Multi-Mycotoxin Contamination of Concentrates Fed to Dairy Calves in Southeast Brazil: A Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Physical Form Characterization
2.2. Mycotoxin Extraction Procedure and Instrumentation
2.3. Statistical Analysis
2.4. Reagents
3. Results
3.1. Characterization of Physical Form of Concentrates
3.2. Mycotoxins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corassin, C.H.; de Oliveira, C.A.F. Mycotoxins in the Dairy Industry. Dairy 2023, 4, 392–394. [Google Scholar] [CrossRef]
- Vedovatto, M.G.; Bento, A.L.; Kiefer, C.; Souza, K.M.R.; Franco, G.L. Micotoxinas Na Dieta de Bovinos de Corte: Revisão. Arch. De Zootec. 2020, 69, 234–244. [Google Scholar] [CrossRef][Green Version]
- Goldblatt, L.A. Implications of Mycotoxins. Clin. Toxicol. 1972, 5, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J. Etiology of Turkey “X” Disease in Retrospect: A Case for the Involvement of Cyclopiazonic Acid. Mycotoxin Res. 1986, 2, 3–7. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Hartinger, T.; Grabher, L.; Pacífico, C.; Angelmayr, B.; Faas, J.; Zebeli, Q. Short-Term Exposure to the Mycotoxins Zearalenone or Fumonisins Affects Rumen Fermentation and Microbiota, and Health Variables in Cattle. Food Chem. Toxicol. 2022, 162, 112900. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. The Role of Mycotoxins in the Health and Performance of Dairy Cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Ganev, K.C.; Khaneghah, A.M.; Portela, J.B.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The Occurrence and Effect of Unit Operations for Dairy Products Processing on the Fate of Aflatoxin M1: A Review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Erickson, P.S.; Kalscheur, K.F. Nutrition and Feeding of Dairy Cattle. In Animal Agriculture: Sustainability, Challenges and Innovations; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–180. ISBN 9780128170526. [Google Scholar]
- Khan, M.A.; Bach, A.; Weary, D.M.; von Keyserlingk, M.A.G. Invited Review: Transitioning from Milk to Solid Feed in Dairy Heifers. J. Dairy. Sci. 2016, 99, 885–902. [Google Scholar] [CrossRef]
- Bittar, C.M.M.; Gallo, M.P.; Silva, J.T.; de Paula, M.R.; Poczynek, M.; Mourão, G.B. Gradual Weaning Does Not Improve Performance for Calves with Low Starter Intake at the Beginning of the Weaning Process. J. Dairy. Sci. 2020, 103, 4672–4680. [Google Scholar] [CrossRef]
- Schöne, F.; Rajendram, R. Iodine in Farm Animals. In Comprehensive Handbook of Iodine; Elsevier: Amsterdam, The Netherlands, 2009; pp. 151–170. [Google Scholar]
- Suárez, B.J.; Van Reenen, C.G.; Gerrits, W.J.J.; Stockhofe, N.; van Vuuren, A.M.; Dijkstra, J. Effects of Supplementing Concentrates Differing in Carbohydrate Composition in Veal Calf Diets: II. Rumen Development. J. Dairy. Sci. 2006, 89, 4376–4386. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.D.; Pereira, J.C.; Bettero, V.P.; de Queiroz, A.C.; Costa, M.G.; de Paula Leonel, F. Níveis de Concentrado Na Dieta de Bezerros. Rev. Bras. De Zootec. 2009, 38, 1133–1141. [Google Scholar] [CrossRef][Green Version]
- Bittar, C.M.M.; Ferreira, L.S.; Santos, F.A.P.; Zopollatto, M. Desempenho e Desenvolvimento Do Trato Digestório Superior de Bezerros Leiteiros Alimentados Com Concentrado de Diferentes Formas Físicas. Rev. Bras. De Zootec. 2009, 38, 1561–1567. [Google Scholar] [CrossRef]
- Van Gastelen, S.; Mens, A.J.W.; Binnendijk, G.P.; Ellis, J.L.; Powell, C.D.; Gerrits, W.J.J. Effect of Solid Feed Level and Types of Roughage on Passage Kinetics of Milk Replacer, Concentrate, and Roughage in Veal Calves. J. Dairy. Sci. 2021, 104, 7871–7887. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Brasil Produção da Pecuária Municipal; Brasil Ministério da Agricultura; IBGE. Diretoria de Agropecuária, Recursos Naturais e Geografia. Prod. Pec. Munic. Rio de Janeiro 2023, 51, 1–12. [Google Scholar]
- Zanotto, D.L.; Bellaver, C. Método de Determinação Da Granulometria de Ingredientes Para Uso Em Rações de Suínos e Aves; EMBRAPA-CNPSA: Concórdia, Brazil, 1996. [Google Scholar]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A Liquid Chromatography/Tandem Mass Spectrometric Multi-Mycotoxin Method for the Quantification of 87 Analytes and Its Application to Semi-Quantitative Screening of Moldy Food Samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Franco, L.T.; Petta, T.; Rottinghaus, G.E.; Bordin, K.; Gomes, G.A.; Oliveira, C.A.F. Co-Occurrence of Mycotoxins in Maize Food and Maize-Based Feed from Small-Scale Farms in Brazil: A Pilot Study. Mycotoxin Res. 2019, 35, 65–73. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the Impact of Mycotoxins on Dairy Cattle Health: Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin Occurrence in Grains and the Role of Postharvest Management as a Mitigation Strategies. A Review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Juraschek, L.M.; Kappenberg, A.; Amelung, W. Mycotoxins in Soil and Environment. Sci. Total Environ. 2022, 814, 152425. [Google Scholar] [CrossRef]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, É.R.; Dias, R.R.S.; Pinheiro, G.R.G.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in Cattle Feed and Feed Ingredients in Brazil: A Five-Year Survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef]
- Custódio, L.; Prados, L.F.; Yiannikouris, A.; Holder, V.; Pettigrew, J.; Kuritza, L.; de Resende, F.D.; Siqueira, G.R. Mycotoxin Contamination of Diets for Beef Cattle Finishing in Feedlot. Rev. Bras. De Zootec. 2019, 48, e20190079. [Google Scholar] [CrossRef]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-Occurrence and Toxicological Relevance of Secondary Metabolites in Dairy Cow Feed from Thailand. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- Feijó Corrêa, J.A.; Orso, P.B.; Bordin, K.; Hara, R.V.; Luciano, F.B. Toxicological Effects of Fumonisin B1 in Combination with Other Fusarium Toxins. Food Chem. Toxicol. 2018, 121, 483–494. [Google Scholar] [CrossRef]
- Osweiler, G.D.; Kehrli, M.E.; Stabel, J.R.; Thurston, J.R.; Ross, P.F.; Wilson, T.M. Effects of Fumonisin-Contaminated Corn Screenings on Growth and Health of Feeder Calves. J. Anim. Sci. 1993, 71, 459–466. [Google Scholar] [CrossRef]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Kennedy, D.G.; Hewitt, S.A.; McEvoy, J.D.G.; Currie, J.W.; Cannavan, A.; Blanchflower, W.J.; Elliot, C.T. Zeranol Is Formed from Fusarium Spp. Toxins in Cattle in Vivo. Food Addit. Contam. 1998, 15, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen Development, Intestinal Growth and Hepatic Metabolism in the Pre- and Postweaning Ruminant. J. Dairy. Sci. 2004, 87, E55–E65. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in Food and Feed. In Advances in Food and Nutrition Research; Academic Press Inc.: New York, NY, USA, 2019; Volume 89, pp. 297–345. ISBN 9780128171714. [Google Scholar]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination Across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Kyprianou, M. European Commission Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding (2006/576/EC). Off. J. Eur. Union 2006, 49, L299. [Google Scholar]
- Oyesigye, E.; Cervini, C.; Oluwakayode, A.; Mahuku, G.; Medina, A. First Evidence on the Occurrence of Multi-Mycotoxins and Dietary Risk Exposure to AFB1 along the Cassava Value Chain in Uganda. Mycotoxin Res. 2024, 40, 693–708. [Google Scholar] [CrossRef]
- Moore, D.A.; Taylor, J.; Hartman, M.L.; Sischo, W.M. Quality Assessments of Waste Milk at a Calf Ranch. J. Dairy. Sci. 2009, 92, 3503–3509. [Google Scholar] [CrossRef]
- Borowsky, A.M.; Rosim, R.E.; Tonin, F.G.; de Oliveira, C.A.F.; Corassin, C.H. Co-Occurrence of Mycotoxins in the Diet and in the Milk of Dairy Cows from the Southeast Region of Brazil. Toxins 2024, 16, 492. [Google Scholar] [CrossRef]
- Frey, M.; Rosim, R.; Oliveira, C. Mycotoxin Co-Occurrence in Milks and Exposure Estimation: A Pilot Study in São Paulo, Brazil. Toxins 2021, 13, 507. [Google Scholar] [CrossRef]
| Mycotoxins | RT (min.) | Mass (g/mol) | Molecular Ion | Transition (m/z) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|
| AFB1 | 4.80 | 312.3 | [M+H]+ | 312.7 > 284.9 a | 0.4 | 0.8 |
| 312.7 > 241.1 b | ||||||
| AFB2 | 4.50 | 314.3 | [M+H]+ | 314.7 > 259.0 a | 0.4 | 0.8 |
| 314.7 > 287.0 b | ||||||
| AFG1 | 4.46 | 328.3 | [M+H]+ | 328.9 > 243.0 a | 0.4 | 0.8 |
| 328.9 > 199.5 b | ||||||
| AFG2 | 4.18 | 330.3 | [M+H]+ | 330.9 > 245.0 a | 0.5 | 1.0 |
| 330.9 > 188.9 b | ||||||
| DON | 1.98 | 296.3 | [M+H]+ | 297.3 > 249.1 a | 6.1 | 18.0 |
| 297.3 > 231.1 b | ||||||
| FB1 | 5.40 | 721.8 | [M+H]+ | 722.5 > 334.0 a | 0.9 | 2.5 |
| 722.5 > 352.1 b | ||||||
| FB2 | 3.74 | 705.8 | [M+H]+ | 706.5 > 336.2 a | 0.7 | 2.0 |
| 706.5 > 318.3 b | ||||||
| T2 | 4.49 | 489.2 | [M+NH4]+ | 484.2 > 541.1 a | 5.1 | 15.0 |
| 484.2 > 542.0 b | ||||||
| ZEN | 5.98 | 318.1 | [M-H]− | 317.1 > 175.1 a | 6.1 | 18.0 |
| 317.1 > 130.9 b |
| Overall | Bran | Multiparticle | Pelleted | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| FM | 3.7 | 0.2 | 3.0 | 0.1 | 4.4 | 0.6 | 4.9 | 0.1 |
| MGD, mm | 1.7 | 2.7 | 0.9 | 0.9 | 2.5 | 7.4 | 3.2 | 0.1 |
| Density, g/L | 591 | 15 | 581 | 18 | 602 | 66 | 606 | 28 |
| Mycotoxin (μg/kg) | Mean ± SE | Minimum | Maximum |
|---|---|---|---|
| FB1 | 2750.1 ± 600.9 | 467.8 | 9443.6 |
| FB2 | 834.9 ± 197.6 | 134.7 | 3428.0 |
| ZEN | 929.9 ± 796.6 | 78.3 | 5708.5 |
| Sample | FB1 (μg/kg) | FB2 (μg/kg) | ZEN (μg/kg) |
|---|---|---|---|
| 1 | 467.8 | 134.7 | <LOQ |
| 2 | 895.7 | 232.3 | <LOQ |
| 3 | 3332.8 | 1242.7 | <LOQ |
| 4 | 4537.9 | 1436.9 | 5708.5 |
| 5 | 9443.6 | 3428.0 | <LOQ |
| 6 | 2480.0 | 699.6 | <LOQ |
| 7 | 4202.6 | 1160.8 | 100.9 |
| 8 | 6814.4 | 1823.3 | <LOQ |
| 9 | 1776.4 | 701.5 | <LOQ |
| 10 | 2159.8 | 410.4 | <LOQ |
| 11 | 1089.3 | 352.2 | <LOQ |
| 12 | 780.9 | 234.5 | 111.3 |
| 13 | 520.7 | 143.4 | 78.3 |
| 14 | 1233.5 | 410.7 | <LOQ |
| 15 | 761.1 | 207.9 | <LOQ |
| 16 | 676.8 | 209.1 | 174.5 |
| 17 | 1471.0 | 497.7 | 135.8 |
| 18 | 7475.4 | 2109.9 | <LOQ |
| 19 | 2132.4 | 427.6 | 200.4 |
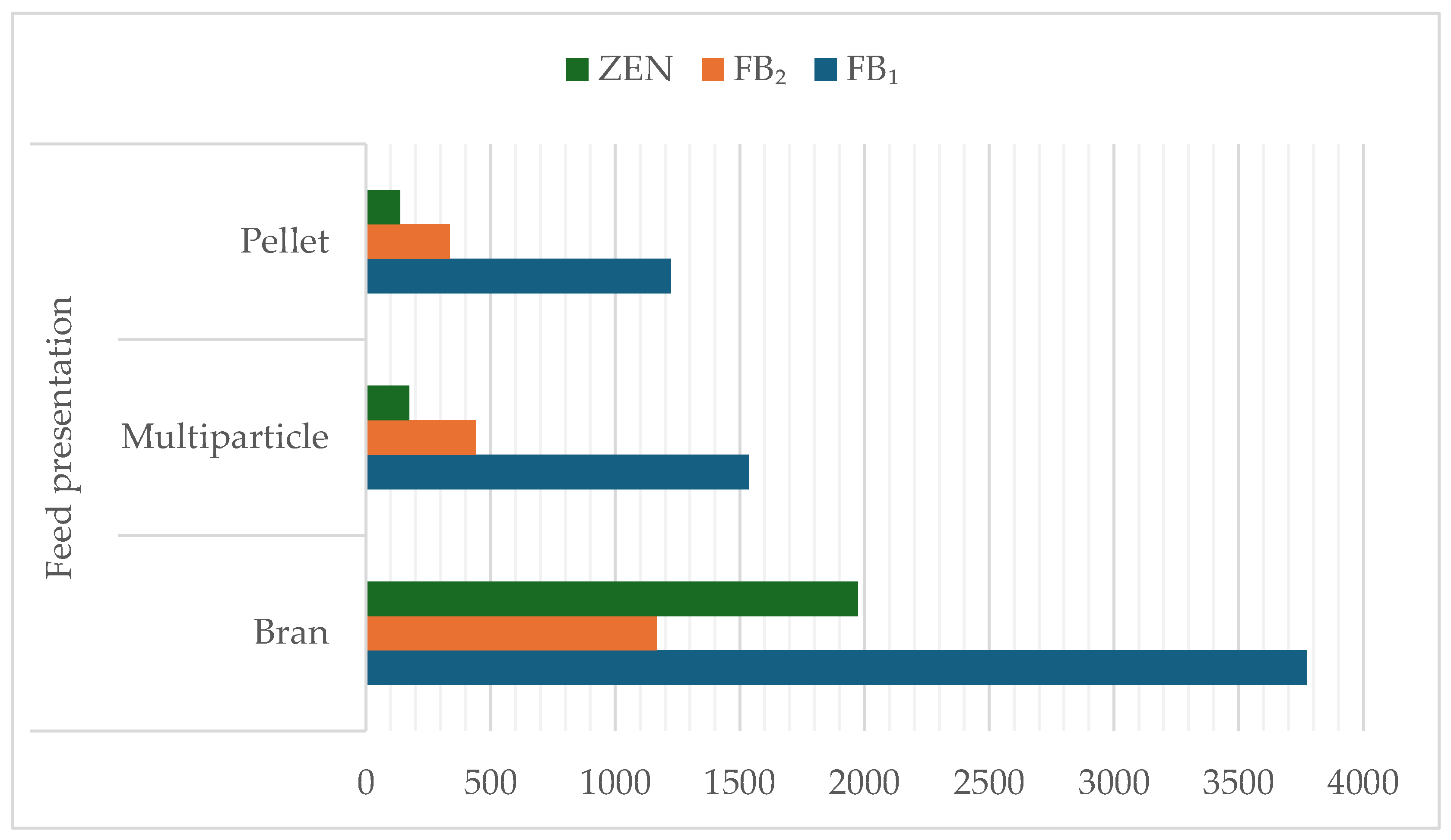
| Mycotoxin (μg/kg) | Feed Presentation | p-Value | ||
|---|---|---|---|---|
| Bran | Multiparticle | Pellet | ||
| FB1 | 3774.57 ± 920.01 | 1537.63 ± 12.41 | 1223.73 ± 282.41 | 0.1321 |
| FB2 | 1168.61 ± 304.53 | 440.32 ± 444.41 | 337.44 ± 68.42 | 0.1378 |
| ZEN | 1973.53 ± 1867.49 | 174.48 ± 142.95 | 138.16 ± 35.26 | 0.6169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, R.D.; Moreira Borowsky, A.; Alves e Silva, T.; Evangelista, G.C.R.C.; Maris Machado Bittar, C.; Corassin, C.H. Multi-Mycotoxin Contamination of Concentrates Fed to Dairy Calves in Southeast Brazil: A Case Report. Dairy 2025, 6, 44. https://doi.org/10.3390/dairy6040044
Pires RD, Moreira Borowsky A, Alves e Silva T, Evangelista GCRC, Maris Machado Bittar C, Corassin CH. Multi-Mycotoxin Contamination of Concentrates Fed to Dairy Calves in Southeast Brazil: A Case Report. Dairy. 2025; 6(4):44. https://doi.org/10.3390/dairy6040044
Chicago/Turabian StylePires, Rogério D’Antonio, Aline Moreira Borowsky, Tobias Alves e Silva, Giovanna Canela Ruiz Castro Evangelista, Carla Maris Machado Bittar, and Carlos Humberto Corassin. 2025. "Multi-Mycotoxin Contamination of Concentrates Fed to Dairy Calves in Southeast Brazil: A Case Report" Dairy 6, no. 4: 44. https://doi.org/10.3390/dairy6040044
APA StylePires, R. D., Moreira Borowsky, A., Alves e Silva, T., Evangelista, G. C. R. C., Maris Machado Bittar, C., & Corassin, C. H. (2025). Multi-Mycotoxin Contamination of Concentrates Fed to Dairy Calves in Southeast Brazil: A Case Report. Dairy, 6(4), 44. https://doi.org/10.3390/dairy6040044






