Relationship Between Hyperkeratosis, Teat Conformation Traits, Microbiological Isolation, and Somatic Cell Count in Milk from Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd and Data Collection
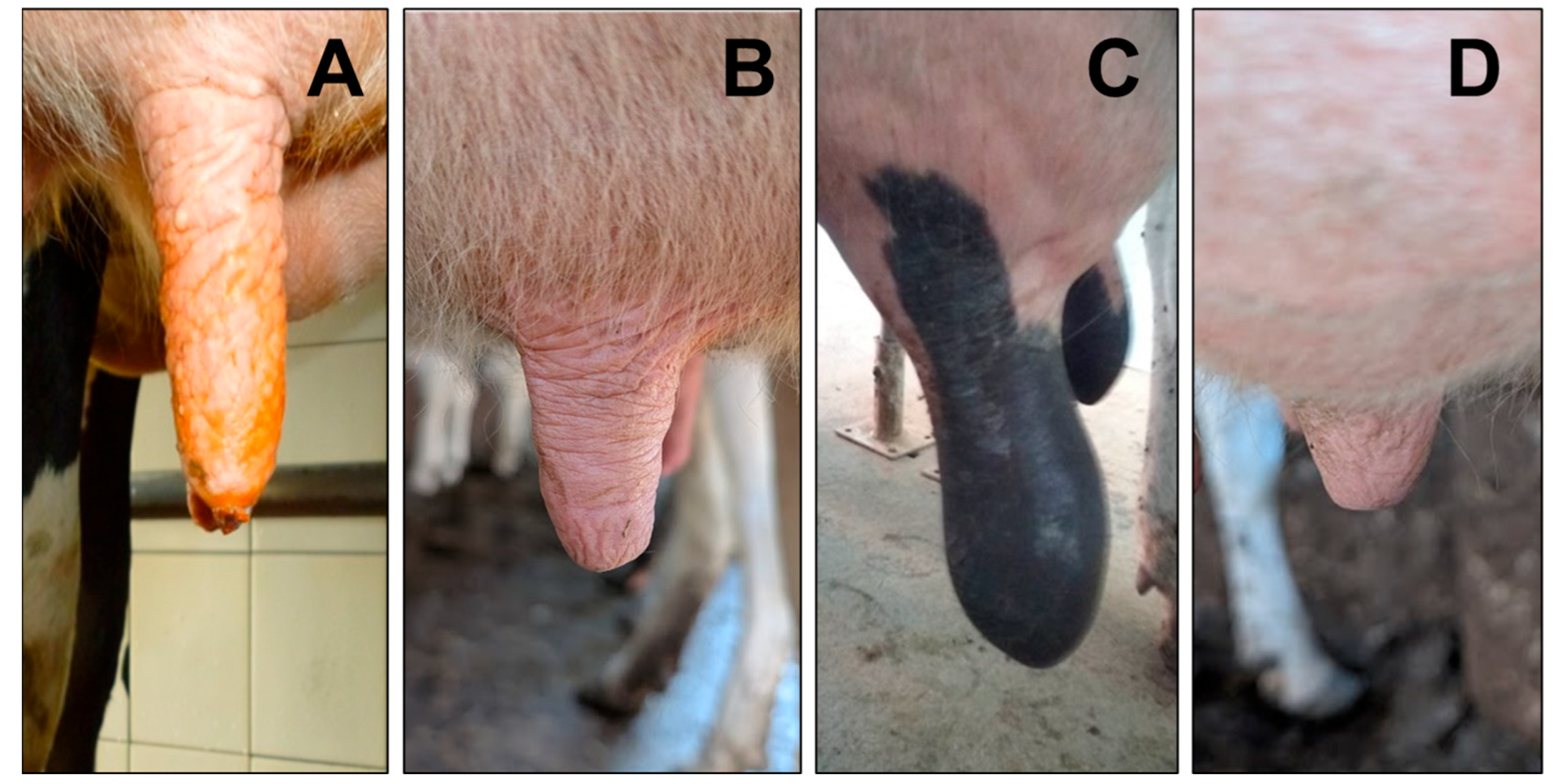
2.2. Evaluation of Hyperkeratosis Severity and Teat Morphometric Parameters
2.3. Sample Collection
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asadpour, R.; Bagherniaee, H.; Houshmandzad, M.; Fatehi, H.; Rafat, A.; Nofouzi, K.; Maftouni, K. Relationship between Teat End Hyperkeratosis with Intra Mammary Infection and Somatic Cell Counts in Lactating Dairy Cattle. Rev. Méd. Vét. 2015, 166, 266–270. [Google Scholar]
- Hogeveen, H.; van der Voort, M. Assessing the Economic Impact of an Endemic Disease: The Case of Mastitis. Rev. Sci. Et Tech. 2017, 36, 217–226. [Google Scholar] [CrossRef]
- Shafeeq, M.; Muneer, A.; Aqib, A.I.; Kirn, N. Economic Impacts of Clinical and Sub Clinical Mastitis on Dairy Farms. Vet. Sci. Res. 2021, 3, 31–39. [Google Scholar] [CrossRef]
- Alessio, D.R.M.; Velho, J.P.; McManus, C.M.; Knob, D.A.; Vancin, F.R.; Antunes, G.V.; Busanello, M.; De Carli, F.; Neto, A.T. Lactose and Its Relationship with Other Milk Constituents, Somatic Cell Count, and Total Bacterial Count. Livest. Sci. 2021, 252, 104678. [Google Scholar] [CrossRef]
- Jashari, R.; Piepers, S.; De Vliegher, S. Evaluation of the Composite Milk Somatic Cell Count as a Predictor of Intramammary Infection in Dairy Cattle. J. Dairy Sci. 2016, 99, 9271–9286. [Google Scholar] [CrossRef]
- de Souza, M.M.S.; Dubenczuk, F.C.; Melo, D.A.; Holmström, T.C.N.; Mendes, M.B.; Reinoso, E.B.; Coelho, S.M.O.; Coelho, I.S. Antimicrobial Therapy Approaches in the Mastitis Control Driven by One Health Insights. Rev. Bras. Med. Vet. 2024, 46, e002624. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, D.A.; Ruegg, P.L. Effects of Tail Docking on Milk Quality and Cow Cleanliness. J. Dairy Sci. 2002, 85, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited Review Mastitis in Dairy Heifers Nature of the Disease. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef]
- Sordillo, L.M. New Concepts in the Causes and Control of Mastitis. J. Mammary Gland Biol. Neoplasia 2011, 16, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Mammary Gland Immunobiology and Resistance to Mastitis. Vet. Clin. North Am. Food Anim. Pract. 2018, 34, 507–523. [Google Scholar] [CrossRef]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of Mastitis: Insights into Disease Recognition and Resolution. J. Mammary Gland Biol. Neoplasia 2011, 16, 291–304. [Google Scholar] [CrossRef]
- Dufour, S.; Dohoo, I.R.; Barkema, H.W.; DesCôteaux, L.; DeVries, T.J.; Reyher, K.K.; Roy, J.P.; Scholl, D.T. Manageable Risk Factors Associated with the Lactational Incidence, Elimination, and Prevalence of Staphylococcus Aureus Intramammary Infections in Dairy Cows. J. Dairy Sci. 2012, 95, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Innate Immune Response of Bovine Mammary Gland to Pathogenic Bacteria Responsible for Mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Ezzat Alnakip, M.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Caamaño-Antelo, S.; Calo-Mata, P.; Barros-Velázquez, J. The Immunology of Mammary Gland of Dairy Ruminants between Healthy and Inflammatory Conditions. J. Vet. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef]
- Neijenhuis, F.; Barkema, H.W.; Hogeveen, H.; Noordhuizen, J.P.T.M. Relationship between Teat-End Callosity and Occurrence of Clinical Mastitis. J. Dairy Sci. 2001, 84, 2664–2672. [Google Scholar] [CrossRef]
- Mein, G.A.; Neijenhuis, F.; Morgan, W.F.; Reinemann, D.J.; Hillerton, J.E.; Baines, J.R.; Ohnstad, I.; Rasmussen, M.D.; Timms, L.; Britt, J.S.; et al. Evaluation of Bovina Teat Condition in Commercial Dairy Herds: Non-Infectious Factors. In Proceedings of the AABP-NMC International Symposium on Mastitis and Milk Quality in Vancouver, Vancouver, BC, Canada, 13–15 September 2001; pp. 13–15. [Google Scholar]
- Cardozo, L.L.; Thaler Neto, A.; Souza, G.N.; Picinin, L.C.A.; Felipus, N.C.; Reche, N.L.M.; Schmidt, F.A.; Werncke, D.; Simon, E.E. Risk Factors for the Occurrence of New and Chronic Cases of Subclinical Mastitis in Dairy Herds in Southern Brazil. J. Dairy Sci. 2015, 98, 7675–7685. [Google Scholar] [CrossRef]
- Abdelghany, S.; Fahim, N.H.; Samir, F.; Radwan, M.A. The Use of Teat-End Hyperkeratosis to Predict Somatic Cell Count and Milk Quality of Holstein Cows Raised in Egypt. Trop. Anim. Sci. J. 2021, 44, 213–221. [Google Scholar] [CrossRef]
- Gouvêa, F.L.R.; Cardozo, L.L.; Canal, J.; Troncarelli, M.Z.; Pantoja, J.C.F. A Descriptive Study of Teat Morphology, Milking Machine Characteristics, and Milking Practices in a Sample of Brazilian Dairy Herds. Livest. Sci. 2020, 241, 104196. [Google Scholar] [CrossRef]
- Emre, B.; Alacam, E. The Occurrence of Teat Hyperkeratosis in Cows and Its Effect on Milk Somatic Cell Counts. Turk. Klin. J. Vet. Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Fonseca, L.H.M.; da Cunha, A.F.; Saraiva, L.H.G.; Coelho, K.S.; Nunes, M.F. Influência Da Sujidade e Hiperqueratose de Tetos Na Ocorrência de Mastite Subclínica Bovina. Acta Vet. Bras. 2016, 10, 233–237. [Google Scholar] [CrossRef][Green Version]
- Zoche-Golob, V.; Haverkamp, H.; Paduch, J.H.; Klocke, D.; Zinke, C.; Hoedemaker, M.; Heuwieser, W.; Krömker, V. Longitudinal Study of the Effects of Teat Condition on the Risk of New Intramammary Infections in Dairy Cows. J. Dairy Sci. 2015, 98, 910–917. [Google Scholar] [CrossRef]
- Guarín, J.F.; Paixão, M.G.; Ruegg, P.L. Association of Anatomical Characteristics of Teats with Quarter-Level Somatic Cell Count. J. Dairy Sci. 2017, 100, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.L.; Araújo, J.P.; Cantalapiedra, J. How Is the Association of Teat-End Severe Hyperkeratosis on Udder Health and Dairy Cow Behavior ? Rev. Med. Vet. 2018, 169, 30–37. [Google Scholar]
- Pantoja, J.C.F.; Correia, L.B.N.; Rossi, R.S.; Latosinski, G.S. Association between Teat-End Hyperkeratosis and Mastitis in Dairy Cows: A Systematic Review. J. Dairy Sci. 2020, 103, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Knob, D.A.; Scholz, A.M.; Alessio, D.R.M.; Mendes, B.P.B.; Perazzoli, L.; Kappes, R.; Thaler Neto, A. Reproductive and Productive Performance, Udder Health, and Conformation Traits of Purebred Holstein, F1, and R1 Crossbred Holstein × Simmental Cows. Trop. Anim. Health Prod. 2019, 52, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Araújo, V.M.; Rangel, A.N.; De Medeiros, H.R.; Moutinho, I.D.D.C.; Alexandre, M.M.; Bezerra, K.C. Relação Entre a Hiperqueratose Dos Tetos e a Ocorrência de Mastite Sub-Clínica. Arch. Vet. Sci. 2012, 17, 73–77. [Google Scholar] [CrossRef]
- Haverkamp, H.; Paduch, J.-H.; Klocke, D.; Hoedemaker, M.; Krömker, V. Prevalence of Teat End Hyperkeratosis in Lactating Dairy Cattle and Their Association with Animal Variables. Int. J. Environ. Agric. Res. 2017, 3, 75–82. [Google Scholar] [CrossRef]
- Stanek, P.; Żółkiewski, P.; Januś, E. A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives. Agriculture 2024, 14, 1292. [Google Scholar] [CrossRef]
- Niero, T.R.; Kappes, R.; Scheid, A.L.; Ramos, A.F.; Ribeiro, E.B.; Cardozo, L.L.; Ferraz, S.M.; Thaler Neto, A. Effect of Double-Premilking Teat Disinfection Protocols on Bacterial Counts on Teat Skin of Cows and Milker Gloves in a Free-Stall-Housed Dairy Herd. J. Dairy Res. 2024, 91, 311–314. [Google Scholar] [CrossRef]
- Duse, A.; Persson-Waller, K.; Pedersen, K. Microbial Aetiology, Antibiotic Susceptibility and Pathogen-Specific Risk Factors for Udder Pathogens from Clinical Mastitis in Dairy Cows. Animals 2021, 11, 2113. [Google Scholar] [CrossRef]
- Paduch, J.H.; Mohr, E.; Krömker, V. The Association between Teat End Hyperkeratosis and Teat Canal Microbial Load in Lactating Dairy Cattle. Vet. Microbiol. 2012, 158, 353–359. [Google Scholar] [CrossRef]
- Hohmann, M.F.; Wente, N.; Zhang, Y.; Krömker, V. Bacterial Load of the Teat Apex Skin and Associated Factors at Herd Level. Animals 2020, 10, 1647. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Kappes, R.; Knob, D.A.; Neto, A.T.; Alessio, D.R.M.; Rodrigues, W.B.; Manfred, A.; Bonotto, R. Cow ’ s Functional Traits and Physiological Status and Their Relation with Milk Yield and Milk Quality in a Compost Bedded Pack Barn System. Rev. Bras. Zootec. 2020, 49, e20190213. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Carter, M.E.; Donnelly, W.J.; Leonard, F.C. Microbiologia Veterinária e Doenças Infecciosas; Artmed: Porto Alegre, Brazil, 2005. [Google Scholar]
- National Mastitis Council. Microbiological Procedures for the Diagnosis of Bovine Udder Infection and Determination of Milk Quality, 4th ed.; National Mastitis Council: New Prague, MN, USA, 2004. [Google Scholar]
- de Oliveria, S.J.; Vaz, A.K. Guia Bacteriológico Prático: Identificação, Patogenicidade e Imunidade, 3rd ed.; Editora da ULBRA: Canoas, Brazil, 2018. [Google Scholar]
- National Mastitis Council. Current Concepts of Bovine Mastitis, 4th ed.; National Mastitis Council: New Prague, MN, USA, 1999. [Google Scholar]
- Middleton, J.R.; Fox, L.K.; Pighetti, G.; Petersson-Wolfe, C. Laboratory Handbook on Bovine Mastitis; National Mastitis Council Inc.: New Prague, MN, USA, 2017. [Google Scholar]
- Ali, A.K.A.; Shook, G.E. An Optimum Transformation for Somatic Cell Concentration in Milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- de Pinho Manzi, M.; Nóbrega, D.B.; Faccioli, P.Y.; Troncarelli, M.Z.; Menozzi, B.D.; Langoni, H. Relationship between Teat-End Condition, Udder Cleanliness and Bovine Subclinical Mastitis. Res. Vet. Sci. 2012, 93, 430–434. [Google Scholar] [CrossRef]
- Guarín, J.F.; Baumberger, C.; Ruegg, P.L. Anatomical Characteristics of Teats and Premilking Bacterial Counts of Teat Skin Swabs of Primiparous Cows Exposed to Different Types of Bedding. J. Dairy Sci. 2017, 100, 1436–1444. [Google Scholar] [CrossRef]
- Derakhshani, H.; Plaizier, J.C.; De Buck, J.; Barkema, H.W.; Khafipour, E. Composition and Co-Occurrence Patterns of the Microbiota of Different Niches of the Bovine Mammary Gland: Potential Associations with Mastitis Susceptibility, Udder Inflammation, and Teat-End Hyperkeratosis. Anim. Microbiome 2020, 2, 11. [Google Scholar] [CrossRef]
- Bhutto, A.L.; Murray, R.D.; Woldehiwet, Z. Udder Shape and Teat-End Lesions as Potential Risk Factors for High Somatic Cell Counts and Intra-Mammary Infections in Dairy Cows. Vet. J. 2010, 183, 63–67. [Google Scholar] [CrossRef]
- Pantoja, J.C.F.; Almeida, A.P.; dos Santos, B.; Rossi, R.S. An Investigation of Risk Factors for Two Successive Cases of Clinical Mastitis in the Same Lactation. Livest. Sci. 2016, 194, 10–16. [Google Scholar] [CrossRef]
- Lage, P.G.; Araújo, A.R.; Teixeira, A.G.; Pinotti, M.; Faleiros, R.R. Dispositivo Fotobiomodulador Para Prevenção e Tratamento de Hiperqueratose de Teto Em Vacas Leiteiras. Pesq. Vet. Bras. 2014, 34, 515–522. [Google Scholar] [CrossRef][Green Version]
- Chegini, A.; Hossein-Zadeh, N.G.; Hosseini-Moghadam, H.; Shadparvar, A.A. Relações Genéticas e Ambientais Entre Produção de Leite e Tipos Diferentes de Mastite e Hiperqueratose Em Vacas Holandesas. Acta Sci. 2016, 38, 191–196. [Google Scholar] [CrossRef][Green Version]
- Wieland, M.; Basran, P.S.; Virkler, P.D.; Heuwieser, W. An Observational Study to Investigate the Association of Teat Skin Condition with Clinical Mastitis Risk. JDS Commun. 2024, 5, 654–658. [Google Scholar] [CrossRef]
- Breen, J.E.; Green, M.J.; Bradley, A.J. Quarter and Cow Risk Factors Associated with the Occurrence of Clinical Mastitis in Dairy Cows in the United Kingdom. J. Dairy Sci. 2009, 92, 2551–2561. [Google Scholar] [CrossRef]
- Brito, J.R.F.; Sales, R. de O. Health of the Ubere. A Revision. Rev. Bras. Hig. E Sanidade Anim. 2007, 1, 67–87. [Google Scholar] [CrossRef]
- Passchyn, P.; Piepers, S.; De Vliegher, S. Pathogen Group-Specific Risk Factors for Intramammary Infection in Treated and Untreated Dairy Heifers Participating in a Prepartum Antimicrobial Treatment Trial. J. Dairy Sci. 2014, 97, 6260–6270. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Pantoja, J.C.F. Understanding and Using Somatic Cell Counts to Improve Milk Quality; TEAGASC: Carlow, Ireland, 2013; Volume 52. [Google Scholar]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring Udder Health and Milk Quality Using Somatic Cell Counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Hamel, J.; Zhang, Y.; Wente, N.; Krömker, V. Heat Stress and Cow Factors Affect Bacteria Shedding Pattern from Naturally Infected Mammary Gland Quarters in Dairy Cattle. J. Dairy Sci. 2021, 104, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.J.; Deng, Y.; Wehri, T.C.; Pena-Mosca, F.; Ray, T.; Crooker, B.A.; Godden, S.M.; Caixeta, L.S.; Noyes, N.R. The Impact of Kit, Environment, and Sampling Contamination on the Observed Microbiome of Bovine Milk. mSystems 2024, 9, e0115823. [Google Scholar] [CrossRef] [PubMed]
- Krebs, I.; Zhang, Y.; Wente, N.; Leimbach, S.; Krömker, V. Bacteremia in Severe Mastitis of Dairy Cows. Microorganisms 2024, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.V.; Cobuci, J.A.; Costa, C.N.; Padilha, A.H.; Kern, E. Correlações Genéticas Entre Características de Tipo e Produtivas Em Vacas Da Raça Holandesa No Brasil. In Proceedings of the 49aReunião Anual da Sociedade Brasileira de Zootecnia, Brasília, Brazil, 23–26 July 2012; pp. 21–23. [Google Scholar]
- Tiezzi, F.; Maisano, A.M.; Chessa, S.; Luini, M.; Biffani, S. Heritability of Teat Condition in Italian Holstein Friesian and Its Relationship with Milk Production and Somatic Cell Score. Animals 2020, 10, 2271. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Bansal, B.K.; Gupta, D.K. Relationship between Teat Morphological Traits and Subclinical Mastitis in Frieswal Dairy Cows. Trop. Anim. Health Prod. 2017, 49, 1623–1629. [Google Scholar] [CrossRef]
- Wendt, K.; Köhler, S.; Sass, D.; Spasovski, S.; Piltz, E. Einflussfaktoren Auf Die Hämatogene Mikrozirkulation an Der Zitze Des Milchrindes. Züchtungskunde 2007, 79, 119–127. [Google Scholar]
- Singh, R.S.; Bansal, B.K.; Gupta, D.K. Udder Health in Relation to Udder and Teat Morphometry in Holstein Friesian × Sahiwal Crossbred Dairy Cows. Trop. Anim. Health Prod. 2014, 46, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Pereira, G.; Bexiga, R. Bimodal Milk Flow and Overmilking in Dairy Cattle: Risk Factors and Consequences. Animal 2023, 17, 100716. [Google Scholar] [CrossRef]
- Masía, F.M.; Lyons, N.A.; Piccardi, M.; Balzarini, M.; Hovey, R.C.; Garcia, S.C. Modeling Variability of the Lactation Curves of Cows in Automated Milking Systems. J. Dairy Sci. 2020, 103, 8189–8196. [Google Scholar] [CrossRef]
- Silva Boloña, P.; Upton, J.; Reinemann, D.J. Effects of Simulated Quarter and Udder Teat Cup Removal Settings on Strip Milk and Milking Duration in Dairy Cows. J. Dairy Sci. 2020, 103, 4446–4454. [Google Scholar] [CrossRef]
- Singh, A.; Spellman, M.E.; Somula, H.; Wieland, M. Effects of Flow-Responsive Pulsation on Teat Tissue Condition and Milking Performance in Holstein Dairy Cows. J. Dairy Sci. 2024, 107, 7337–7351. [Google Scholar] [CrossRef]
| Sample Origin | Variable | Category | Teat-End Condition | p > χ2 * | |||
|---|---|---|---|---|---|---|---|
| With Hyperkeratosis | Without Hyperkeratosis | ||||||
| N | % | N | % | ||||
| Milk | Growth | Negative | 114 | 50 | 114 | 50 | =1.0000 |
| Positive | 56 | 50 | 56 | 50 | |||
| Classification | Major Microorganisms | 27 | 55.1 | 22 | 44.9 | =0.3835 | |
| Minor Microorganisms | 29 | 46.8 | 33 | 53.2 | |||
| Transmission | Environmental | 39 | 59.1 | 27 | 40.9 | =0.0778 | |
| Contagious | 9 | 34.6 | 17 | 65.4 | |||
| Coinfection | 8 | 42.1 | 11 | 57.9 | |||
| Swab | Growth | Negative | 66 | 49.6 | 67 | 50.4 | =0.9542 |
| Positive | 30 | 49.2 | 31 | 50.9 | |||
| Classification | Major Microorganisms | 14 | 53.8 | 12 | 46.1 | =0.5221 | |
| Minor Microorganisms | 15 | 45.4 | 18 | 54.5 | |||
| Transmission | Environmental | 4 | 33.3 | 8 | 66.7 | =0.4625 | |
| Contagious | 15 | 53.6 | 13 | 46.4 | |||
| Mixed infections | 10 | 52.6 | 9 | 47.4 | |||
| Sample Origin | Explanatory Variable | Class | Number of Samples | X ± SEM |
|---|---|---|---|---|
| Milk * | Growth | Negative | 228 | 2.27 ± 0.191 b |
| Positive | 112 | 4.24 ± 0.273 a | ||
| Classification | Major Microorganisms | 49 | 5.36 ± 0.441 a | |
| Minor Microorganisms | 62 | 3.86 ± 0.385 b | ||
| Swab ** | Growth | Negative | 133 | 3.06 ± 0.254 b |
| Positive | 61 | 3.98 ± 0.435 a | ||
| Classification | Major Microorganisms | 26 | 3.91 ± 0.571 a | |
| Minor Microorganisms | 33 | 3.70 ± 0.516 a |
| Microorganisms Isolated in Milk | Number of Samples (%) | Microorganisms Isolated in Swab | Number of Samples (%) |
|---|---|---|---|
| Contagious microorganisms: | 28 (15.3) | Contagious microorganisms: | 19 (17.6) |
| SCP | 13 (7.1) | SCP | 8 (7.4) |
| Staphylococcus aureus | 9 (4.9) | Corynebacterium spp. | 8 (7.4) |
| Corynebacterium sp. | 6 (3.3) | Staphylococcus aureus | 3 (2.8) |
| Environmental microorganisms: | 16 (8.7) | Environmental microorganisms: | 16 (14.8) |
| Bacillus spp. | 4 (2.2) | Trueperella pyogenes | 4 (3.7) |
| Nocardia spp. | 3 (1.6) | Bacillus spp. | 4 (3.7) |
| Streptococcus equi | 3 (1.6) | Serratia spp. | 2 (1.9) |
| Streptococcus spp. | 2 (1.1) | Yersinia spp. | 2 (1.9) |
| Streptococcus dysgalactiae | 1 (0.5) | Nocardia spp. | 1 (0.9) |
| SBSEC | 1 (0.5) | Streptococcus uberis | 1 (0.9) |
| Streptococcus uberis | 1 (0.5) | Streptococcus equi | 1 (0.9) |
| Yersinia spp. | 1 (0.5) | Enterobacter spp. | 1 (0.9) |
| SCN | 18 (9.8) | SCN | 2 (1.9) |
| Others | 9 (4.9) | Others | 5 (4.6) |
| No growth | 113 (61.4) | No growth | 66 (61.1) |
| Teat Conformation | Front Quarter | Rear Quarter |
|---|---|---|
| N (%) | N (%) | |
| Shape | ||
| Pointed | 43 (25.4) | 44 (26.0) |
| Round | 10 (5.9) | 17 (10.0) |
| Cylindrical | 113 (66.9) | 94 (55.7) |
| Small | 10 (1.8) | 14 (8.3) |
| Placement | ||
| 1 | 56 (33.1) | 8 (4.8) |
| 5 | 89 (52.7) | 45 (26.9) |
| 9 | 24 (14.2) | 114 (68.3) |
| Variable | Number of Samples | Mean ± SEM |
|---|---|---|
| Shape | ||
| Pointed | 87 | 2.41 ± 0.081 |
| Round | 27 | 2.62 ± 0.151 |
| Cylindrical | 207 | 2.43 ± 0.052 |
| Small | 17 | 2.62 ± 0.244 |
| Total | 338 | - |
| Placement | ||
| 1 | 64 | 2.46 ± 0.141 |
| 5 | 134 | 2.55 ± 0.068 |
| 9 | 138 | 2.34 ± 0.084 |
| Total | 336 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardozo, L.L.; Knob, D.A.; Santos, P.T.d.; Pelizza, A.; Mori, A.P.; Camera, M.; Ferraz, S.M.; de Assis, M.Z.; Thaler Neto, A. Relationship Between Hyperkeratosis, Teat Conformation Traits, Microbiological Isolation, and Somatic Cell Count in Milk from Dairy Cows. Dairy 2025, 6, 45. https://doi.org/10.3390/dairy6040045
Cardozo LL, Knob DA, Santos PTd, Pelizza A, Mori AP, Camera M, Ferraz SM, de Assis MZ, Thaler Neto A. Relationship Between Hyperkeratosis, Teat Conformation Traits, Microbiological Isolation, and Somatic Cell Count in Milk from Dairy Cows. Dairy. 2025; 6(4):45. https://doi.org/10.3390/dairy6040045
Chicago/Turabian StyleCardozo, Leonardo Leite, Deise Aline Knob, Pauline Thais dos Santos, Angela Pelizza, Ana Paula Mori, Mauricio Camera, Sandra Maria Ferraz, Marcella Zampoli de Assis, and André Thaler Neto. 2025. "Relationship Between Hyperkeratosis, Teat Conformation Traits, Microbiological Isolation, and Somatic Cell Count in Milk from Dairy Cows" Dairy 6, no. 4: 45. https://doi.org/10.3390/dairy6040045
APA StyleCardozo, L. L., Knob, D. A., Santos, P. T. d., Pelizza, A., Mori, A. P., Camera, M., Ferraz, S. M., de Assis, M. Z., & Thaler Neto, A. (2025). Relationship Between Hyperkeratosis, Teat Conformation Traits, Microbiological Isolation, and Somatic Cell Count in Milk from Dairy Cows. Dairy, 6(4), 45. https://doi.org/10.3390/dairy6040045






