiemann, F.; Wagener, A.; Welker, C. Fachkräftebedarf in der Landwirtschaft im Land Brandenburg bis 2030, Berlin. Ministerium für Arbeit, Soziales, Gesundheit, Frauen und Familie des Landes Brandenburg in Kooperation mit dem Ministerium für Ländliche Entwicklung, Umwelt und Landwirtschaft des Landes Brandenburg. 2018. Available online: https://mluk.brandenburg.de/cms/media.php/lbm1.a.3310.de/Fachkraeftestudie-Landwirtschaft2030.pdf (accessed on 19 August 2025).
- Middleton, G.E.; Overton, M.W. Case report: Use of a transition cow risk assessment tool and economic assessment tool to determine areas of opportunity in a herd with high incidence of transition cow diseases. Bov. Pract. 2018, 52, 173–181. [Google Scholar] [CrossRef]
- Dirksen, G.; Gründer, H.-D.; Stöber, M. Die Klinische Untersuchung des Rindes; Enke: Stuttgart, Germany, 2012. [Google Scholar]

| Herd size and structure | 504 cows, 131 calves, 100 heifers (6–12 m), 187 heifers (12–24 m), 24 heifers (>24 m); cow/young stock ratio: 0.88; mainly German Holstein breed |
| The facility continuously increased its total number of cows between 2010 and 2015 from 430 to 494 cows and stayed constant at ~500 cows thereafter. | |
| 35.4% 1st lactation, 25.5% 2nd lactation, 19.0% 3rd lactation, and 11.3% 4th lactation animals; average lactation: 2.3 | |
| Milk production and content | Milk production per cow and year (test-day data): 10,397 kg, Marketable milk per cow and year: 10,012 kg (of which 66 kg are fed to calves) = 96.3% of milk produced |
| 305 d milk production: 1st lactation: 8920 kg, 2nd lactation: 10,616 kg, ≥3rd lactation: 11,151 kg | |
| Test-data (average (±SD) of last 12 months): milk kg per cow and day (dry cows not included): 32.1 (±1.4) kg, fat%: 3.95 (±0.17)%, protein%: 3.34 (±0.11)%, urea: 200 (±17) mg/mL, SSC: 177 (±26) thousand cells/mL, average days in milk (DIM): 180 (±7) | |
| SCC analysis revealed high SCC at beginning of lactation, especially in older animals: 132, 137 and 349 thousand cells/mL in mean in the first 50 DIM in the 1st, 2nd and 3rd lactation, respectively. In the ≥3rd lactation animals 11% exhibited ≥750 thousand cells/mL in the first 50 DIM. | |
| Culling (last 12 months) | Culling rate: 36.3%, a total of 181 animals of which 87% slaughtered, 7% died, 4% euthanized; 4% mortality rate (% of the rolling herd average that died on-site within one year) |
| Culling reasons: 32% mastitis, 20% lameness, 16% fertility, 11% metabolic disorders, 21% other (low production, milkability, and other reasons) | |
| Percentage culled in the first 30 DIM: heifers: 4.3%, 2nd lactation: 4.9%, 3rd lactation: 14.0% | |
| Production of culled animals: 30,207 kg lifetime production | |
| Slaughter weight and revenue of cows sold for slaughter: 276 kg and 677 €; extrapolated on all animals that were culled (slaughter and mortalities on farm) in that period: 566 € per cow. | |
| Fertility/Calvings (last 12 months) | Days between calvings: 395 d, voluntary waiting period: 78 d, first service conception rate: 35.6%, insemination index (cows): 2.6, pregnancy index (=insemination index excl. culled animals): 2.1 |
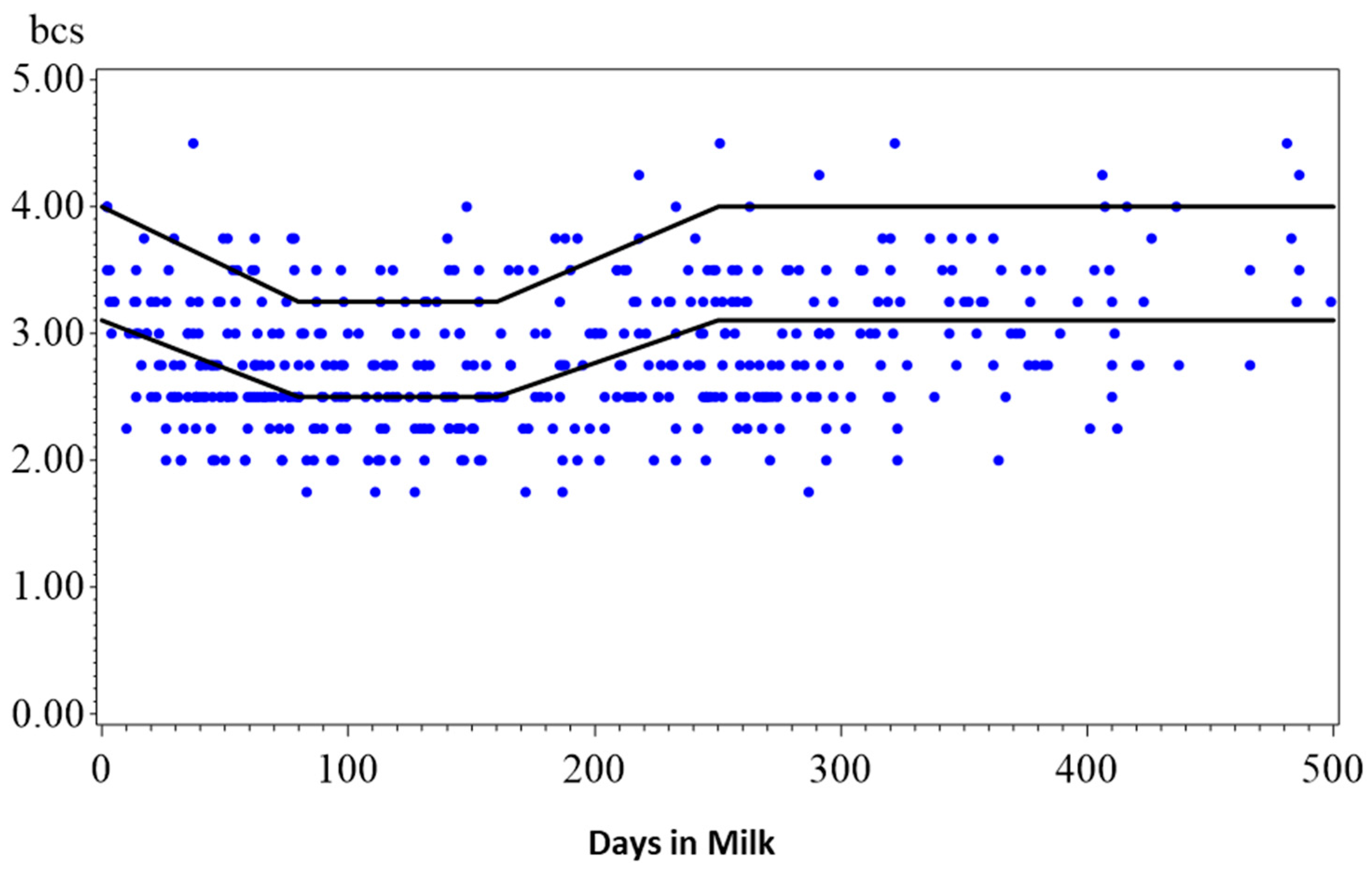
| Average dry cow period: 58 days (intended: 8 weeks, however high variation: many animals with a longer dry period in ≥3rd lactation, Figure A1) | |
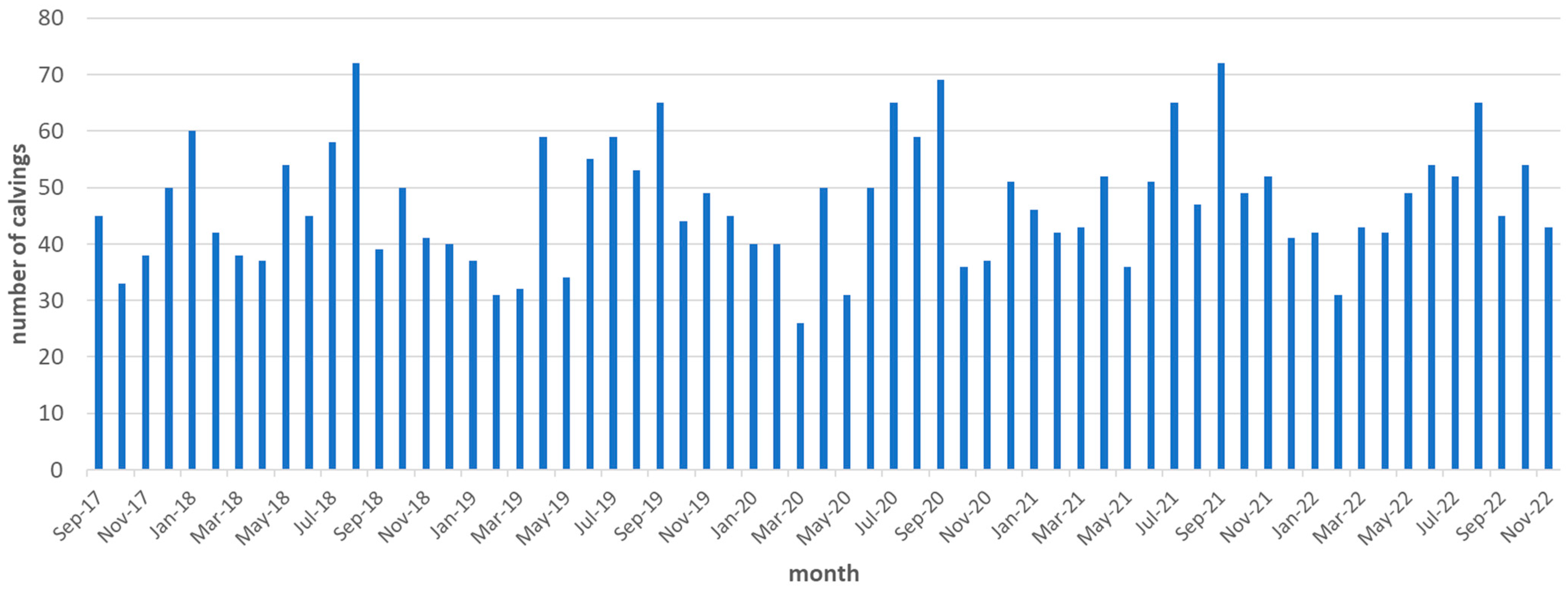
| Number of calvings per month between October 2017 and September 2018 show a non-continuous calving pattern (Figure A2) | |
| Stillbirth rate: 6.7% (heifers: 4.8%, cows: 7.5%), excl. twins: 4.7%; highest stillbirth rates in 4th lactation animals (13.3%, excl. twins: 6.0%; 5.6% twins with a stillbirth rate of 75%; stillbirth defined as calf born dead or dying within 24 h after birth and born >240 d of gestation) | |
| Calving ease (and resp. stillbirth rate): 16.0% not observed (9.4%), 63.6% easy (1.2%), 17.4% medium (11.1%), 2.5% heavy (15.4%), 0.5% caesarean section (50.0%) | |
| Weights of stillbirths markedly lower than life born animals: 1st lactation: 35.5 vs. 39.4 kg (n = 8), 2nd lactation: 30.9 vs. 40.3 kg (n = 7), 3rd lactation: 20.0 vs. 41.3 kg (n = 2), 4th lactation: 24.8 vs. 42.6 kg (n = 4, twins excluded); in ≥2nd lactation animals, 6 of the stillbirths were born ≤260 d of gestation. |
| Dry Cows | Close-Up | Fresh Cows | First Lactation Heifers | High- Yielding 1 | High- Yielding 2 | Mid Yielding/Lactation | |
|---|---|---|---|---|---|---|---|
| Jan 2019—Jun 2019 | 10.8 | 9.8 | 17.3 | 20.6 | 23.9 | 23.9 | 17.9 |
| Jul 2019—Dec 2021 | 12.4 | 16.7 | 19.3 | 20.6 | 24.2 | 24.0 | 19.3 |
| Jan 2022—Jun 2022 | 13.4 | 18.7 | 21.3 | 21.6 | 24.8 | 25.5 | 22.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schären-Bannert, M.; Waurich, B.; Rachidi, F.; Wöckel, A.; Wippermann, W.; Wittich, J.; Hermenau, G.; Bannert, E.; Hufe, P.; May, D.; et al. Metabolic Disorders in Transition Dairy Cows in a 500-Cow Herd—Analysis, Prevention and Follow-Up. Dairy 2025, 6, 49. https://doi.org/10.3390/dairy6050049
Schären-Bannert M, Waurich B, Rachidi F, Wöckel A, Wippermann W, Wittich J, Hermenau G, Bannert E, Hufe P, May D, et al. Metabolic Disorders in Transition Dairy Cows in a 500-Cow Herd—Analysis, Prevention and Follow-Up. Dairy. 2025; 6(5):49. https://doi.org/10.3390/dairy6050049
Chicago/Turabian StyleSchären-Bannert, Melanie, Benno Waurich, Fanny Rachidi, Adriana Wöckel, Wolf Wippermann, Julia Wittich, Guntram Hermenau, Erik Bannert, Peter Hufe, Detlef May, and et al. 2025. "Metabolic Disorders in Transition Dairy Cows in a 500-Cow Herd—Analysis, Prevention and Follow-Up" Dairy 6, no. 5: 49. https://doi.org/10.3390/dairy6050049
APA StyleSchären-Bannert, M., Waurich, B., Rachidi, F., Wöckel, A., Wippermann, W., Wittich, J., Hermenau, G., Bannert, E., Hufe, P., May, D., Dänicke, S., Swalve, H., & Starke, A. (2025). Metabolic Disorders in Transition Dairy Cows in a 500-Cow Herd—Analysis, Prevention and Follow-Up. Dairy, 6(5), 49. https://doi.org/10.3390/dairy6050049







